Abstract
Severe acute pancreatitis (SAP) is normally related to multiorgan dysfunction and local complications. Studies have found that local pancreatic renin-angiotensin system (RAS) was significantly upregulated in drug-induced SAP. The present study aimed to investigate the effects of angiotensin II receptors inhibitor valsartan on dual role of RAS in SAP in a rat model and to elucidate the underlying mechanisms. 3.8% sodium taurocholate (1 ml/kg) was injected to the pancreatic capsule in order for pancreatitis induction. Rats in the sham group were injected with normal saline in identical locations. We also investigated the regulation of experimentally induced SAP on local RAS expression in the pancreas through determination of the activities of serum amylase, lipase and myeloperoxidase, histological and biochemical analysis, radioimmunoassay, fluorescence quantitative PCR and Western blot analysis. The results indicated that valsartan could effectively suppress the local RAS to protect against experimental acute pancreatitis through inhibition of microcirculation disturbances and inflammation. The results suggest that pancreatic RAS plays a critical role in the regulation of pancreatic functions and demonstrates application potential as AT1 receptor antagonists. Moreover, other RAS inhibitors could be a new therapeutic target in acute pancreatitis.
Keywords: ICAM-1, MDA, P-selectin, Renin-angiotensin System, Severe acute pancreatitis
INTRODUCTION
Acute pancreatitis (AP), a pancreatic inflammatory disease, is one of the most catastrophic upper abdominal disorders [1,2,3,4,5,6]. The incident rate of this inflammatory disease is about 150 to 420 and 700 to 800 per million/year in United Kingdom and United States, respectively, whereas it ranges from 106 to 205 per million/year in Japan [7]. AP is associated with parenchymaledema, tissue necrosis, hemorrhage, and inflammatory cell infiltration [1,2,3,4,5,6,7]. Severe acute pancreatitis (SAP) with a mortality approaching 30% occurred in approximately 20% of the patients with AP because of multiorgan dysfunction and local complications [4,6]. The recent studies evidenced that the pancreatic acinar cell which secret the digestive enzymes into the gastrointestinal tract could initiate of AP [8]. Activation of intra-acinar enzyme results in increased levels of blood pancreatic enzymes, multiple organ failure, activation of inflammation and other immune responses [2,9,10]. Activation of the exocrine enzymes, especially trypsin, induces various activation of protease within the pancreas which would degrade lots of cellular protein and eventually result in pancreatic lesion [9,10]. In addition, the autoantigens in injured cells trigger immune system, leading to the aseptic inflammation of the pancreas, and eventually tissue damage and necrosis [9,10]. Until now, although several studies have paid attention to the pancreatitis pathophysiology, effective and ideal therapy has still not been demonstrated for SAP. In clinical treatment, the aseptic inflammation-mediated damage-associated molecular patterns (DAMPs) could be prevented and controlled.
The classical renin-angiotensin system (RAS), a circulating hormonal system, is essential for blood pressure regulation, extracellular fluid volume, absorption of electrolytes and homeostasis [11,12,13,14,15,16]. A local function-independent RAS in the pancreas without association in blood circulating hormones bioavailability was previously proposed in the dogs, rodents and human [15,16]. Pancreatic RAS plays different roles in the pancreatic physiology and pathophysiology regulation as recently reviewed [17]. For example, the pancreatic RAS may play a critical role for the regulation of pancreatic microcirculation and ductal secretion [18]. Overexpression of the local RAS components including angiotensinogen, renin and angiotensin-converting enzyme (ACE) suggest a potential role of the pancreatic RAS in AP [13,14,15,16]. ACE plays a role in converting angiotensinogen into angiotensin II (Ang II), a physiologically active product which performs activity by binding mainly to its specific receptors called angiotensin II type 1 receptors (AT1R) and angiotensin II type 2 receptors (AT2R) [13,14,15,16]. AT1 and AT2 receptor expression was mainly detected in blood vessels endothelia and pancreatic ductal system epithelia, and at a smaller intensity in acini [19,20]. As pancreatic microcirculatory changes like vasoconstriction, capillary stasis, decreased oxygen tension, and progressive ischaemia were demonstrated to occur in the early stage of AP, Ang II, AT1R and AT2R are responsible for pancreatic microcirculatory regulation, which may in turn cause pancreatic tissue injury in AP [21]. Therefore, pancreatic microcirculation in the local RAS plays an essential role in pancreatitis. Recent study also demonstrated the association and the important role of RAS/vitamin D in the genesis or severity of AP of 2 RAS polymorphisms with AP, which suggest the ready potential for pharmacological manipulation of this system using existing marketed agents [22]. Tsang et al. [23] also showed the existence of an acinar RAS in the pancreas of potential importance in the physiological regulation of digestive enzyme secretion. The results supported that the differential actions of AT1 and AT2 receptors and their upregulation may have clinical relevance to the pathogenesis and management of acute pancreatitis.
Moreover, Ip et al. supported that the potential mechanisms of RAS-mediated oxidative stress in AP involved ROS generation [24]. Apart from pancreatic microcirculation, Ang II has biological function for the induction of inflammation in pancreas. For activation of RAS, Ang II-induced increases in ROS levels, and then promotes the expression of proinflammatory cytokines [7]. Indeed, ROS can promote leukocyte activation, cytokine production, and result in pancreatic microcirculation dysfunction [7]. For instance, concentration of superoxide dismutase (SOD), an antioxidative enzyme which converts superoxide radicals to H2O2 in pancreatic tissue, shows negative correlation with lipid peroxidation products in the gland of rats with acute pancreatitis [1]. Recent studies also have been proved that the blood level of myeloperoxidase (MPO) is dependent on the severity of AP and on cytokine blood levels. Additionally, the level of malondialdehyde (MDA) is commonly used as an indicator of ROS formation in AP [1]. Pancreatic inflammation leaded to sudden increase of MDA. As a result, these suggest that RAS activation may relate to aseptic inflammation-mediated DAMPS [7,24]. Previous investigation indicated that the local pancreatic RAS was upregulated in experimental AP [11,12,13,14,15,16]. In other words, RAS inhibition could reduce the effects of pancreatic inflammation and fibrosis, which casts a new insight on the role of the pancreatic RAS in pancreatitis.
