Abstract
Tuberculosis (TB) remains a major global health problem, and the incidence of TB cases has not significantly decreased over the past decade in Korea. The standard short course regimen is highly effective against TB, but requires multiple TB-specific drugs and a long treatment duration. Recent studies using late-generation fluoroquinolones and/or high-dose rifapentine-containing regimens to shorten the duration of TB treatment showed negative results. Extending the treatment duration may be considered in patients with cavitation on the initial chest radiograph and positivity in sputum culture at 2 months of treatment for preventing TB relapse. Current evidence does not support the use of fixed-dose combinations compared to separate drugs for the purpose of improving treatment outcomes. All patients receiving TB treatment should be monitored regularly for response to therapy, facilitation of treatment completion, and management of adverse drug reactions. Mild adverse effects can be managed with symptomatic therapy and changing the timing of the drug administration, but severe adverse effects require a discontinuation of the offending drugs.
Keywords: Tuberculosis, Antitubercular Agents, Fluoroquinolones, Combination Therapy, Drug-Related Side Effects and Adverse Reactions
Introduction
Tuberculosis (TB) remains a major global health problem, with an estimated 9 million incident cases and 1.5 million deaths in 2013 according to the World Health Organization (WHO)1. In Korea, the prevalence of bacteriologically or radiologically active TB (>5 years old) decreased dramatically from 5,168/100,000 persons in 1965 to 767/100,000 persons in 19952. However, TB remains a current major health concern in Korea, since new cases have plateaued at approximately 100/100,000 persons over the past decade3. One possible explanation for the steady rate of new TB cases in Korea is an increasing proportion of the elderly, who may be at a higher risk for TB development3. TB also has the highest mortality rate among the infectious diseases; it was 4.9/100,000 persons in 20123,4,5,6,7. In pulmonary TB patients, the mortality rate during TB treatment reached 3.4% in a recent multicenter study8.
Current TB drugs were developed 40 years ago and have a high treatment success rate if patients take TB drugs throughout the course of treatment9. However, current regimens include many TB drugs and require lengthy treatment durations. Therefore, there are challenges in the current TB treatment such as shortening the treatment duration, increasing adherence, and managing adverse drug effects. This article covers current TB treatment in patients with newly diagnosed drug-susceptible pulmonary TB based on the Korean Guidelines for Tuberculosis, second edition, 201410 and recent clinical studies for TB treatment8,11,12,13,14,15,16,17,18,19,20.
Principles of TB Chemotherapy
The overall goals for treatment of TB are (1) to cure the disease that could improve quality of life for individual patients, (2) to minimize transmission of Mycobacterium tuberculosis to other persons, and (3) to prevent a relapse of this disease and spread of drug-resistant TB10,21.
M. tuberculosis has characteristics distinct from other bacteria, including slow growth, with generation times of 15 to 20 hours and a capacity for dormancy that requires prolonged treatment to confirm successful eradication22. Moreover, this organism can actively multiply in the liquid caseous debris of pulmonary cavities, which may contain up to 108 organisms 22. Drug resistance could be achieved by random genetic point mutation; it is also possible that the large population of organisms in such cavities could have the potential for drug resistance22. Therefore, a combination of highly active agents against M. tuberculosis is needed for the successful eradication of these organisms in their various states, and for preventing drug resistance. However, the most important cause of TB therapy treatment failure is the development of drug resistance, which can be developed by irregular drug-taking, inadequate dosage, and single-drug treatment. Sufficiently long periods of TB treatment are also needed to eradicate the slow growing mycobacteria. Therefore, to achieve the overall TB treatment goal, the following principles should be followed: (1) combination therapy of more than three TB drugs for prevention of drug resistance, (2) once-daily administration of TB drugs and exact dosage for optimizing efficacy, and (3) taking TB drugs regularly for at least 6 months.
