Abstract
Background
A relationship between low vitamin D levels and the development or outcomes of respiratory diseases has been identified. However, there is no data on the vitamin D status in patients with acute eosinophilic pneumonia (AEP). We evaluated the vitamin D status in patients with AEP among South Korean military personnel.
Methods
We prospectively compared the serum levels of total 25-hydroxyvitamin D [25(OH)D], 25(OH)D3, and 25(OH)D2 among patients with AEP, pulmonary tuberculosis (PTB), and community-acquired pneumonia (CAP).
Results
In total, 65 patients with respiratory diseases, including AEP (n=24), PTB (n=19), and CAP (n=22), were identified. Of the 24 patients with AEP, 2 (8%) had deficient total 25(OH)D levels (<10 ng/mL), 17 (71%) had insufficient total 25(OH)D levels (≥10 to <30 ng/mL), and only 5 (21%) had sufficient total 25(OH)D levels (≥30 to <100 ng/mL). The difference in the total 25(OH)D levels among patients with AEP, PTB, and CAP was not statistically significant (p=0.230). The median levels of total 25(OH)D, 25(OH)D3, and 25(OH)D2 were 22.84, 22.84, and 0.00 ng/mL, respectively, and no differences in the 25(OH)D level were present among patients with AEP, PTB, and CAP with the exception of the total 25(OH)D level between patients with AEP and PTB (p=0.042).
Conclusion
We have shown that low vitamin D levels are frequently found in patients with AEP and are comparable with those in patients with PTB and CAP.
Keywords: Deficiency, Pulmonary Eosinophilia, Pneumonia, Vitamin D
Introduction
Vitamin D is a prohormone important in bone mineralization and calcium homeostasis. Dermal synthesis after ultraviolet radiation exposure is the major source of vitamin D; a minority of vitamin D comes from dietary sources. Vitamin D3, or cholecalciferol, is formed when ultraviolet radiation converts 7-dehydrocholesterol in epidermal keratinocytes and dermal fibroblasts to previtamin D. Vitamin D2, or ergocalciferol, is formed when ergosterol in plants is exposed to irradiation and is frequently found in plant dietary sources. Both of these forms of vitamin D are converted in the liver to 25-hydroxyvitamin D [25(OH)D], the major circulating form of vitamin D and a good indicator of an individual's vitamin D status. It is then converted in the kidney to 1,25(OH)D, the biologically active form1.
Recent studies on the role of vitamin D in human disease have consistently suggested the presence of an association between low vitamin D levels and the development of various respiratory diseases, including pulmonary tuberculosis (PTB)2,3,4, respiratory tract infections5,6,7,8, chronic obstructive lung disease9,10, and asthma11. In addition, molecular studies have also suggested that the vitamin D may be a potent regulator in the inflammatory process in various diseases such as autoimmune encephalomyelitis12 and inflammatory bowel disease13, and it may repress production of inflammatory cytokines in T helper cells, including interferon-γ, interleukin (IL)-2, and IL-514.
Acute eosinophilic pneumonia (AEP)15,16,17 is an uncommon inflammatory lung disease of unknown etiology characterized by acute respiratory or systemic symptoms, diffuse radiographic pulmonary infiltrates, and infiltration of eosinophils into the lung18, and specific cytokines including IL-5 plays important roles in the eosinophil recruitment and in maintaining active inflammatory processes19. However, no data on the vitamin D status in patients with AEP is currently available. Therefore, we prospectively compared the serum levels of vitamin D in South Korean military personnel with AEP with those of patients with PTB and community-acquired pneumonia (CAP), both of which are known to be associated with low vitamin D levels. Our goal was to identify a possible association between low vitamin D levels and the development of AEP.
Materials and Methods
1. Study patients
Consecutive adult patients newly diagnosed with AEP, PTB, or CAP were prospectively screened and recruited from 1 May 2014 to 1 August 2014 at the Armed Forces Capital Hospital (874-bed military referral hospital) in Gyeonggi Province, South Korea.