A hallmark of the inflammatory process in AP was leucocyte infiltration into the pancreas, which may cause tissue damage [7]. Leucocyte accumulation at inflammation locations is a multistep process mediated by expression of adhesion molecules of the selectin and integrin families on leucocytes and endothelial cells [7]. The study of Hartman et al. revealed that P-selectin play a crucial role in neutrophil rolling regulation and extravascular infiltration regulation in AP [3]. In experimental pancreatitis, inhibiting P-selectin protected against pancreatic tissue injury [3]. Targeting P-selectin may be an effective way to reduce inflammation in AP. However, the role of specific adhesion molecules in mediating leucocyte accumulation in AP was not yet completely understood.
Neutrophil recruitment also plays an essential role in inflammatory responses development in AP [25]. A number of adhesion molecules and inflammatory mediators are involved in the rolling, adhesion, and transmigration mechanisms. Intercellular adhesion molecule-1 (ICAM-1), a glycoprotein which mainly expressed on the surface of endothelial cells, has been demonstrated to play a vital role in the development of AP [25]. The recent research showed that the expression of ICAM-1is significantly upregulated in the serum of AP patients and in the pancreas of mice with caerulein-induced AP [25].
Angiotensin II AT1 receptor blocker valsartan is non-peptide competitive antagonist that is mediated by highly selective blockage of AT1 receptors, which causes the most detrimental effects of Ang II [26,27,28]. The specificity of antagonism of ATI receptors is greater about 20,000 times than that of AT2 receptors [29]. Due to AT 1 receptor blockage, Ang II level rises and overactivation of AT2 receptors occurs, which promotes apoptosis, inhibits proliferation and hypertrophy, inhibits release of aldosterone, improves ventricular and vascular remodeling, improves apotassium-sparing and natriuretic activity, and improves vascular tone through vasodilation [26,27,28,29]. Valsartan is widely used in treatment of and preventing high blood pressure, coronary heart diseases, and other vascular mediated diseases because of many advantages of using valsartan such as high safety, high efficacy, high tolerability, long action time but few side effects which have been supported by clinical investigation [24]. For instance, recent studies indicated that pulmonary injury with valsartan ameliorated bleomycin induction, hepatic fibrosis with carbon tetrachloride induction, tubulointerstitial fibrosis with 5/6 nephrectomy induction, and Dahl salt-sensitive induced concentric left ventricular hypertrophy in rats by decreased TGF-b1 production. In the present study, we aimed to examine the effects of valsartan on the dual role of RAS in SAP in a rat model and elucidate the underlying mechanisms [26]. The results showed that valsartan could activate the local RAS to protect against experimental acute pancreatitis, according to inhibition of microcirculation disturbances and inflammation. Thus pancreatic renin-angiotensin system was supported to play a vital role in pancreatic functions regulation and the potential application of AT1 receptor antagonists and other RAS inhibitors could be a new therapeutic target in acute pancreatitis.
METHODS
Materials
Sodium taurocholate and valsartan were purchased from Sigma. The kits for detection of serum amylase (AMY), malondialdehyde (MDA), and myeloperoxidase (MPO) were obtained from Nanjing Jiancheng Bioengineering Institute. Angiotensin (Ang II) ELISA kit was acquired from Wuhan ColorfulGene Biological Technology Co.,LTD. Moreover, the primers of ICAM-1 were synthesized by Invitrogen Biotechnology Co.,LTD. The FITC labeled P-selectin antibodies were obtained from Beijing Biosynthesis Biotechnology Co., Ltd.
Animals and animal procedures
Male Wistar rats with SPF class were provided by Hubei Provincial Center For Disease Control and Prevention, China. The Wistar rats were randomly divided into 3 groups (n=18 for each group) consisting of sham operation (SO) group, SAP group (SAP-induced), valsartan treatment group (SAP induced plus valsartan treatment; SAP+V). Average weight of them in experiment was about 200~250 g. Anesthesia was showed with intraperitoneal injection of 10% chloral hydrate 3 ml/kg. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85~23, Revised 1985), and has been approved by the Animal Care and Use Committee, Fifth Hospital of Wuhan.
Model induction procedure
Wistar rats were fasted for 12 h and prevented drinking for 4 h before the operation. Laparotomy was performed through a midline incision. A microaneurysm clip was placed around the common biliopancreatic duct which entry into the duodenum in the attempt to prevent reflux of enteric contents into the duct. After preparation of a 1-ml U-40 insulin syringe with a 28-gauge, 1/2-inch microfine intravenous needle, 1 ml/kg 5% sodium taurocholate (Sigma, St. Louis, MO, USA) was slowly infused into the common biliopancreatic duct at a volume of 2.0 ml/kg at 0.1 ml/min. After several minutes, the pancreatic tissue would become dark purple, swell, and bleed locally and then the abdominal organs were gently repositioned, and the peritoneal cavity was douched with about 2 ml/kg of normal saline. The abdominal wall was sutured in the end.
Valsartan was dissolved in DMSO (dimethyl sulfoxide) before use. In the SAP group, 10% DMSO soulution (2 ml/kg) was injected into the femoral vein of rats. In the valsartan treatment group, injection of constant volume of 10% DMSO solution with valsartan (5 mg/kg) was also performed in rats after SAP induction for 30 min. In the SO group, laparotomy was performed after anesthesia and then the abdomen was closed after flipping the pancreas. After 30 min, 2 ml/kg 10% DMSO solution was injected into femoral vein of these rats. The animals were sacrificed 3 h, 6 h and 12 h after surgery respectively (n=6 for each group per each time). The ascitic fluid volume was recorded. Moreover, blood samples were drawn into tubes without anticoagulant (for preparation of serum). Then, the whole blood was put at room temperature for approximately 1 h. Centrifugation was then performed to get the serum fraction. Serum aliquots were stored frozen at -20℃ until analysis. The pancreatic tissues were removed and immediately frozen in liquid nitrogen and then stored at -80℃ until assay. Portions of this organ were also fixed in paraformaldehyde for histological examination.
Histopathologic analysis
A part of the pancreas including the main pancreatic duct from the same anatomical location in each rat was fixed in 4% paraformalin for 24 h and embedded in paraffin. Hematoxylin and eosin (H&E) were used to stain each paraffin section to examine each pancreas. According to scores for histological examination summed by Schmidt et al., the tissues were scored with respect to edema, acinar necrosis, inflammatory infiltrate, hemorrhage, fat necrosis and perivascular inflammation in 20 fields [30].