TB drugs are classified into five groups according to efficacy, potency, and drug class (Table 1)23. First-line TB drugs comprising group 1-except streptomycin, which is classified with the other injectable agents in group 2-are the most potent, have the best tolerance of all TB drugs, and are currently recommended in a four-drug combination for the treatment of drug-susceptible TB. A lower degree of efficacy and a higher degree of intolerability and toxicity have limited the use of second-line TB drugs for patients with drug-resistant TB or intolerance with first-line drugs.
Table 1. Groups, recommended dosage for daily administration, and adverse effects of tuberculosis drugs for adults23.
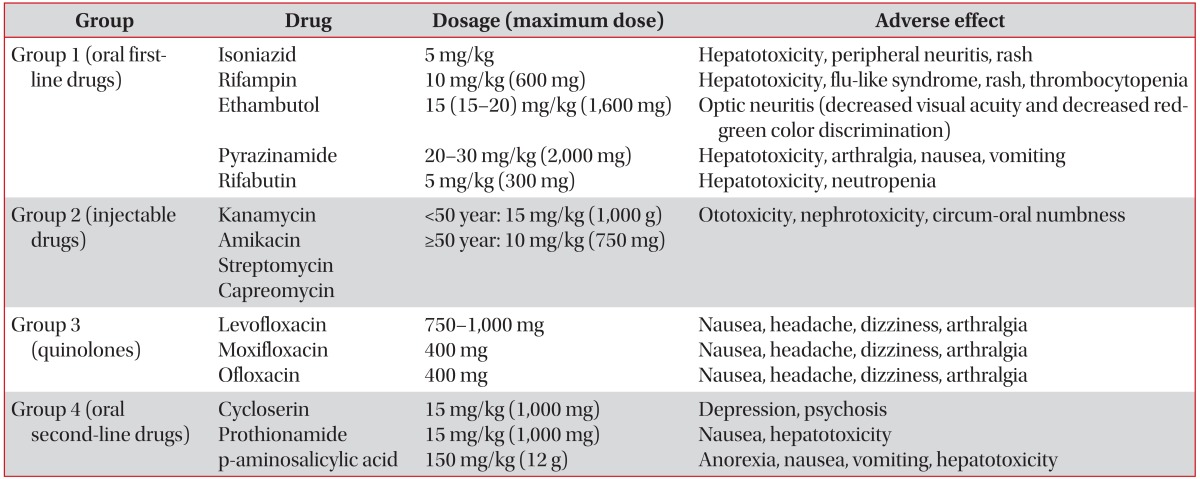
The standard short course regimen is a 2-month period of isoniazid (INH), rifampin (RIF), ethambutol (EMB), and pyrazinamide (PZA), followed by a 4-month period of INH, RIF, and EMB (2HREZ/4HR[E]). If the organism is susceptible to INH and RIF upon drug susceptibility testing, EMB can be discontinued. If PZA cannot be included in the initial phase of treatment, a combination of INH, RIF, and PZA should be administered for 9 months (9HRE).
The standard four-drug (2HREZ/4HR) regimen has shown high efficacy in achieving cure rates around 90%-95%, both in treatment under oversight of TB control programs and trial conditions24. A recent multicenter retrospective cohort study was conducted in Korea, including a total of 2,481 patients with pulmonary TB and excluding patients with treatment default, those who were transferred to another hospital, had unknown treatment outcome, and had positive human immunodeficiency virus antibodies; successful treatment was achieved for 2,333 patients (94%), who comprised those being cured (45.5%) and completing treatment (48.5%)8. However, the treatment success fate of all reported TB patients in Korea is still low, that is, less than 70%; this low success rate is mainly caused by patients with treatment default and for whom there is an unknown treatment outcome5. Therefore, to improve the treatment success rate in Korea, more efforts are necessary to reduce the number of patients with default and to improve the collection of accurate data regarding treatment outcomes in TB patients.
