Abstract
Here, we investigated the role of zerumbone, a natural cyclic sesquiterpene of Zingiber zerumbet Smith, on angiogenesis using human umbilical vein endothelial cells (HUVECs). Zerumbone inhibited HUVECs proliferation, migration and tubule formation, as well as angiogenic activity by rat aorta explants. In particular, zerumbone inhibited phosphorylation of vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1, which are key regulators of endothelial cell function and angiogenesis. In vivo matrigel plug assay in mice demonstrated significant decrease in vascularization and hemoglobin content in the plugs from zerumbone-treated mice, compared with control mice. Overall, these results suggest that zerumbone inhibits various attributes of angiogenesis, which might contribute to its reported antitumor effects.
Keywords: Angiogenesis, Zerumbone, Vascular endothelial growth factor receptor, Human umbilical vein endothelial cells
INTRODUCTION
Phytochemicals are non-nutritive compounds found in plants. They are attracting research interest because of their health-promoting effects [1]. Epidemiological studies have consistently indicated a relationship between copious consumption of fruits and vegetables and reduction in the risk of developing several chronic diseases [2]. These health benefits have spurred the widespread purchase and consumption of phytochemical-enriched foods. Most of the populace in developing countries mainly relies on herbs to treat disease, which are considered to be safer and more effective than synthetic drugs [3,4]. Still, how phytochemicals beneficially affect physiological conditions remains unclear.
Angiogenesis is the development of new blood vessels from pre-existing vessel. These complex and multistep process includes proliferation, migration and tube formation of endothelial cells [5]. The angiogenic process involves the activation, proliferation and migration of endothelial cells toward various angiogenic stimuli, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) [6,7,8,9]. Interestingly, many cancer cells can induce secretion of several pro-angiogenic factors, which is commonly observed in most aggressive tumors [8]. Also, a tumor creates a favorable microenvironment that induces angiogenesis and facilitates cell invasion to promote continued tumor growth and metastasis [10,11]. Since angiogenesis is essential for tumor development and tumor vasculature is an optimal target for anti-cancer strategies, anti-angiogenic compounds may act as cancer treatment agents or adjuncts to standard chemotherapeutic regimens [12]. In this regard, non-toxic anti-angiogenic phytochemicals could have greater practical significance compared to non-selective cytotoxic therapies to control the tumor growth and metastasis by targeting angiogenesis.
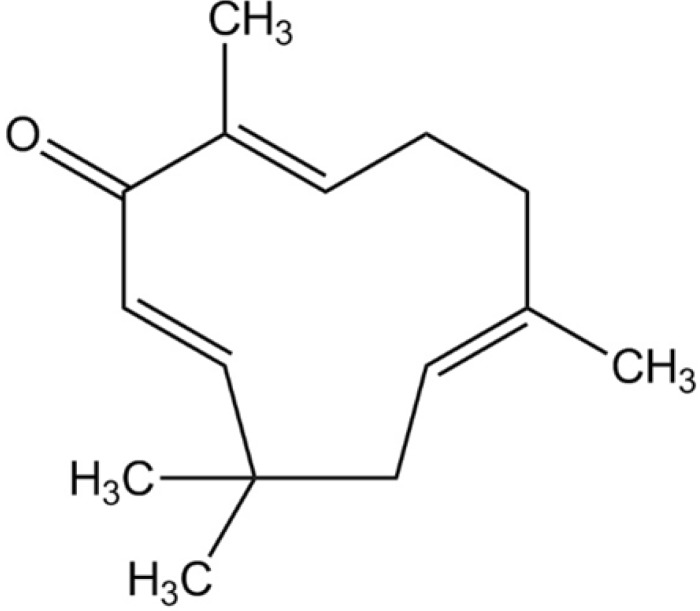
Zerumbone ((2E, 6E, 10E)-2, 6, 9, 9-tetramethylcycloundeca-2, 6, 10-trien-1-one, Fig. 1) is a phytochemical that was first isolated from the essential volatile oil of rhizomes of Zingiber zerumbet [13]. Its potentially broad use in the treatment of cancers, leukemia and virus infection has been indicated [14,15,16]. In addition, zerumbone has a variety of pharmacological effects, which include anti-oxidant, anti-inflammatory and anti-bacterial activity [17,18]. More recently, zerumbone was demonstrated to inhibit angiogenesis by blocking nuclear factor-κB in pancreatic and gastric cancer [19,20]. But, the detail mechanisms of its anti-angiogenic effect on human endothelial cells remain unclear.
Fig. 1. Structure of Zerumbone.
The present study reports that zerumbone can suppress the proliferation, migration and tube formation of endothelial cells in vitro and angiogenesis in vivo by blocking essential angiogenic signaling pathways.
METHODS
Materials
Zerumbone, heparin, dimethylsulfoxide (DMSO), HEPES solution and anti-β-actin antibody were obtained from Sigma-Aldrich (St. Louis, MO, USA). Medium 199 (M199), fetal bovine serum (FBS), penicillin and streptomycin were obtained from Hyclone (Logan, UT, USA). Endothelial cell growth supplement (ECGS) and matrigel were purchased from BD Bioscience (Becton, Dickinson, USA). Cell Titer 96® AQueous One Solution Cell Proliferation assay kits and CytoTox 96® Non-Radioactive Cytotoxicity assay kits were purchased from Promega (Madison, MI, USA). Anti-phospho-VEGFR2 antibody, VEGFR2 antibody, FGFR antibody, recombinant mouse VEGF164 and bFGF were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-phospho-FGFR antibody was obtained from R&D systems (Minneapolis, MN, USA). Recombinant human VEGF165 and bFGF were purchased from Pepprotech (Rocky Hill, NJ, USA).