Determination of serum amylase (AMY) and pancreas-specific serum lipase
AMY level in serum and lipase level were determined by Starch-iodine assay and nephelometry respectively. The kits were purchased from Nanjing Jiancheng Bioengineering Institute. The experiments were done according to the instruction.
Biochemical analysis
Superoxide dismutase (SOD) activity was measured as previously described by Aydin et al. Briefly, a mixture with 50 ml supernatant and 850 ml of substrate solution containing 0.05 mmol/L xanthine sodium and 0.025 mmol/L 2-(4-iodophen-yl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride in a buffer solution which contains 50 mmol/L 3-(cyclohexylaminol)-1-propanesulfonic acid and 0.094 mmol/L ethylene-diaminetetraacetic acid (pH 10.2) was prepared. Xanthine oxidase was then added to the mixture and the increase in absorbance was followed at 505 nm for 3 min.
Lipid peroxidation was estimated by the measurement of thiobarbituric acid reactive substances in the supernatant. Briefly, malondialdehyde (MDA) was mixed with thiobarbituric acid, the solution was measured spectrophotometrically at 532 nm.
Tissue myeloperoxidase determination
Pancreas tissue myeloperoxidase (MPO) activity was determined by employing a quantitative indicator of neutrophil infiltration. Briefly, 0.5 g homogenized pancreas tissues were obtained in a solution containing 0.5% hexa-decyltrimethyl-ammonium bromide dissolved in phosphate saline buffer (PBS) (pH 6.0). After centrifugation, the supernatant was collected for the assay. After mixing the sample with TMB chromogen substrate solution, the mixture incubated for 110s. H2SO4 was added to stop the reaction. The MPO activity was analyzed with the absorbance at 450 nm The assay was carried out by using a commercial kit (Nanjing Jiancheng Bioengineering Institute, China), according to the manufacturer's instructions.
Radioimmunoassay
The Ang II and renin were detected by radioimmunoassay following the manufacturer's instructions.
Fluorescence quantitative PCR
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA depending on the manufacturer's protocol. cDNA was formed from reverse-transcription of 2 µg of total RNA by using Super Script III first strand cDNA synthesis Kit (Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed with the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The SYBR Green PCR Master Mix kits (Applied Biosystems, Foster City, CA, USA) were used according to the supplier's and the primers please Supplemental Table 1.
Thermal cycling conditions were: 95℃ for 2 min; 40 cycles of 10 s denaturation at 94℃, 10 s annealing at 54℃, and 20 s extension at 72℃; and 1 cycle of 5 min at 72℃. The relative mRNA levels of Ang II, AT1 receptor and ICAM-1 were expressed as ratios compared with GAPDH mRNA levels, respectively.
Western blot analysis
Total cellular proteins were extracted by incubating cells in lysis buffer (1X PBS, 1% NP-40, 0.1% sodium dodecylsulfate (SDS), 5 mM EDTA, 0.5% sodium deoxycholate, and 1 mM sodium orthovanadate with protease inhibitors). bicinchoninic acid assay (BCA) (Pierce) was used to determine the protein concentrations. Equal amount of proteins was loaded in 8% glycine gel-SDS-PAGE per lane. After electrophoresis, nitrocellulose membrane was used for transferring separated proteins. Then, 5% non-fat milk in TBST buffer was used to blocke with the membrane for 1 h. After that, the membranes were incubated with primary antibodies of AngII, AT1 receptor, ICAM-1 and β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 1:1000 dilutions in 5% non-fat milk overnight at 4℃, and then anti-rabbit IgG monoclonal antibody conjugated with horseradish peroxidase (Southern Biotech) at 1:2000 dilution for 1 h at room temperature. Protein bands were detected using the West Femto system (PIERCE).
Measurement of the P-selectin
We used a standard cytometric assay to measure P-selectin surface expression in the blood. Within 15 min after blood sample collection (3.8% sodium citrate), 100 µl of blood sample was fixed with 1 ml of 1% paraformaldehyde (pH7.2, 0.2 µm micron membrane filtered) for ranging from 1 to 6 h.
Then, 0.5 ml blood sample were transferred into polyethylene test tube and settled down without centrifugation. The supernatant with rich platelet was collected and washed with PBS solution 3 times. The platelets were resuspended in PBS solution with 2% BSA to regulate the concentration of platelets to be 0.5~1.0×109/ml. Then, the platelets were stained with 10 µl of FITC-conjugated anti-human P-selectin and, after a 20-min incubation in the dark, were mixed with 1 ml of 1% freshly prepared paraformaldehyde. Finally, the samples were analyzed by flow cytometry.
Statistical analysis
The results are expressed as the mean±standard error of the mean (x̄±s). The significance of differences in total histopathologic scores, serum amylase activities and cytokine levels were assessed using one-way analysis of variance. p<0.05 was considered to indicate a statistically significant result. All statistical measurements were performed using SPSS PC version 13.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Effects of valsartan on pancreatic histology
In this study, SAP model was set up by injecting 10% DMSO solution (2 ml/kg) into femoral vein of rats. For the valsartan treatment group, injection of constant volume of 10% DMSO solution with valsartan (5 mg/kg) was also performed in rats after SAP induction for 30 min. In this model, valsartan didn't cause the mortality of SAP rats after 72 h. Therefore, further studies on the effects of valsartan on pancreatic histology were carried out. The morphology of pancreas samples were examined and compared among the treatment groups with an attempt to assess the effects of valsartan on local pancreatic injury. As shown in Fig. 1, under the light microscope, histological examination demonstrated that pancreatic tissues in SAP rats exhibited severe edema with large interstitial spaces, vascular congestion, necrosis, and inflammation (Fig. 1B). Part or all of the acini in the SAP group were showed a high degree of destruction on the histoarchitecture (Fig. 1B). Valsartan was indicated to improve the integrity and architecture of the acini (Fig. 1C). There was no significant necrosis and the degree of pancreatic tissue hyperemia, edema and inflammatory cell infiltration in these rats was reduced. In the SO group, there was no obvious histological change, based on complete pancreatic lobules and clear interstitial space observed. However, further studies are needed to clarify the changes in the pancreatic histology by using immunohistochemistry and/or some other molecular techniques in the future.