A definitive diagnosis of AEP was based on a modification of criteria proposed by Philit et al.18 as previously reported16: (1) acute onset of febrile respiratory manifestations <1 month in duration regardless of hypoxemia, (2) bilateral diffuse infiltrates on chest radiography or computed tomography, (3) >25% eosinophils on bronchoalveolar lavage or eosinophilic pneumonia on lung biopsy, and (4) absence of known causes of pulmonary eosinophilia, including drugs, toxins, and infections.
The diagnosis of PTB was considered to be definitive if (1) microbiological testing (acid-fast bacillus staining, polymerase chain reaction, or culture) using lower respiratory tract specimens confirmed infection with Mycobacterium tuberculosis or (2) the patient's clinical presentation met the criteria for clinical active PTB established by the World Health Organization20,21. CAP was defined as the presence of a new infiltrate on chest radiography plus at least one of the following: (1) fever (body temperature of ≥38.0℃) or hypothermia (body temperature of <35.0℃); (2) altered breath sounds on auscultation; or (3) respiratory symptoms including cough, sputum, pleuritic chest pain, or dyspnea22.
Samples for measurement of the serum 25(OH)D level was performed after the study patients had provided written informed consent. The present study was approved by the Armed Forces Capital Hospital Institutional Review Board, which permitted review and publication of patient records. This work was supported by the Korean Military Medical Research Project by ROK Ministry of National Defense (ROK-MND-2014-KMMRP-021).
2. Patient management and data collection
All study patients were treated appropriately according to their respiratory disease: a 2-week course of corticosteroids was given to patients with AEP16,17, anti-tuberculosis drugs were given to patients with PTB, and empirical antibiotics were given to patients with CAP. Peripheral blood samples for measurement of the 25(OH)D level were obtained from patients diagnosed with AEP, PTB, and CAP before starting treatment, and the total levels of 25(OH)D, 25(OH)D3, and 25(OH)D2 were evaluated. The degree of total 25(OH)D was classified as deficient (<10 ng/mL), insufficient (≥10 to <30 ng/mL), or sufficient (≥30 to <100 ng/mL). The serum levels of total 25(OH)D, 25(OH)D3, and 25(OH)D2 were compared among patients with AEP, PTB, and CAP.
Data on the patients' baseline characteristics, body mass index, underlying conditions, and laboratory findings including the white blood cell count, C-reactive protein level, and erythrocyte sedimentation rate were also collected. Follow-up data were last obtained on 1 August 2014.
3. Measurement of serum 25(OH)D level
1) Reagents and calibration materials
We used a multilevel serum calibrator set as a calibrator and MassCheck 25(OH)D3/D2 serum control as the internal quality control material (Chromsystems, Munich, Germany). 25(OH)D2/D3 (IsoScience, Trevose, PA, USA) was used as the internal standard and was diluted with methanol: isopropanol (8:2, v:v). Other reagents and organic solvents were of high-performance liquid chromatography grade.
2) Sample processing
In total, 150 µL of blood samples, quality control materials, or correction material and 20 µL of internal standard were mixed; 150 µL of 0.2 M ZnSO4 were then added, and the mixture was shaken in a vortex mixer for 10 seconds. Next, 300 µL of methanol were added and the serum proteins were precipitated. Hexane (750 µL) was added and shaken in a vortex mixer for 30 seconds. After 5 minutes of centrifugation, the supernatant was transferred to a Waters Maximum Recovery Vial (Waters, Manchester, UK) and dried at 50℃ in nitrogen. Finally, 75 µL of 70% methanol were added and dissolved, and 20 µL of the mixture were injected into a liquid chromatography-mass spectrometry (LC-MS/MS) system.
3) Ultra performance LC-MS/MS
An ACQUITY ultra-performance LC system (Waters, Milford, MA, USA) with a BEH phenyl column (2.1×50.0 mm, 1.7 µm) was used, and the test was performed under the following conditions: dose, 20 µL; analysis time, 5 minutes; flow rate, 0.45 mL/min; mobile phase A, 2 mM ammonium acetate with 0.1% formic acid solution (distilled water); and mobile phase B, 2 mM ammonium acetate with 0.1% formic acid (methanol). The ACQUITY TQD was used, and the test was performed under the following conditions: electrospray ionization, positive ion mode, and multiple reaction monitoring. All analyses of the measured data were performed using the QuanLynx 4.0 software.