Optimal Duration of TB Treatment
The relapse rates for standard 6-month treatment of pulmonary TB were 1%-2% at 24 months after treatment, although the current TB regimen showed high efficacy25. It has been recommended that patients with risk factors for relapse including cavitation on chest radiography, extensive disease, immunosuppression, and positive sputum culture at 2 months of treatment may need a treatment extension of up to 9 months9. However, the evidence regarding efficacy of treatment extension is weak and there are no defined criteria for prolonging treatment to prevent relapse of TB. In a recent study performed in Korea, the combination of cavitation on initial chest radiography and positive culture after 2 months was associated with an increased risk of 1-year relapse, with the authors suggesting a treatment extension in these patients17. However, this was a single center retrospective study that included only 6 patients with TB relapse, and there was no proven efficacy of prolonged TB treatment for preventing relapse of TB in these patients. Further studies are needed to evaluate the efficacy of extending standard treatments. In the Korean Guidelines for Tuberculosis, based on the previous study, in cases with cavitations on the chest radiograph at the start of treatment and a positive culture after 2 months of treatment, extending standard treatment may be considered, but the total duration of treatment should be individualized according to the patient's condition10.
Shortening the treatment duration could be a big advancement in TB control. It could enhance adherence to TB treatment, decrease side effect rates, and reduce costs. A randomized, open-label equivalence trial evaluating the shortening of a standard treatment continuation period for 2 months (2 months of HREZ+2 months of HR) in patients with non-cavitary TB and negative sputum culture after 2 months showed significantly higher rates of relapse in a 4-month arm than a standard 6-month arm (7.0% vs. 1.6%; risk difference, 0.054; 95% confidence interval with Hauck-Anderson correction, 0.01-0.10)26. Failure to eradicate dormant bacilli in the use of current standard regimens might result in a higher relapse rate. Therefore, regimens successfully used to shorten the treatment duration need to focus on the introduction of new drugs with novel mechanisms of action11. Recently, three non-inferiority trials were performed to shorten standard therapy to 4 months using fluoroquinolones or higher doses of rifapentine (Table 2)11,12,13,14.
Table 2. Comparisons of current trials which aimed to shorten duration of TB treatment.
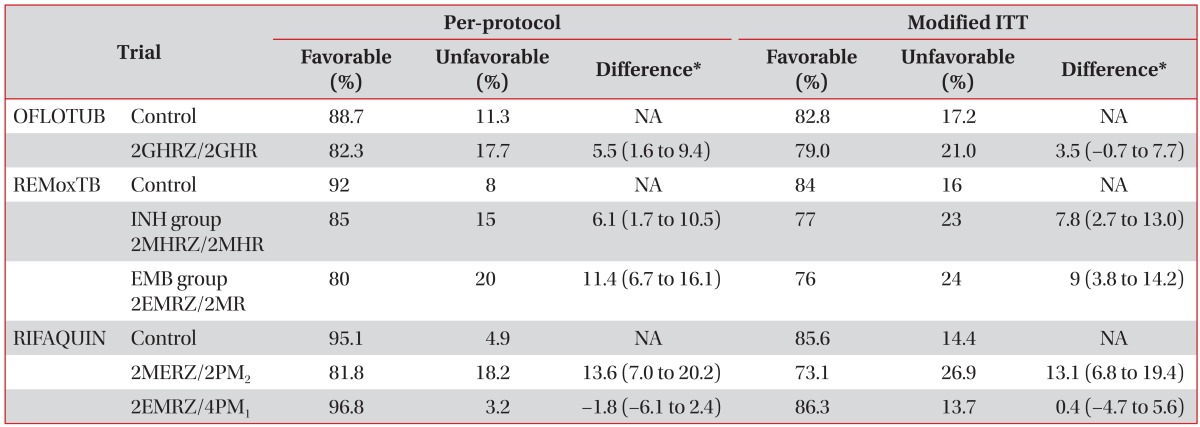
*Adjusted difference, experimental group-control group, percentage points (OFLOTUB, 95% CI; REMoxTB, 97.5% CI; RIFAQAUIN, 90% CI). TB: tuberculosis; ITT: intention-to-treat; Control: 2HREZ/4HR; G: gatifloxacin; H: isoniazid; R: rifampin; Z: pyrazinamide; NA: not applicable; INH: isoniazid; EMB: ethambutol; M: moxifloxacin; E: ethambutol; P: rifapentine; PM2: twice weekly moxifloxacin and rifapentine; PM1: once-weekly moxifloxacin and rifapentine; CI: confidence interval.