Fig. 1. Histological changes in pancreatic tissue sections under the light microscopy (magnification, ×200). (A) Sham-operated group, pancreatic tissue section shows normal acinar structure. (B) SAP group, massive destruction was observed in acinar glandular structure and islet cells of pancreatic tissue. (C) SAP plus valsartan treatment group, relative preservation in pancreatic structure is seen compared with the SAP group. The black arrow indicates necrotic cells, the red arrow indicates edema, and the blue arrow indicates inflammatory cell infiltrations.
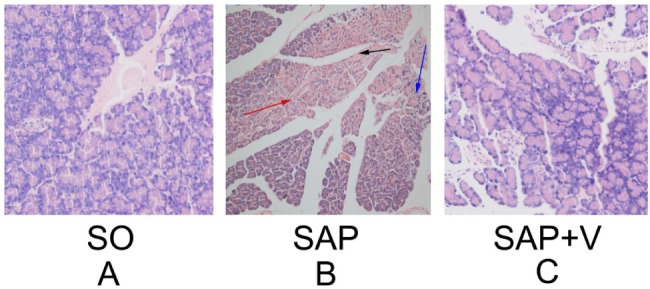
Table 1 summarized the scoring of the histological evaluations from three independent assessors. The mean histopathological score of SAP and SAP+V group were remarkably higher than that of SO group (p<0.05). On the other hand, the mean histopathological score of SAP group was lower (p<0.05) than that of SAP+V groups. In other words, valsartan treatment caused a clear reduction in the severity of pancreatitis.
Table 1. Histopathological scores in pancreatic tissues in all groups (x̄±s, score).
arepresents compared with the SO group, p<0.05.
brepresents compared with the SAP group, p<0.05.
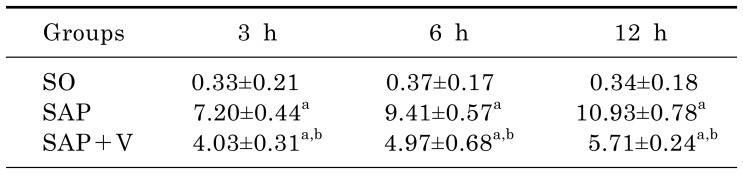
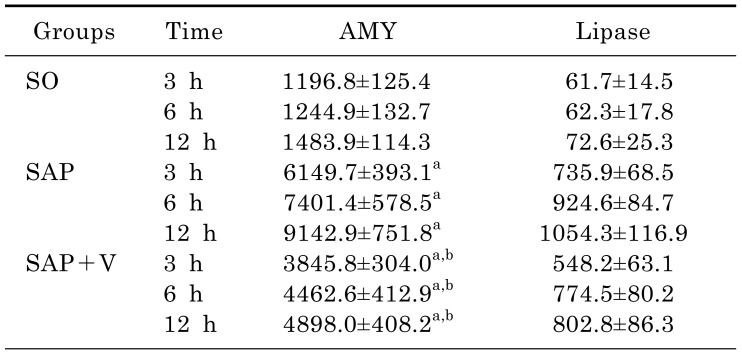
Effects of valsartan on the levels of serum amylase and lipase
Compared with that of SO group, the levels of serum amylase and pancreatic lipase significantly increased in a time-dependent manner (p<0.05). However, the levels of serum amylase and pancreatic lipase in SAP+V group were lower than that of SAP group at the same time after operation (p<0.05) (Table 2).
Table 2. Levels of serum amylase and pancreatic lipase in 3 experimental groups at different time (x̄±s, U/dL).
arepresents compared with the SO group, p<0.05.
brepresents compared with the SAP group, p<0.05.
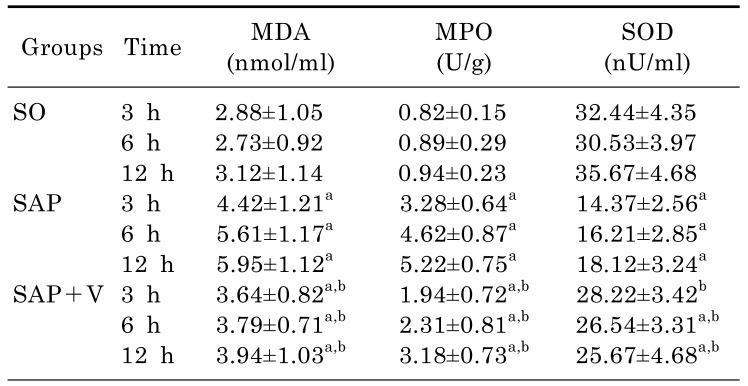
Effects of valsartan on serum MDA, pancreatic SOD, and MPO activity
The effects of valsartan on the enzyme activities of serum MDA, pancreatic SOD, and MPO were also examined in this study. As shown in Table 3, compared with that of SO group, there was a greater significant difference in serum MDA and pancreatic MPO levels (p<0.05) in the SAP and SAP+V groups. Moreover, the levels of serum MDA and pancreatic MPO increased with time. On the other hand, valsartan treatment significantly decreased serum MDA and pancreatic MPO activities in SAP+V group compared with the SAP group (p<0.05) at different time in the experiments. In addition, in the SAP group, the serum SOD activities were significantly lower than the SO and SAP+V groups (p<0.05). However, the serum SOD activities has elevated due to valsartan treatment in the SAP+V group compared with SAP group.
Table 3. The activities of pancreatic MDA, MPO and SOD enzyme activities in 3 experimental groups at different time (x̄±s, n=18).
arepresents compared with the SO group, p<0.05.
brepresents compared with the SAP group, p<0.05.
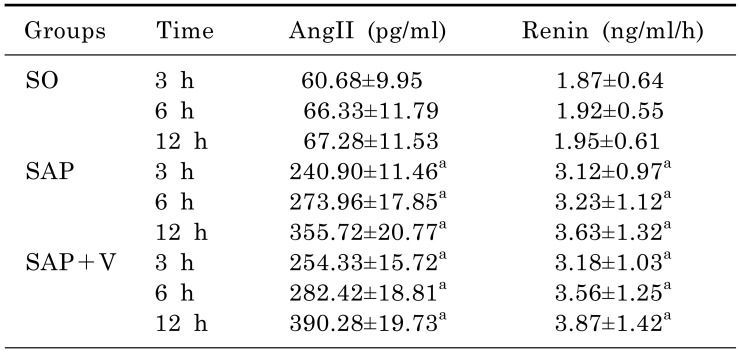
Effects of valsartan on levels of Ang II and renin
The effects of valsartan on the levels of serum Ang II and renin were detected by using radioimmunoassay. As shown in Table 4, in SAP and SAP+V groups, the levels of Ang II and renin were significantly elevated (p<0.05) when compared to SO group in a time-dependent manner. There was no significant difference between the SAP and SAP+V groups in terms of Ang II and renin.