4. Statistical analysis
Data are presented as medians (interquartile range, IQR) for continuous variables and as numbers (percentage) for categorical variables. Data were compared using the Mann-Whitney U test for continuous variables and the chi-squared or Fisher exact test for categorical variables. All statistical analyses were performed using the PASW software ver. 18.0 (SPSS Inc., Chicago, IL, USA). A two-sided p-value of <0.05 was considered to indicate statistical significance.
Results
1. Baseline patient characteristics
In total, 65 patients with respiratory diseases, including AEP (n=24), PTB (n=19), and CAP (n=22) were identified during the study period. The infectious etiologies of CAP and PTB were microbiologically identified in 14 (64%) and 11 (58%) patients, respectively. Baseline characteristics of the study patients are shown in Table 1. All patients were young males. The median ages were 20, 21, and 21 years in patients with AEP, PTB, and CAP, respectively, and the median age of patients with AEP tended to be higher than that of patients with PTB and CAP. All patients with AEP were current smokers, and their median body mass index was highest among all three respiratory disease groups. Among the patients with CAP, one patient had asthma and one had bronchiectasis; one patient with PTB had concurrent intestinal tuberculosis. The white blood cell count and C-reactive protein level were significantly higher in patients with AEP, with a median of 15,675/µL (IQR, 12,705-18,765/µL) and 9.35 mg/dL (5.27-13.69 mg/dL), respectively. The results of the electrolyte tests, including the phosphate and calcium levels, in all study patients were within the reference ranges.
Table 1. Baseline characteristics of the study patients.
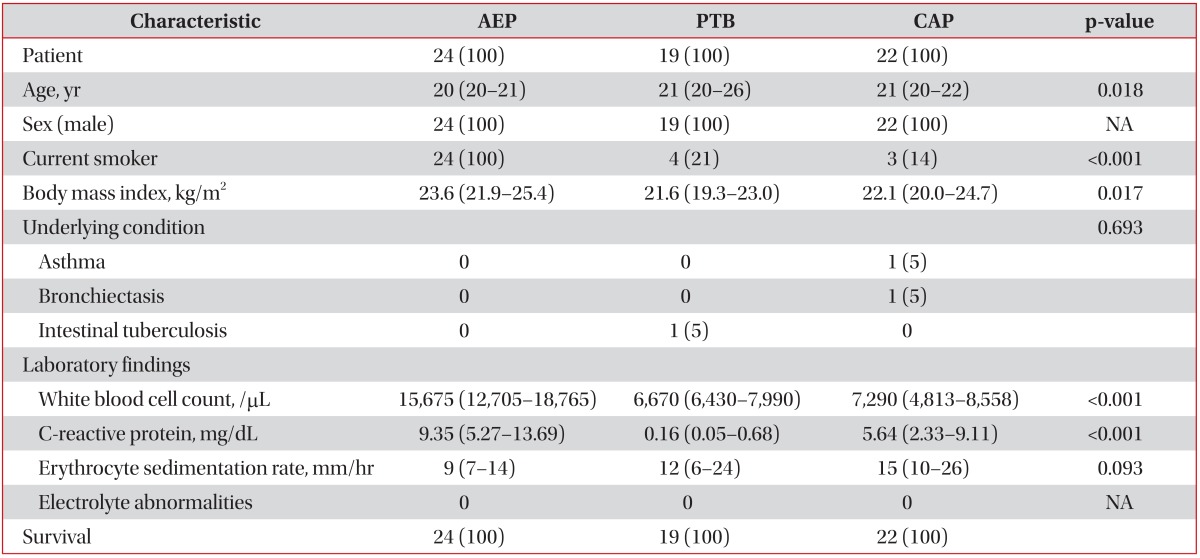
Values are presented as median (interquartile range) or number (%).
AEP: acute eosinophilic pneumonia; PTB: pulmonary tuberculosis; CAP: community acquired pneumonia; NA: not applicable.
