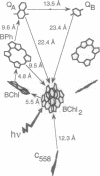
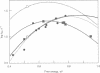
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5730–5734. doi: 10.1073/pnas.84.16.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashnagar A., Bruce J. M., Dutton P. L., Prince R. C. One- and two-electron reduction of hydroxy-1,4-naphthoquinones and hydroxy-9,10-anthraquinones. The role of internal hydrogen bonding and its bearing on the redox chemistry of the anthracycline antitumour quinones. Biochim Biophys Acta. 1984 Oct 16;801(3):351–359. doi: 10.1016/0304-4165(84)90138-7. [DOI] [PubMed] [Google Scholar]
- Bylina E. J., Youvan D. C. Directed mutations affecting spectroscopic and electron transfer properties of the primary donor in the photosynthetic reaction center. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7226–7230. doi: 10.1073/pnas.85.19.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., el-Kabbani O., Tiede D., Norris J., Schiffer M. Structure of the membrane-bound protein photosynthetic reaction center from Rhodobacter sphaeroides. Biochemistry. 1991 Jun 4;30(22):5352–5360. doi: 10.1021/bi00236a005. [DOI] [PubMed] [Google Scholar]
- DeVault D., Chance B. Studies of photosynthesis using a pulsed laser. I. Temperature dependence of cytochrome oxidation rate in chromatium. Evidence for tunneling. Biophys J. 1966 Nov;6(6):825–847. doi: 10.1016/s0006-3495(66)86698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangiacomo K. M., Dutton P. L. In photosynthetic reaction centers, the free energy difference for electron transfer between quinones bound at the primary and secondary quinone-binding sites governs the observed secondary site specificity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2658–2662. doi: 10.1073/pnas.86.8.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Murchison H. A., Nagarajan V., Parson W. W., Allen J. P., Williams J. C. Specific alteration of the oxidation potential of the electron donor in reaction centers from Rhodobacter sphaeroides. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10265–10269. doi: 10.1073/pnas.91.22.10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Michel H., Epp O., Deisenhofer J. Pigment-protein interactions in the photosynthetic reaction centre from Rhodopseudomonas viridis. EMBO J. 1986 Oct;5(10):2445–2451. doi: 10.1002/j.1460-2075.1986.tb04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser C. C., Keske J. M., Warncke K., Farid R. S., Dutton P. L. Nature of biological electron transfer. Nature. 1992 Feb 27;355(6363):796–802. doi: 10.1038/355796a0. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warncke K., Dutton P. L. Experimental resolution of the free energies of aqueous solvation contributions to ligand-protein binding: quinone-QA site interactions in the photosynthetic reaction center protein. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2920–2924. doi: 10.1073/pnas.90.7.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warncke K., Gunner M. R., Braun B. S., Gu L., Yu C. A., Bruce J. M., Dutton P. L. Influence of hydrocarbon tail structure on quinone binding and electron-transfer performance at the QA and QB sites of the photosynthetic reaction center protein. Biochemistry. 1994 Jun 28;33(25):7830–7841. doi: 10.1021/bi00191a010. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Alden R. G., Murchison H. A., Peloquin J. M., Woodbury N. W., Allen J. P. Effects of mutations near the bacteriochlorophylls in reaction centers from Rhodobacter sphaeroides. Biochemistry. 1992 Nov 17;31(45):11029–11037. doi: 10.1021/bi00160a012. [DOI] [PubMed] [Google Scholar]