Table 4. Levels of Ang II and renin in 3 experimental groups at different time (x̄±s, ng/l, n=18).
arepresents compared with the SO group, p<0.05.
Effects of valsartan on the expression levels of pancreatic Ang II, AT1 receptor and ICAM-1
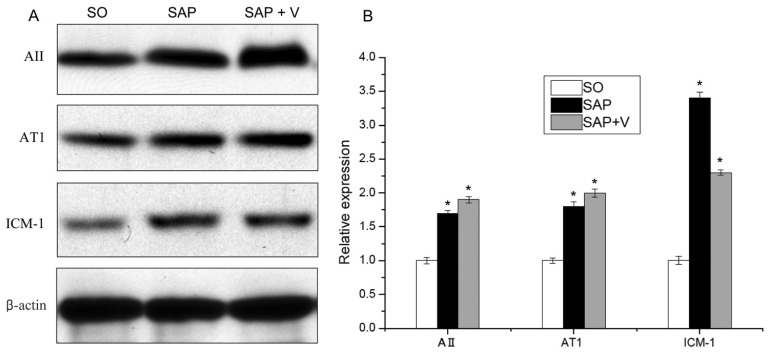
As shown in Table 5, the results of fluorescence quantitative PCR revealed that mRNA levels of pancreatic Ang II, AT1 receptor and ICAM-1 significantly elevated in the SAP and SAP+V groups. There was no significant difference of mRNA levels of pancreatic Ang II and AT1 receptor between SAP and SAP+V groups. However, the mRNA levels of pancreatic ICAM-1 in the SAP-induced rats with valsartan treatment were lower than that of SAP-induced rats.
Table 5. Fluorescence quantitative PCR of Ang II , AT1 receptor and ICAM-1 (n=18).
arepresents compared with the SO group, p<0.05.
brepresents compared with the SAP group, p<0.05.
The protein levels of Ang II, AT1 receptor and ICAM-1 were detected through Western blot analysis. As shown in Fig. 2, the protein level of β-actin indicated that the amount of protein in each band was equal. According to the results of the analysis, protein levels of Ang II, AT1 receptor and ICAM-1 significantly increased in SAP and SAP+V groups compared with SO group. In contrast, valsartan treatment caused a significant increase in the expression levels of Ang II, AT1 receptor and ICAM-1 in SAP+V groups when compared to SAP group.
Fig. 2. Western blot analysis of the expression levels of Ang II, AT1 receptor and ICAM-1. Each bar represents the mean±SD from three samples (*p<0.05 vs. control).
Effects of valsartan on the level of platelet P-selectin
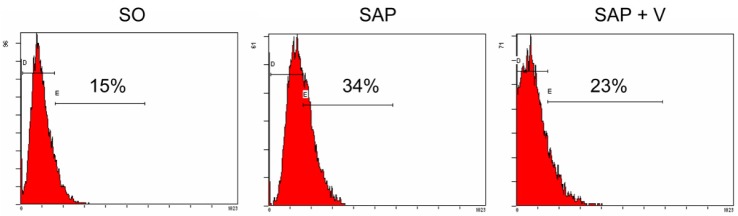
In the present study, P-selectin expression in platelets in different groups by using flow cytometry. As shown in Fig. 3, in the SO, SAP and SAP+V groups, the level of P-seletin were 15%, 34%, and 23% respectively. The level of P-selectin was significantly higher in SAP and SAP+V groups (p<0.05), according to the results of SO, SAP, and SAP+V groups. However, valsartan treatment significantly reduced the P-selectin level in SAP-induced rats (p<0.05).
Fig. 3. P-selectin expression on platelets in three experimental groups by using flow cytometric analysis. The values represent the percentages of P-selectin positive cells.
DISCUSSION
In the past years, a number of researches about the initiation and propagation of AP have been done, but to date no fully effective drugs or treatment is available. Studies have found that local pancreatic RAS was significantly upregulated in drug-induced SAP [11,12,13,14,15,16]. Ang II may be responsible for the pancreatic ductal adenocarcinoma development, through stimulation of angiogenesis and prevention of chemotherapy toxicity, and AP initiation and propagation [31]. The present study aimed to investigate the effects of Ang II receptors inhibitor valsartan on dual role of RAS in SAP in a rat model and to elucidate the underlying mechanisms. Our results suggest that pancreatic RAS plays a critical role in the regulation of pancreatic functions and demonstrates application potential as AT1 receptor antagonists.
Key RAS components expression and localization in the acinar and endothelial cells of the exocrine pancreas are found in recent studies. Moreover, these RAS components are responsible to ROS generation responding to chronic hypoxia and acute pancreatitis [32,33,34,35,36]. For instance, Greenstein et al. reported increased plasma RAS activity in patients with acute pancreatitis [32]. They evidenced that normal plasma renin content in the patients with AP is 5 times higher than that of normal people [32]. renin Pupilli et al. found thatAng II contributed to the inflammatory edema by promoting the synthesis and secretion of vascular permeability factor of the vascular smooth muscle cells [33]. Additionally, Schieffer et al. found that Ang II can induce oxygen free radicals and activation of JAKs and STAT, which increased the synthesis of proinflammatory cytokines like IL-6 TNF-a, IL-1b and so on [35]. Indeed, severe ischemia and/or hypoxia conditions could be induced by an increased sensitivity of Ang II-mediated vasoconstriction in pancreatic microcirculation, which could result in oxidative stress, overexpression of proinflammatory factors and migration/adhesion of monocytes and leukocytes, ultimately leading to pancreatic cell inflammation and injury [11,12]. Based on the information mentioned above, RAS plays an essential role in inducing inflammation and microcirculatory regulation in the pancreas, which may in turn lead to pancreatic tissue injury in AP. Therefore, we hypothesized that RAS inhibition may be a new therapeutic target in AP. For example, prophylactic administration of saralasin, a nonspecific antagonist for AT1 and AT2 receptors, was protective against AP [35]. At the same time, pancreatic and systemic inflammation could be improved by the AT1 receptor antagonist losartan [36]. In the present study, we showed that, valsartan could be used as a therapeutic agent against SAP-induced damage. In detail, the expression levels of serum Ang II, renin and AT1 receptors were significantly increased in SAP group.