2. Comparisons of serum 25(OH)D levels in the study patients
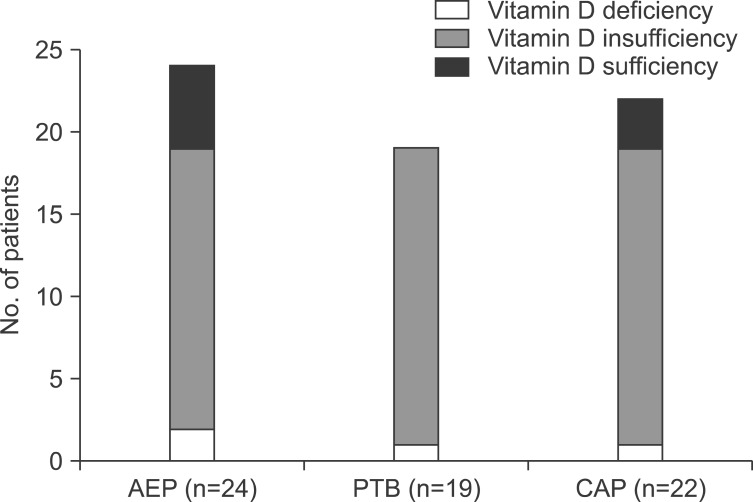
Serum 25(OH)D levels according to the different respiratory diseases are shown in Table 2. Of the 24 patients with AEP, 2 (8%), 17 (71%), and five (21%) had deficient, insufficient, and sufficient total 25(OH)D levels, respectively. Of the 19 patients with PTB, one (5%) had deficient total 25(OH)D levels and the others (n=18, 95%) had insufficient levels. Of the 22 patients with CAP, one (4%), 18 (82%), and three (14%) had deficient, insufficient, and sufficient total 25(OH)D levels, respectively (Figure 1). The differences in the proportions of degree of the serum total 25(OH)D levels among patients with AEP, PTB, and CAP were not statistically significant (p=0.230).
Table 2. Comparisons of serum 25(OH)D levels in the study patients.
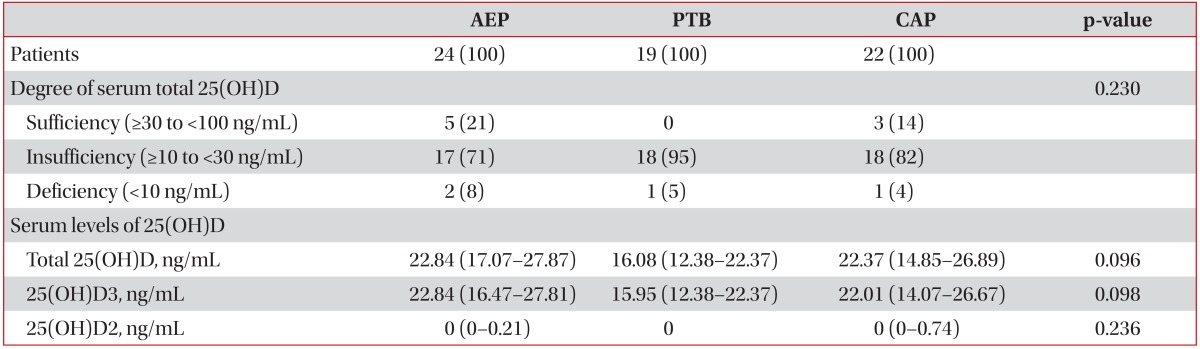
Values are presented as median (interquartile range) or number (%).
AEP: acute eosinophilic pneumonia; PTB: pulmonary tuberculosis; CAP: community acquired pneumonia.
Figure 1. Degree of serum total 25(OH)D levels among patients with acute eosinophilic pneumonia (AEP), pulmonary tuberculosis (PTB), and community-acquired pneumonia (CAP).