OFLOTUB, a randomized, open-label, controlled trial, compared the standard 6-month regimen (2HREZ/4HR) with a regimen in which gatifloxacin (400 mg once daily) was substituted for EMB during 4 months of treatment comprised of a 2-month intensive phase of gatifloxacin, INH, RIF, and PZA, followed by a 2-month maintenance phase of gatifloxacin, INH, and RIF (2GHRZ/2GHR)14. The primary endpoints at 24 months after the end of treatment were unfavorable outcomes including treatment failure, recurrence, and death or dropout during treatment, which did not meet the non-inferiority of the 4-month regimen compared to the standard regimen.
REMoxTB (a randomized, double-blind, placebo-controlled trial) is comparing standard 6-month therapy (control group) with two study groups: the INH group, in which EMB was replaced with moxifloxacin for 17 weeks, and the EMB group, in which INH was replaced with moxifloxacin for 17 weeks12. The primary outcome that included treatment failure or relapse within 18 months after randomization of this study also did not meet the non-inferiority of the 4-month trial regimens compared to the standard regimen. However, trial regimens showed a more rapid sputum-negative conversion in both solid and liquid culture media. There was no significant difference in the incidence of grade 3 or 4 adverse events, as reported in 127 patients (19%) in the INH group, 111 patients (17%) in the EMB group, and 123 patients (19%) in the control group.
RIFAQUIN (a randomized, controlled trial) compared the standard 6-month regimen with two other study regimens (4-month regimen: 2 months of daily EMB, moxifloxacin, RIF, and PZA followed by 2 months of twice-weekly moxifloxacin and rifapentine [900 mg]; 6-month regimen: 2 months of daily EMB, moxifloxacin, RIF, and PZA followed by 4 months of once-weekly moxifloxacin and rifapentine [1,200 mg])13. The 6-month regimen was as effective as the standard 6-month regimen, but the 4-month regimen was inferior to the standard.
Based on these three clinical trials, recommendations could not be made related to the use of fluoroquinolone and/or high-dose rifapentine-containing regimens in shortening the TB treatment duration by 2 months.
Fixed Dose Combination
One of the most important causes for TB treatment failure or development of drug resistance is non-adherence to TB drugs. It is reported that 20% to 50% of patients with TB treatment fail to complete treatment, and the consequences contribute to prolonged therapy, drug resistance, and death27. To improve adherence for TB treatment, direct observed treatment (DOT) is recommended21. DOT includes provision of anti-TB drugs directly to the patient and observation for patient intake. When DOT is not available, fixed dose combinations (FDCs) might be expected to improve a compliance of TB treatment and reduce a risk of drug resistance by preventing inappropriate drug selection and treatment of a single regimen23. The WHO and the International Union against Tuberculosis and Lung Disease (IUATLD) have recommended the use of these drugs since 199428,29. In a randomized study comparing FDCs and separate drugs, the use of FDCs is preferred because of the potential advantages associated with FDC administration compared with separate-drug formulations 15,18. However, these results do not fully demonstrate the non-inferiority of the FDCs compared with separate drugs using strict pre-trial definitions at both 18 months post-randomization and 30 months after treatment initiation. Moreover, the efficacy of FDCs is still controversial owing to the decreased bioavailability of rifampicin in FDCs30. A recent meta-analysis showed a trend toward higher risk of treatment failure or relapse with FDCs (pooled relative risk, 1.28; 95% confidence interval, 0.99-1.7) compared with separate drugs; there was no evidence that FDCs could improve patient compliance for TB drugs16. Given the possible risks, additional studies evaluating the efficacy of FDCs are warranted.