Nowadays, many studies proved that vasoconstriction plays a vital in the pathological process of AP [26,27,28,29]. Pancreatic blood flow dramatically reduced in the acute necrotizing pancreatitis. As a result, persistent pancreatic ischemia, reduction of blood perfusion and microcirculation disturbances promote the development of edema, hemorrhage, necrosis and eventual AP. Our results showed that, the pathological features of SAP changed from edema to hemorrhage and necrosis after preparation of SAP models. In addition, the trend of pathological damage and the change of plasma Ang II level were consistent. Interestingly, comparing with SAP group, valsartan suppressed the level of serum amylase that is the most common indicator of trypsin during SAP exposure. Moreover, comparing with the SAP group, the pancreatic histopathological scores were significantly decreased in SAP+V group. These results suggested that valsartan exhibits the therapeutic potential against SAP.
The occurrence of local inflammation and systemic complications of the AP has been found associated with ROS and reactive nitrogen species [1]. In the present study, MDA concentration markedly increased, while SOD activities was markedly decreased after 3, 6 and 12 h of SAP treatment (Table 3). Moreover, SOD level were inversely proportional with AMY level, but opposite trend was found for MDA level. These results revealed that production of ROS remarkably increased in SAP group, on the other hand, the ability of scavenging ROS dramatically decreased since levels of SOD and MDA related to ROS production leading to development of SAP. Furthermore, comparing with SAP group, the activity of SOD was higher and the concentration of MDA was lower in the SAP+V group. The results proved that valsartan inhibited ROS generation and reduce cellular membrane damage induced by SAP.
The extent of the invasion of neutrophils into the pancreatic tissue was shown by measuring the MPO activity in tissue samples [1]. Under inflammatory conditions, valsartan acts as an important immunoregulatory factor that affects the synthesis of cytokines. In our study, the pancreatic MPO levels were significantly elevated in rats with SAP in a time-dependent manner. However, the levels of MPO were dramatically decreased after co-treatment with valsartan. It may be because that Ang II can be able to stimulate the synthesis and secretion of VPF, leading to cell invasion and exudation of plasma macromolecules. Also, Ang II can induce the synthesis of adhesion molecules and chemokines, promoting the migration and adhesion of monocytes and neutrophils [25]. These factors led to the formation of edema and inflammation of the pancreas. Studies also showed that selective AT 1 receptor antagonists could be used to reduce the inflammation and damage of the pancreatic cells [26,27,28]. In this study, it was found that MPO activity in pancreatic tissue decreased after valsartan treatment. Therefore, valsartan may demonstrate anti-inflammatory effect to prevent the infiltration of neutrophils and consequently reducing the release of inflammatory cytokines in pancreatic tissue.
A number of proinflammatory mediators, such as adhesion molecules, chemokines, cytokines and transcription factors were significantly associated with RAS [3,25,37]. Thanks to neutrophils invasion into inflamed tissue, foreign antigens were destructed and the injured tissue were broken down and remodeled [3,25]. ICAM-1, one of these adhesion molecules, has been showed to be upregulated in AP. ICAM-1 exhibits a crucial role in binding white blood cells to endothelial cells lining the blood vessels, therefore the blood cells are able to migrate out of the bloodstream into a tissue which is closely related to SAP [25]. In this study, the results of fluorescence quantitative PCR and Western blot analysis revealed that, the expression level of tissue ICAM-1 in SAP groupwas increased and was higher than those of other groups. Consistently, Wei et al. demonstrated that the extremely high levels of serum ICAM-1 in SD rats with AP associated lung injury.
Apart from ICAM-1, the adhesion molecule P-selectin is known to be upregulated in AP, and they also play a deleterious role if the disease by neutrophil recruitment into pancreas and distant organs. Folch et al. have demonstrated that, in rats with AP, the expression levels of P-selectin in lung and pancreas remarkably increased, and the tissue infiltration of leucocytes were observed and showed high MPO activity [38]. However, the MPO activities in lung and pancreas were inhibited by anti-P-selectin antibody [38]. Telek et al. indicated that intense, heterogeneous acinar oxygen free radicals (OFR) production, strong P-selectin, and increasing NF-kappa B activation were showed in early AP samples [39]. Tsang et al. also explored the antagonism of AngII receptors on the AP-induced pancreatic injury severity [16]. They showed that, cerulein-induced pancreatic injury could be dramatically improved by losartan which blocked the AT1 receptor. In the present study, P-selectin expression was decreased in the SAP+V group by comparing with SAP group. blockade of the AT1 receptor by valsartan could markedly inhibit the ROS generation. Possibly, the blockage of the AT1 receptor inhibited the activation of RAS mediated AT1 receptor-ROS-NFκB pathway and ICAM-1 expression, the accumulation of inflammatory cells decreased. It could reduce the oxidative stress and control the pancreatic injury caused by aseptic inflammation. Studies have showed that, inflammation can influence the balance between NO and ROS, which may lead to dysfunction of microcirculation [40,41]. The consumption of a large amount of NAPDH was caused by inflammation in the patients with SAP. Inhibition of superoxide and ROS generation resulted in suppression of NO production, exacerbating local microcirculation disturbances [42,43]. Then, leukocyte adhesion increased due to local microcirculatory hemodynamic changes [42,43]. Ischemia and reperfusion could cause oxidative stress, release of inflammatory cytokines, leukocyte adhesion, and other inflammatory reactions.
In conclusion, the present study has clearly evidenced that, the inhibition of AT1 receptor by valsartan can activate the local RAS to protect against experimental acute pancreatitis through inhibition of microcirculation disturbances and inflammation. As an alternative approach, the AT1 receptor antagonist and other RAS inhibitors exhibit application potential in clinical treatment of AP in further research.