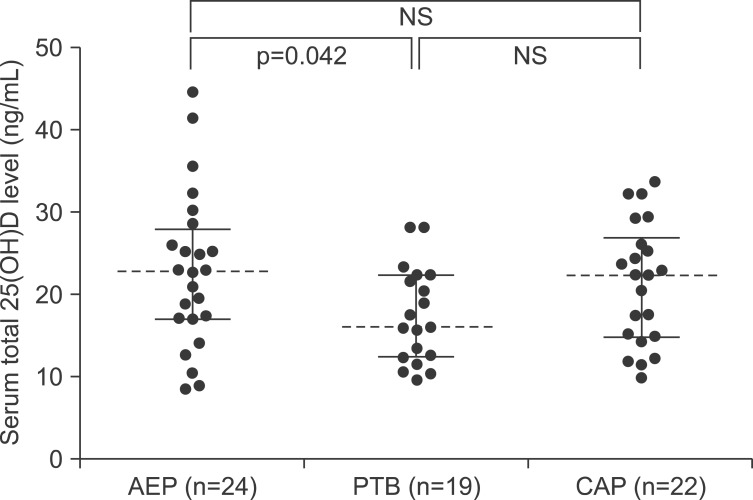
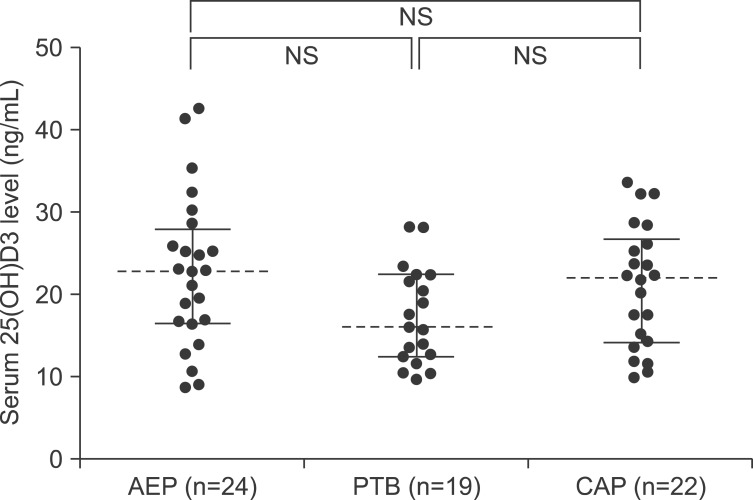
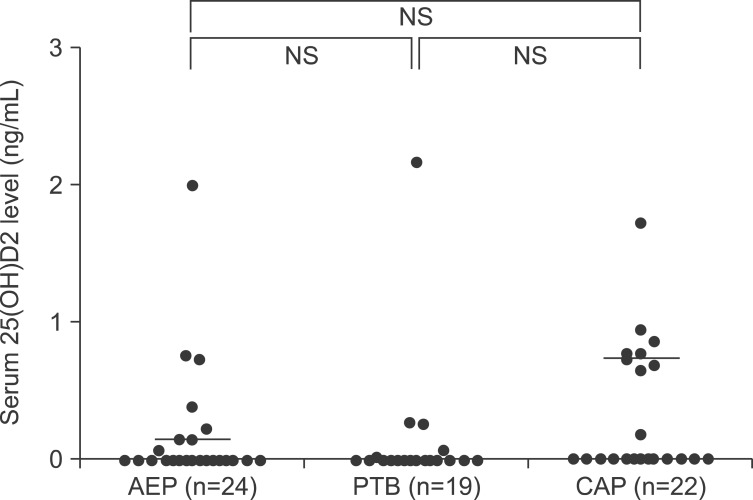
The median serum levels of total 25(OH)D were 22.84 ng/mL (IQR, 17.07-27.87), 16.08 ng/mL (IQR, 12.38-22.37), and 22.37 ng/mL (IQR, 14.85-26.89) in patients with AEP, PTB, and CAP, respectively. The median serum levels of 25(OH)D3 were 22.84 ng/mL (IQR, 16.47-27.81), 15.95 ng/mL (IQR, 12.38-22.37), and 22.01 ng/mL (IQR, 14.07-26.67) in patients with AEP, PTB, and CAP, respectively. The median serum levels of 25(OH)D2 were 0.00 ng/mL (IQR, 0.00-0.21), 0.00 ng/mL (IQR, 0.00-0.00), and 0.00 ng/mL (IQR, 0.00-0.74) in patients with AEP, PTB, and CAP, respectively. The serum 25(OH)D2 levels were below the detection limit in 15 (63%), 15 (79%), and 13 (59%) of patients with AEP, PTB, and CAP, respectively.