Monitoring of Treatment Response and Definition of Treatment Outcome
All patients who received TB treatment are recommended to undergo regular monitoring, not only for observing the response to therapy but also for facilitating treatment completion, and identifying and managing adverse drug reactions10,21,23. Monthly monitoring of patient weight and accordingly adjusting TB drug dosage upon weight change is also recommended10,23. To monitor pulmonary TB patients, sputum smears and culture examinations are recommended at monthly intervals until two consecutive culture specimens are negative at the end of treatment10,21. However, the response using sputum studies has its limitations, particularly in patients with smear- and culture-negative pulmonary TB. In these patients, chest radiography can provide information on the treatment response, but this alone is not recommended for monitoring the treatment response in pulmonary TB patients due to non-responsive fibrotic lesions to TB treatment and possible other causes of lung infiltration such as lung cancer, pneumonia, bronchiectasis, and hemorrhage10. Chest computed tomography (CT) has been acknowledged to be a more sensitive and specific tool for pulmonary TB compared to chest radiography; it is suggested as an appropriate tool for evaluating treatment responses and complications during treatment31,32,33,34,35,36. However, in a recent study including 66 pulmonary TB patients who had chest CT scans before and after TB treatment, 50% of all patients had significant residual lesions in chest CT scans after TB treatment, although these lesions had no evidence of persistent activity or the possibility of early relapse during the median 15 months of follow-up19.
The treatment outcome definitions were revised recently by WHO: "default" and "transfer out" were removed, and "lost to follow-up" and "not evaluated" were inserted37. Lost to follow-up included cases with no start of TB treatment in addition to cases with interrupting TB treatment for 2 consecutive months or more, and not evaluated included cases with unknown outcome in addition to cases where the patient was transferred to another treatment unit (Table 3)37.
Table 3. Revised definitions of treatment outcomes for drug-susceptible tuberculosis patients37.
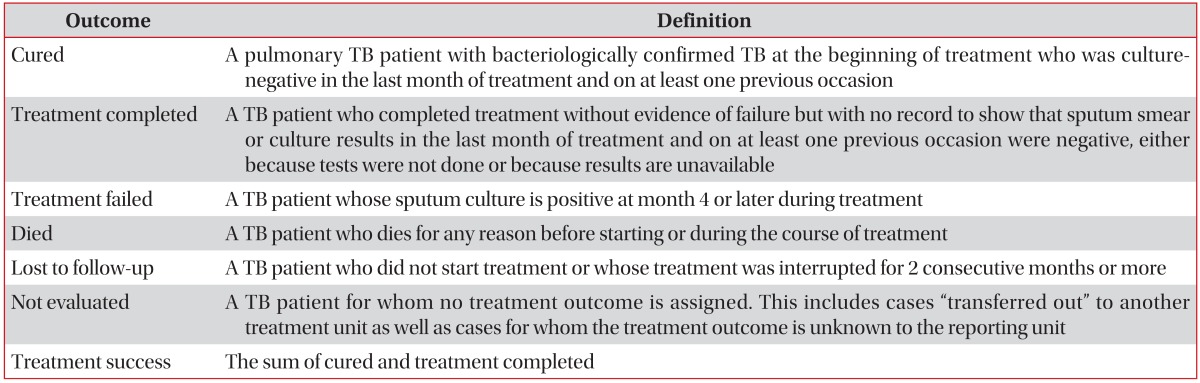
Adopted from World Health Organization (2013). Definitions and reporting framework for tuberculosis, 2013 revision: WHO/HTM/TB/2013.2 (available from: http://apps.who.int/iris/bit stream/10665/79199/1/9789241505345_eng.pdf), with permission of World Health Organization37.
TB: tuberculosis.
Management of Common Adverse Effects
Current combination chemotherapy for curing TB requires a lengthy treatment duration, and many TB drugs are associated with a high incidence of adverse effects. Mild adverse effects such as minor rash with itch, non-gouty polyarthralgia, and mild gastrointestinal upset including nausea, vomiting, poor appetite, and abdominal pain can generally be managed with symptomatic therapy and changing the timing of the drug administration. However, in severe adverse effects such as drug-induced hepatitis, RIF induced severe immunologic reactions including thrombocytopenia, hemolytic anemia, acute renal failure, and thrombotic thrombocytopenic purpura, EMB-induced optic toxicity, and PZA-induced acute gouty arthritis, offending drugs should be discontinued10,21.