ABBREVIATIONS
- SAP
Severe acute pancreatitis
- RAS
renin-angiotensin system
- DAMPs
damage-associated molecular patterns
- ACE
angiotensin-converting enzyme
- Ang II
angiotensin II
- AT1R
angiotensin II type 1 receptors
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- MPO
myeloperoxidase
- MDA
malondialdehyde
- ICAM-1
Intercellular adhesion molecule-1
- DMSO
dimethyl sulfoxide
- PBS
phosphate saline buffer
SUPPLEMENTARY MATERIALS
Supplementary data including one figure can be found with this article online at http://pdf.medrang.co.kr/paper/pdf/Kjpp/Kjpp019-04-01-s001.pdf.
Primers for quantitative PCR
References
- 1.Akay C, Yaman H, Oztosun M, Cakir E, Yildirim AO, Eyi YE, Agilli M, Akgul EO, Aydin I, Kaldirim U, Tuncer SK, Eken A, Oztas E, Poyrazoglu Y, Yasar M, Ozkan Y. The protective effects of taurine on experimental acute pancreatitis in a rat model. Hum Exp Toxicol. 2013;32:522–529. doi: 10.1177/0960327113482692. [DOI] [PubMed] [Google Scholar]
- 2.Galuppo M, Nocentini G, Mazzon E, Ronchetti S, Esposito E, Riccardi L, Sportoletti P, Di Paola R, Bruscoli S, Riccardi C, Cuzzocrea S. The glucocorticoid-induced TNF receptor family-related protein (GITR) is critical to the development of acute pancreatitis in mice. Br J Pharmacol. 2011;162:1186–1201. doi: 10.1111/j.1476-5381.2010.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartman H, Abdulla A, Awla D, Lindkvist B, Jeppsson B, Thorlacius H, Regnér S. P-selectin mediates neutrophil rolling and recruitment in acute pancreatitis. Br J Surg. 2012;99:246–255. doi: 10.1002/bjs.7775. [DOI] [PubMed] [Google Scholar]
- 4.Liu M, Shi L, Chen M, Chen S, Zou X. Effects of c-Jun N-terminal kinase signaling pathway on severe acute pancreatitis-associated lung injury. Pancreas. 2012;41:358–366. doi: 10.1097/MPA.0b013e3182297f09. [DOI] [PubMed] [Google Scholar]
- 5.Yubero S, Manso MA, Ramudo L, Vicente S, De Dios I. Dexamethasone down-regulates the inflammatory mediators but fails to reduce the tissue injury in the lung of acute pancreatitis rat models. Pulm Pharmacol Ther. 2012;25:319–324. doi: 10.1016/j.pupt.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Yang B, Bai B, Liu CX, Wang SQ, Jiang X, Zhu CL, Zhao QC. Effect of umbilical cord mesenchymal stem cells on treatment of severe acute pancreatitis in rats. Cytotherapy. 2013;15:154–162. doi: 10.1016/j.jcyt.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Chan YC, Leung PS. The Renin-angiotensin system and reactive oxygen species: implications in pancreatitis. Antioxid Redox Signal. 2011;15:2743–2755. doi: 10.1089/ars.2011.4071. [DOI] [PubMed] [Google Scholar]
- 8.Kingsnorth A, O'Reilly D. Acute pancreatitis. BMJ. 2006;332:1072–1076. doi: 10.1136/bmj.332.7549.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickartz T, Mayerle J, Lerch MM. Autoimmune pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2007;4:314–323. doi: 10.1038/ncpgasthep0837. [DOI] [PubMed] [Google Scholar]
- 10.Hoque R, Malik AF, Gorelick F, Mehal WZ. Sterile inflammatory response in acute pancreatitis. Pancreas. 2012;41:353–357. doi: 10.1097/MPA.0b013e3182321500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung PS, Chan WP, Nobiling R. Regulated expression of pancreatic renin-angiotensin system in experimental pancreatitis. Mol Cell Endocrinol. 2000;166:121–128. doi: 10.1016/s0303-7207(00)00275-6. [DOI] [PubMed] [Google Scholar]
- 12.Leung PS, Carlsson PO. Tissue renin-angiotensin system: its expression, localization, regulation and potential role in the pancreas. J Mol Endocrinol. 2001;26:155–164. doi: 10.1677/jme.0.0260155. [DOI] [PubMed] [Google Scholar]
- 13.Oruc N, Ozutemiz O, Nart D, Yuce G, Celik HA, Ilter T. Inhibition of renin-angiotensin system in experimental acute pancreatitis in rats: a new therapeutic target? Exp Toxicol Pathol. 2010;62:353–360. doi: 10.1016/j.etp.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Skipworth JR, Szabadkai G, Olde Damink SW, Leung PS, Humphries SE, Montgomery HE. Review article: pancreatic renin-angiotensin systems in health and disease. Aliment Pharmacol Ther. 2011;34:840–852. doi: 10.1111/j.1365-2036.2011.04810.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsang SW, Cheng CH, Leung PS. The role of the pancreatic renin-angiotensin system in acinar digestive enzyme secretion and in acute pancreatitis. Regul Pept. 2004;119:213–219. doi: 10.1016/j.regpep.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Tsang SW, Ip SP, Leung PS. Prophylactic and therapeutic treatments with AT 1 and AT 2 receptor antagonists and their effects on changes in the severity of pancreatitis. Int J Biochem Cell Biol. 2004;36:330–339. doi: 10.1016/s1357-2725(03)00257-7. [DOI] [PubMed] [Google Scholar]
- 17.Leung PS. Current research of the RAS in pancreatitis and pancreatic cancer. Adv Exp Med Biol. 2010;690:179–199. doi: 10.1007/978-90-481-9060-7_10. [DOI] [PubMed] [Google Scholar]
- 18.Tahmasebi M, Puddefoot JR, Inwang ER, Vinson GP. The tissue renin-angiotensin system in human pancreas. J Endocrinol. 1999;161:317–322. doi: 10.1677/joe.0.1610317. [DOI] [PubMed] [Google Scholar]