We also compared the total 25(OH)D (Figure 2), 25(OH)D3 (Figure 3), and 25(OH)D2 (Figure 4) levels among the study patients with AEP, PTB, and CAP. There were no significant differences in the 25(OH)D levels among the study patients, with the exception of the total 25(OH)D level between patients with AEP and PTB (p=0.042) (Figure 2).
Figure 2. Comparisons of serum total 25(OH)D levels among patients with acute eosinophilic pneumonia (AEP), pulmonary tuberculosis (PTB), and community-acquired pneumonia (CAP). NS: not significant.
Figure 3. Comparisons of serum 25(OH)D3 levels among patients with acute eosinophilic pneumonia (AEP), pulmonary tuberculosis (PTB), and community-acquired pneumonia (CAP). NS: not significant.
Figure 4. Comparisons of serum 25(OH)D2 levels among patients with acute eosinophilic pneumonia (AEP), pulmonary tuberculosis (PTB), and community-acquired pneumonia (CAP). NS: not significant.
Discussion
In the present study, we compared the vitamin D status among South Korean military personnel with AEP, PTB, and CAP. Most patients with AEP (n=19, 79%) had insufficient or deficient serum total 25(OH)D levels, and the serum levels of both 25(OH)D3 and 25(OH)D2 in patients with AEP were as low as those of patients with PTB and CAP, without significant differences. This result was observed despite the fact that all patients with AEP were young, previously healthy males with high physical activity levels and that it was late spring and summer in South Korea, which would have allowed for adequate ultraviolet radiation exposure.
Studies on the role of vitamin D in respiratory diseases have consistently indicated that low vitamin D levels may be associated with a higher incidence, greater severity, or poorer treatment responses in various respiratory diseases, including PTB2,3,4,23, respiratory tract infections5,6,7,8,24, chronic obstructive lung disease9,10, and asthma11. In PTB, for example, several candidate polymorphisms of the vitamin D receptor and vitamin D binding protein that modulate the development of tuberculosis have been identified. Another study of 259 patients with PTB showed that supplementation with high doses of vitamin D accelerated clinical and radiographic improvement3, suggesting that vitamin D supplementation enhances primarily innate responses to mycobacterial infection. Furthermore, an association between a higher risk of upper respiratory infections and low vitamin D levels has been identified5,8,25, and a recent clinical investigation showed that high vitamin D levels are associated with better lung function, less airway hyper-responsiveness, and improved corticosteroid responses in patients with asthma11. To our knowledge, however, no reported studies have focused on the serum vitamin D levels of patients with AEP. In this context, our data suggest a possible association between low vitamin D levels and the development of or increased vulnerability to AEP.
AEP is an uncommon inflammatory lung disease of unknown etiology. All patients exhibit typical clinical features including acute-onset respiratory distress with fever, diffuse pulmonary infiltration, and elevated proportions of eosinophils in bronchoalveolar lavage fluid, but the detailed pathophysiology of the disease remains unknown. Some previous studies suggested that a hypersensitivity reaction to an unidentified inhaled antigen could be a part of the inflammatory process26,27,28,29 and demonstrated that several cytokines are associated with eosinophil recruitment in AEP30,31. However, no risk factors for the development of, or increased susceptibility to, AEP have been identified. Given the possible roles of vitamin D in inflammation and immune modulation in patients with respiratory diseases32,33, our data on the high incidence of low vitamin D levels in patients with AEP suggest that low vitamin D levels are associated with AEP. However, data on the association between vitamin D levels and the inflammatory process of AEP are lacking; further studies are needed.