Drug-induced hepatotoxicity is the most frequent adverse effect limiting the use of effective first-line TB drugs and inducing transfer to other TB drugs38. Alanine aminotransferase (ALT) level has been revised as more specific for drug-induced hepatotoxicity than aspartate aminotransferase, which can be also increased in abnormal conditions in muscle, heart, or kidney 38. If the ALT level is more than three times the upper limit of normal with symptoms, or more than five times the upper limit of normal without symptoms, three of the first-line anti-TB drugs, INH, RIF, and PZA should be discontinued; other causes of hepatitis such as viral hepatitis A, B, and C should be evaluated, in addition to intake of drugs other than those for TB, herbal medications, and alcohol. After the ALT has decreased to less than two times the upper limit of normal, first-line drugs can be reinitiated one at a time. Underlying chronic liver diseases have been contributing risk factors for TB drug-induced hepatitis, and patients with this disease might receive less effective non-hepatotoxic second-line TB drugs38,39. However, the relative risks according to various etiologies of chronic liver disease such as hepatitis A, B, and C infections, and degree of liver cirrhosis on the development of drug-induced hepatitis have not been well studied. Research conducted in Korea to address these issues revealed that patients with hepatitis B or C seropositivity, or without advanced liver cirrhosis (Child-Pugh score less than B), could be used for standard short course regimens including INH and RIF20,40,41.
Conclusion
Since the distinctive characteristics of M. tuberculosis include acquired drug resistance, slow growth rates, and dormancy, TB treatment requires a combination of many TB drugs and a lengthy treatment duration. However, most patients with drug-sensitive TB could be cured by a highly effective standard short course regimen if patients received a full course of TB therapy. Therefore, a clinician's efforts regarding appropriate management of adverse drug effects and treatment response monitoring are needed to prevent drug interruption. Recently, new regimens such as late-generation quinolones and high-dose rifapentine-containing regimens have been administered to reduce the duration of TB treatment, but the results of these studies were not successful. Further studies using new drugs with novel mechanisms of action are critical for shortening the treatment duration.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.World Health Organization. Global tuberculosis report 2014 [Internet] Geneva: World Health Organization; 2014. [cited 2014 Mar 1]. Available from: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf. [Google Scholar]
- 2.Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998;2:27–36. [PubMed] [Google Scholar]
- 3.Park YK, Park YS, Na KI, Cho EH, Shin SS, Kim HJ. Increased tuberculosis burden due to demographic transition in Korea from 2001 to 2010. Tuberc Respir Dis. 2013;74:104–110. doi: 10.4046/trd.2013.74.3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis patients in Korea 2012 [Internet] Cheongwon: Korea Centers for Disease Control and Prevention; 2013. [cited 2014 Mar 1]. Available from: http://tbfree.cdc.go.kr/tbfree/cmm/BoardList.do?boardType=REPORT&id=4500. [Google Scholar]
- 5.Park YS, Hong SJ, Boo YK, Hwang ES, Kim HJ, Cho SH, et al. The national status of tuberculosis using nationwide medical records survey of patients with tuberculosis in Korea. Tuberc Respir Dis. 2012;73:48–55. doi: 10.4046/trd.2012.73.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ. Current status of tuberculosis in Korea. Korean J Med. 2012;82:257–262. [Google Scholar]