- 19.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 20.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhou ZG, Chen YD, Sun W, Chen Z. Pancreatic microcirculatory impairment in experimental acute pancreatitis in rats. World J Gastroenterol. 2002;8:933–936. doi: 10.3748/wjg.v8.i5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skipworth JR, Nijmeijer RM, van Santvoort HC, Besselink MG, Schulz HU, Kivimaki M, Kumari M, Cooper JA, Acharya J, Shankar A, Malago M, Humphries SE, Olde Damink SW, Montgomery HE. The effect of Renin Angiotensin system genetic variants in acute pancreatitis. Ann Surg. 2015;261:180–188. doi: 10.1097/SLA.0000000000000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang SW, Cheng CH, Leung PS. The role of the pancreatic renin-angiotensin system in acinar digestive enzyme secretion and in acute pancreatitis. Regul Pept. 2004;119:213–219. doi: 10.1016/j.regpep.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Ip SP, Tsang SW, Wong TP, Che CT, Leung PS. Saralasin, a nonspecific angiotensin II receptor antagonist, attenuates oxidative stress and tissue injury in cerulein-induced acute pancreatitis. Pancreas. 2003;26:224–229. doi: 10.1097/00006676-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Tamizhselvi R, Koh YH, Sun J, Zhang H, Bhatia M. Hydrogen sulfide induces ICAM-1 expression and neutrophil adhesion to caerulein-treated pancreatic acinar cells through NF-kappaB and Src-family kinases pathway. Exp Cell Res. 2010;316:1625–1636. doi: 10.1016/j.yexcr.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 26.Subeq YM, Ke CY, Lin NT, Lee CJ, Chiu YH, Hsu BG. Valsartan decreases TGF-β1 production and protects against chlorhexidine digluconate-induced liver peritoneal fibrosis in rats. Cytokine. 2011;53:223–230. doi: 10.1016/j.cyto.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension. 2010;55:715–721. doi: 10.1161/HYPERTENSIONAHA.109.148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu B, Lin R, Dai R, Chen C, Wu H, Hong M. Valsartan attenuates oxidative stress and NF-κB activation and reduces myocardial apoptosis after ischemia and reperfusion. Eur J Pharmacol. 2013;705:140–147. doi: 10.1016/j.ejphar.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 29.Lee BJ, Kim CD, Jung SW, Kwon YD, Kim YS, Yim HJ, Jeen YT, Lee HS, Kim JS, Chun HJ, Um SH, Lee SW, Choi JH, Ryu HS. Analysis of the factors that affect the mortality rate in severe acute pancreatitis. Korean J Gastroenterol. 2008;51:25–33. [PubMed] [Google Scholar]
- 30.Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaiswal N, Tallant EA, Jaiswal RK, Diz DI, Ferrario CM. Differential regulation of prostaglandin synthesis by angiotensin peptides in porcine aortic smooth muscle cells: subtypes of angiotensin receptors involved. J Pharmacol Exp Ther. 1993;265:664–673. [PubMed] [Google Scholar]
- 32.Greenstein RJ, Krakoff LR, Felton K. Activation of the renin system in acute pancreatitis. Am J Med. 1987;82:401–404. doi: 10.1016/0002-9343(87)90437-2. [DOI] [PubMed] [Google Scholar]
- 33.Pupilli C, Lasagni L, Romagnani P, Bellini F, Mannelli M, Misciglia N, Mavilia C, Vellei U, Villari D, Serio M. Angiotensin II stimulates the synthesis and secretion of vascular permeability factor/vascular endothelial growth factor in human mesangial cells. J Am Soc Nephrol. 1999;10:245–255. doi: 10.1681/ASN.V102245. [DOI] [PubMed] [Google Scholar]
- 34.Schieffer B, Luchtefeld M, Braun S, Hilfiker A, Hilfiker-Kleiner D, Drexler H. Role of NAD (P)H oxidase in angiotensin IIinduced JAK/STAT signaling and cytokine induction. Circ Res. 2000;87:1195–1201. doi: 10.1161/01.res.87.12.1195. [DOI] [PubMed] [Google Scholar]
- 35.Tsang SW, Ip SP, Wong TP, Che CT, Leung PS. Differential effects of saralasin and ramiprilat, the inhibitors of reninangiotensin system, on cerulein-induced acute pancreatitis. Regul Pept. 2003;111:47–53. doi: 10.1016/s0167-0115(02)00226-4. [DOI] [PubMed] [Google Scholar]
- 36.Chan YC, Leung PS. Involvement of redox-sensitive extracellular-regulated kinases in angiotensin II-induced interleukin-6 expression in pancreatic acinar cells. J Pharmacol Exp Ther. 2009;329:450–458. doi: 10.1124/jpet.108.148353. [DOI] [PubMed] [Google Scholar]
- 37.Yu JH, Lim JW, Kim H. Altered gene expression in ceruleinstimulated pancreatic acinar cells: pathologic mechanism of acute pancreatitis. Korean J Physiol Pharmacol. 2009;13:409–416. doi: 10.4196/kjpp.2009.13.6.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folch E, Salas A, Panés J, Gelpí E, Roselló-Catafau J, Anderson DC, Navarro S, Piqué JM, Fernández-Cruz L, Closa D. Role of P-selectin and ICAM-1 in pancreatitis-induced lung inflammation in rats: significance of oxidative stress. Ann Surg. 1999;230:792–798. doi: 10.1097/00000658-199912000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Telek G, Ducroc R, Scoazec JY, Pasquier C, Feldmann G, Rozé C. Differential upregulation of cellular adhesion molecules at the sites of oxidative stress in experimental acute pancreatitis. J Surg Res. 2001;96:56–67. doi: 10.1006/jsre.2000.6052. [DOI] [PubMed] [Google Scholar]
- 40.Kvietys PR, Granger DN. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med. 2012;52:556–592. doi: 10.1016/j.freeradbiomed.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park MY, Jeong YJ, Kang GC, Kim MH, Kim SH, Chung HJ, Jung JY, Kim WJ. Nitric oxide-induced apoptosis of human dental pulp cells is mediated by the mitochondria-dependent pathway. Korean J Physiol Pharmacol. 2014;18:25–32. doi: 10.4196/kjpp.2014.18.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obermaier R, von Dobschuetz E, Muhs O, Keck T, Drognitz O, Jonas L, Schareck W, Hopt UT, Benz S. Influence of nitric oxide on microcirculation in pancreatic ischemia/reperfusion injury: an intravital microscopic study. Transpl Int. 2004;17:208–214. doi: 10.1007/s00147-004-0702-y. [DOI] [PubMed] [Google Scholar]
- 43.De Gasparo M. Angiotensin II and nitric oxide interaction. Heart Fail Rev. 2002;7:347–358. doi: 10.1023/a:1020714518246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers for quantitative PCR