We also evaluated the serum levels of 25(OH)D3, 25(OH)D2, and total 25(OH)D to determine the proportions from dietary sources; we then compared the 25(OH)D3 and 25(OH)D2 levels among the study patients with various respiratory diseases. Interestingly, the serum 25(OH)D2 levels were below the detection limit in most patients regardless of the specific respiratory disease (15 [63%], 15 [79%], and 13 [59%] patients with AEP, PTB, and CAP, respectively), and there were no significant differences in the 25(OH)D3 and 25(OH)D2 levels among the patient groups (Figures 3, 4). Although these findings may be understandable given that dermal synthesis is the major natural source of vitamin D and <10% of vitamin D comes from dietary sources, limited data exist on the 25(OH)D3 and 25(OH)D2 levels or differences between the 25(OH) D3 and 25(OH)D2 levels in patients with respiratory disease. Thus, further studies on the clinical implications of 25(OH)D3 and 25(OH)D2 in patients with respiratory disease may help to understand the mechanisms of vitamin D in respiratory disease.
This study had important limitations. First, there was no healthy control group. Although recent one study have indicated vitamin D deficiency rate of 47.3% in 3,047 males and 65.0% in age of 20-29 in Korean populations34, which were lower than that of our pilot study patients, clinical characteristics of our study patients may be different in terms of age, physical activity levels, ultraviolet radiation exposure, and diet, because our study was conducted at a military hospital. Thus, based on the present study, further well-controlled studies are needed. Second, only a small number of patients were enrolled because of the rarity of the diseases studied and the nature of pilot studies.
In summary, we investigated the vitamin D status in patients with AEP and showed that low vitamin D levels are frequently found in patients with AEP and are comparable with those in patients with PTB and CAP. Further large-scale studies on the relationship between vitamin D deficiency and AEP are needed.
Acknowledgements
This work was supported by the Korean Military Medical Research Project by ROK Ministry of National Defense (ROK-MND-2014-KMMRP-021).
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Park JS, Cho YJ, Yoon HI, Song JH, Lee CT, et al. Low serum 25-hydroxyvitamin D level: an independent risk factor for tuberculosis? Clin Nutr. 2014;33:1081–1086. doi: 10.1016/j.clnu.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis'. BMC Infect Dis. 2013;13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong JY, Kim SY, Chung KS, Kim EY, Jung JY, Park MS, et al. Association between vitamin D deficiency and tuberculosis in a Korean population. Int J Tuberc Lung Dis. 2014;18:73–78. doi: 10.5588/ijtld.13.0536. [DOI] [PubMed] [Google Scholar]
- 5.Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol. 2013;136:321–329. doi: 10.1016/j.jsbmb.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Bergman P, Lindh AU, Bjorkhem-Bergman L, Lindh JD. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8:e65835. doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aregbesola A, Voutilainen S, Nurmi T, Virtanen JK, Ronkainen K, Tuomainen TP. Serum 25-hydroxyvitamin D3 and the risk of pneumonia in an ageing general population. J Epidemiol Community Health. 2013;67:533–536. doi: 10.1136/jech-2012-202027. [DOI] [PubMed] [Google Scholar]
- 8.Remmelts HH, van de Garde EM, Meijvis SC, Peelen EL, Damoiseaux JG, Grutters JC, et al. Addition of vitamin D status to prognostic scores improves the prediction of outcome in community-acquired pneumonia. Clin Infect Dis. 2012;55:1488–1494. doi: 10.1093/cid/cis751. [DOI] [PubMed] [Google Scholar]
- 9.Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179:630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 10.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 11.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126:52–58.e5. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 14.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 15.Lee JE, Rhee CK, Lim JH, Lee SM, Shim YS, Lee CT, et al. Fraction of exhaled nitric oxide in patients with acute eosinophilic pneumonia. Chest. 2012;141:1267–1272. doi: 10.1378/chest.11-1303. [DOI] [PubMed] [Google Scholar]