- 7.Statistics Korea. Annual report of deaths by cause 2012 [Internet] Daejeon: Statistics Korea; 2013. [cited 2014 Mar 1]. Available from: http://kosis.kr/ups/ups_01List01.jsp?grp_no=1005&pubcode=YD&type=F. [Google Scholar]
- 8.Kwon YS, Kim YH, Song JU, Jeon K, Song J, Ryu YJ, et al. Risk factors for death during pulmonary tuberculosis treatment in Korea: a multicenter retrospective cohort study. J Korean Med Sci. 2014;29:1226–1231. doi: 10.3346/jkms.2014.29.9.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med. 2013;368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 10.Joint Committee for the Revision of Korean Guidelines for Tuberculosis; Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed. Seoul and Cheongwon: Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korean Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 11.Kwon YS, Jeong BH, Koh WJ. Tuberculosis: clinical trials and new drug regimens. Curr Opin Pulm Med. 2014;20:280–286. doi: 10.1097/MCP.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med. 2014;371:1577–1587. doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jindani A, Harrison TS, Nunn AJ, Phillips PP, Churchyard GJ, Charalambous S, et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med. 2014;371:1599–1608. doi: 10.1056/NEJMoa1314210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merle CS, Fielding K, Sow OB, Gninafon M, Lo MB, Mthiyane T, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med. 2014;371:1588–1598. doi: 10.1056/NEJMoa1315817. [DOI] [PubMed] [Google Scholar]
- 15.Lienhardt C, Cook SV, Burgos M, Yorke-Edwards V, Rigouts L, Anyo G, et al. Efficacy and safety of a 4-drug fixed-dose combination regimen compared with separate drugs for treatment of pulmonary tuberculosis: the Study C randomized controlled trial. JAMA. 2011;305:1415–1423. doi: 10.1001/jama.2011.436. [DOI] [PubMed] [Google Scholar]
- 16.Albanna AS, Smith BM, Cowan D, Menzies D. Fixed-dose combination antituberculosis therapy: a systematic review and meta-analysis. Eur Respir J. 2013;42:721–732. doi: 10.1183/09031936.00180612. [DOI] [PubMed] [Google Scholar]
- 17.Jo KW, Yoo JW, Hong Y, Lee JS, Lee SD, Kim WS, et al. Risk factors for 1-year relapse of pulmonary tuberculosis treated with a 6-month daily regimen. Respir Med. 2014;108:654–659. doi: 10.1016/j.rmed.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Nunn AJ, Cook SV, Burgos M, Rigouts L, Yorke-Edwards V, Anyo G, et al. Results at 30 months of a randomised trial of FDCs and separate drugs for the treatment of tuberculosis. Int J Tuberc Lung Dis. 2014;18:1252–1254. doi: 10.5588/ijtld.14.0281. [DOI] [PubMed] [Google Scholar]
- 19.Seon HJ, Kim YI, Lim SC, Kim YH, Kwon YS. Clinical significance of residual lesions in chest computed tomography after anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2014;18:341–346. doi: 10.5588/ijtld.13.0565. [DOI] [PubMed] [Google Scholar]
- 20.Shin HJ, Lee HS, Kim YI, Lim SC, Jung JP, Ko YC, et al. Hepatotoxicity of anti-tuberculosis chemotherapy in patients with liver cirrhosis. Int J Tuberc Lung Dis. 2014;18:347–351. doi: 10.5588/ijtld.13.0545. [DOI] [PubMed] [Google Scholar]
- 21.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 22.Schlossberg D. Tuberculosis and nontuberculosis mycobacterial infections. Washington, DC: ASM Press; 2011. [Google Scholar]
- 23.World Health Organization. Treatment of tuberculosis: guidelines for national programmes. 4th ed. Geneva: World Health Organization; 2009. WHO/HTM/TB/2009.420. [Google Scholar]
- 24.Combs DL, O'Brien RJ, Geiter LJ. USPHS Tuberculosis Short-Course Chemotherapy Trial 21: effectiveness, toxicity, and acceptability. The report of final results. Ann Intern Med. 1990;112:397–406. doi: 10.7326/0003-4819-76-3-112-6-397. [DOI] [PubMed] [Google Scholar]