- 16.Rhee CK, Min KH, Yim NY, Lee JE, Lee NR, Chung MP, et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013;41:402–409. doi: 10.1183/09031936.00221811. [DOI] [PubMed] [Google Scholar]
- 17.Jhun BW, Kim SJ, Kim K, Lee JE. Clinical implications of initial peripheral eosinophilia in acute eosinophilic pneumonia. Respirology. 2014;19:1059–1065. doi: 10.1111/resp.12342. [DOI] [PubMed] [Google Scholar]
- 18.Philit F, Etienne-Mastroianni B, Parrot A, Guerin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med. 2002;166:1235–1239. doi: 10.1164/rccm.2112056. [DOI] [PubMed] [Google Scholar]
- 19.Jhun BW, Kim SJ, Kim K, Lee JE, Hong DJ. Clinical implications of correlation between peripheral eosinophil count and serum levels of IL-5 and tryptase in acute eosinophilic pneumonia. Respir Med. 2014;108:1655–1662. doi: 10.1016/j.rmed.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Treatment of tuberculosis: guidelines for national programmes. 3rd ed. Geneva: World Health Organization; 2003. [Google Scholar]
- 21.Lee SW, Jang YS, Park CM, Kang HY, Koh WJ, Yim JJ, et al. The role of chest CT scanning in TB outbreak investigation. Chest. 2010;137:1057–1064. doi: 10.1378/chest.09-1513. [DOI] [PubMed] [Google Scholar]
- 22.Carratala J, Fernandez-Sabe N, Ortega L, Castellsague X, Roson B, Dorca J, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med. 2005;142:165–172. doi: 10.7326/0003-4819-142-3-200502010-00006. [DOI] [PubMed] [Google Scholar]
- 23.Ganmaa D, Giovannucci E, Bloom BR, Fawzi W, Burr W, Batbaatar D, et al. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. Am J Clin Nutr. 2012;96:391–396. doi: 10.3945/ajcn.112.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergman P, Norlin AC, Hansen S, Rekha RS, Agerberth B, Bjorkhem-Bergman L, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663. doi: 10.1136/bmjopen-2012-001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quraishi SA, Bittner EA, Christopher KB, Camargo CA., Jr Vitamin D status and community-acquired pneumonia: results from the third National Health and Nutrition Examination Survey. PLoS One. 2013;8:e81120. doi: 10.1371/journal.pone.0081120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badesch DB, King TE, Jr, Schwarz MI. Acute eosinophilic pneumonia: a hypersensitivity phenomenon? Am Rev Respir Dis. 1989;139:249–252. doi: 10.1164/ajrccm/139.1.249. [DOI] [PubMed] [Google Scholar]
- 27.Imokawa S, Sato A, Hayakawa H, Toyoshima M, Taniguchi M, Chida K. Possible involvement of an environmental agent in the development of acute eosinophilic pneumonia. Ann Allergy Asthma Immunol. 1996;76:419–422. doi: 10.1016/S1081-1206(10)63457-6. [DOI] [PubMed] [Google Scholar]
- 28.Rom WN, Weiden M, Garcia R, Yie TA, Vathesatogkit P, Tse DB, et al. Acute eosinophilic pneumonia in a New York City firefighter exposed to World Trade Center dust. Am J Respir Crit Care Med. 2002;166:797–800. doi: 10.1164/rccm.200206-576OC. [DOI] [PubMed] [Google Scholar]
- 29.Uchiyama H, Suda T, Nakamura Y, Shirai M, Gemma H, Shirai T, et al. Alterations in smoking habits are associated with acute eosinophilic pneumonia. Chest. 2008;133:1174–1180. doi: 10.1378/chest.07-2669. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimoto T, Mizutani H, Tsutsui H, Noben-Trauth N, Yamanaka K, Tanaka M, et al. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat Immunol. 2000;1:132–137. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- 31.Mato N, Bando M, Kusano A, Hirano T, Nakayama M, Uto T, et al. Clinical significance of interleukin 33 (IL-33) in patients with eosinophilic pneumonia. Allergol Int. 2013;62:45–52. doi: 10.2332/allergolint.12-OA-0439. [DOI] [PubMed] [Google Scholar]
- 32.Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. Vitamin D in defense of the human immune response. Ann N Y Acad Sci. 2007;1117:94–105. doi: 10.1196/annals.1402.036. [DOI] [PubMed] [Google Scholar]
- 33.Bartley J. Vitamin D, innate immunity and upper respiratory tract infection. J Laryngol Otol. 2010;124:465–469. doi: 10.1017/S0022215109992684. [DOI] [PubMed] [Google Scholar]
- 34.Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, et al. Vitamin D insufficiency in Korea--a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab. 2011;96:643–651. doi: 10.1210/jc.2010-2133. [DOI] [PubMed] [Google Scholar]