- 25.Chang KC, Leung CC, Yew WW, Ho SC, Tam CM. A nested case-control study on treatment-related risk factors for early relapse of tuberculosis. Am J Respir Crit Care Med. 2004;170:1124–1130. doi: 10.1164/rccm.200407-905OC. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JL, Hadad DJ, Dietze R, Maciel EL, Sewali B, Gitta P, et al. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. Am J Respir Crit Care Med. 2009;180:558–563. doi: 10.1164/rccm.200904-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuneo WD, Snider DE., Jr Enhancing patient compliance with tuberculosis therapy. Clin Chest Med. 1989;10:375–380. [PubMed] [Google Scholar]
- 28.The promise and reality of fixed-dose combinations with rifampicin. A joint statement of the International Union Against Tuberculosis and Lung Disease and the Tuberculosis Programme of the World Health Organization. Tuber Lung Dis. 1994;75:180–181. doi: 10.1016/0962-8479(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 29.Blomberg B, Spinaci S, Fourie B, Laing R. The rationale for recommending fixed-dose combination tablets for treatment of tuberculosis. Bull World Health Organ. 2001;79:61–68. [PMC free article] [PubMed] [Google Scholar]
- 30.Milan-Segovia RC, Dominguez-Ramirez AM, Jung-Cook H, Magana-Aquino M, Romero-Mendez MC, Medellin-Garibay SE, et al. Relative bioavailability of rifampicin in a three-drug fixed-dose combination formulation. Int J Tuberc Lung Dis. 2010;14:1454–1460. [PubMed] [Google Scholar]
- 31.Im JG, Itoh H, Shim YS, Lee JH, Ahn J, Han MC, et al. Pulmonary tuberculosis: CT findings--early active disease and sequential change with antituberculous therapy. Radiology. 1993;186:653–660. doi: 10.1148/radiology.186.3.8430169. [DOI] [PubMed] [Google Scholar]
- 32.Kim WS, Moon WK, Kim IO, Lee HJ, Im JG, Yeon KM, et al. Pulmonary tuberculosis in children: evaluation with CT. AJR Am J Roentgenol. 1997;168:1005–1009. doi: 10.2214/ajr.168.4.9124105. [DOI] [PubMed] [Google Scholar]
- 33.McGuinness G, Naidich DP, Jagirdar J, Leitman B, McCauley DI. High resolution CT findings in miliary lung disease. J Comput Assist Tomogr. 1992;16:384–390. doi: 10.1097/00004728-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Pastores SM, Naidich DP, Aranda CP, McGuinnes G, Rom WN. Intrathoracic adenopathy associated with pulmonary tuberculosis in patients with human immunodeficiency virus infection. Chest. 1993;103:1433–1437. doi: 10.1378/chest.103.5.1433. [DOI] [PubMed] [Google Scholar]
- 35.Hulnick DH, Naidich DP, McCauley DI. Pleural tuberculosis evaluated by computed tomography. Radiology. 1983;149:759–765. doi: 10.1148/radiology.149.3.6647852. [DOI] [PubMed] [Google Scholar]
- 36.Lee JJ, Chong PY, Lin CB, Hsu AH, Lee CC. High resolution chest CT in patients with pulmonary tuberculosis: characteristic findings before and after antituberculous therapy. Eur J Radiol. 2008;67:100–104. doi: 10.1016/j.ejrad.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Definitions and reporting framework for tuberculosis, 2013 revision: WHO/HTM/TB/2013.2 [Internet] Geneva: World Health Organization; 2013. [cited 2015 Mar 3]. Available from: http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf. [Google Scholar]
- 38.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 39.Kang YA. Tuberculosis treatment in patients with comorbidities. Tuberc Respir Dis. 2014;76:257–260. doi: 10.4046/trd.2014.76.6.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee BH, Koh WJ, Choi MS, Suh GY, Chung MP, Kim H, et al. Inactive hepatitis B surface antigen carrier state and hepatotoxicity during antituberculosis chemotherapy. Chest. 2005;127:1304–1311. doi: 10.1378/chest.127.4.1304. [DOI] [PubMed] [Google Scholar]
- 41.Kwon YS, Koh WJ, Suh GY, Chung MP, Kim H, Kwon OJ. Hepatitis C virus infection and hepatotoxicity during antituberculosis chemotherapy. Chest. 2007;131:803–808. doi: 10.1378/chest.06-2042. [DOI] [PubMed] [Google Scholar]