Cell culture
Human umbilical vein endothelia cells (HUVECs) were obtained from Merck Millipore (Darmstadt, Deutschland, Germany) and maintained in M199 medium containing 20% FBS, 50 µg/ml ECGS, 100 µg/ml heparin, 25 mM HEPES solution, 100 U/ml penicillin and 100 vg/ml streptomycin (complement M199 medium) at 37℃ in 5% CO2. HUVECs were used at passages 3~5 for all the experiments.
Cell proliferation assay
The effect of zerumbone on the proliferation of HUVECs was measured by Cell Titer 96 Aqueous One solution cell proliferation assay (MTS assay). HUVECs were seeded at 5×103 per well in 96-well plate with 200 µl of complete M199 medium for 24 h before treatment. Fresh complete M199 medium (200 µl) containing various concentrations of zerumbone was added into each well to treat cells for 24 h. Cell Titer 96® AQueous One Solution Cell Proliferation assay kits was used according to manufacturer's protocol to determine the number of viable cells. Absorbance was read at 490 nm using a microplate reader (Tecan, San Jose, CA, USA). Percentage of cell proliferation was determined using the following formula: (Absorbance test well/ Absorbance 0 µM-treated well)×100.
Lactate dehydrogenase (LDH) toxicity assay
To determine of cytotoxicity of zerumbone on HUVECs, the LDH release assay was performed using a CytoTox 96® Non-Radioactive Cytotoxicity assay kits according to the manufacturer's instructions.
Wound healing migration assay
HUVECs were seeded into 48-well dishes and grew until 80~90% confluence. Sterilized one-milliliter pipette tip was used to generate wounding across the cell monolayer, and the debris was washed with PBS. Fresh complete M199 medium containing different concentrations of zerumbone was added to the scratched monolayers. Cells migrated into the wounded area were taken using an inverted microscope (Leica DM500, Leica Microsystems, Heerbrugg, Switzerland) at 40×magnification after 18 h of incubation. A total of nine areas were selected randomly in three wells of each group were quantified in each experiment. The assay was repeated three times independently. Data are expressed as the percent of control migration (0 µM-treated cells). The assay was repeated three times independently.
Western blot analysis
HUVECs were treated with indicated concentration of zerumbone and recombinant human VEGF165 (10 ng/ml) or bFGF (50 ng/ml) for 15 min. Whole cell lysates were extracted using PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Seongnam-Si, Korea) according to the manufacturer's instructions and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were electrophoretically transferred onto nitrocellulose membranes. Immunoreactive bands were detected by incubating the samples with horseradish peroxidase-conjugated secondary antibodies and visualized using a Femto Supersignal Chemiluminescent Substrate (Thermo, Rockford, USA) and Davinch-Chemi CAS-400SM Western Imaging System (Davinch-K, Seoul, Korea). The density of bands was assessed by Total Lab software (Davinch-K).
Capillary tube formation assay
Matrigel was thawed at 4℃ overnight, after which each well of 48-well plates were coated with 150 µl matrigel and incubated at 37℃ for 1 h. HUVECs (5×104 cells) were added in 500 µl complete M199 medium with zerumbone at 37℃ in a humidified 5% CO2 atmosphere. Images of the formation of capillary-like structures were obtained after 18 h with a computer-assisted Leica DM500 microscope microscope at ×40 magnification. Tubular structures were quantified by manually counting the numbers of connected cells in randomly selected fields; the total tube number in the control group (0 µM-treated cells) was designated as 100%. Tube formation was measured by taking photographs with the Leica DM500 microscope using a ×40 objective. The assay was repeated three times independently.
Rat aortic ring assay
48-well tissue culture plates were covered with 150 µl matrigel and allowed to gel for 30 to 45 min at 37℃, 5% CO2. Thoracic aortas were excised from 8-week-old male Sprague-Dawley rats (Hyochang Science, Deagu, Korea), and the fibroadipose tissue was removed. The aortas were sectioned into 1 mm to 1.5 mm-long cross sections, rinsed several times with PBS, placed on matrigel in wells and covered with an additional 100 µl of matrigel. The rings were cultured in 1.5 ml of M199 medium without serum for 24 h, and then the medium was replaced with 1.5 ml of complete M199 medium with indicated concentration of zerumbone. The medium was changed every 2 days. After 7 days of incubation, the rings were fixed with 4% PFA. The microvessel growth was measured in photographs taken with a Leica DM500 microscope using a ×40 objective.
Matrigel plug assay
Four-week-old C57BL/6 male mice were obtained from Hyochang Science and were subcutaneously injected with 500 µl of matirgel containing 100 ng/ml of recombinant mouse VEGF164, 100 ng/ml of recombinant mouse bFGF and 25 U heparin with indicated concentration of zerumbone. The mice were sacrificed after 7 days and the matrigel plugs were immediately photographed and weighed. To quantitate the vascularisation of the plug, the amount of hemoglobin (Hb) accumulated in the plug was measured using Drabkin's reagent (Sigma) following the manufacturer's protocol.
In addition, the removed matrigel plugs were fixed in 4± PFA followed by embedding in paraffin. Sections were then stained with hematoxylin-eosin (H&E) staining. Red blood cells were measured in photographs taken with a Leica DM500 microscope using a ×40 objective. The protocol was reviewed by the Institutional Animal Care and Use Committee (IACUC) of Catholic University of Daegu (Accredited Unit No. CUD-2013-024).
Statistical analysis
The data are means±SEM of at least three independent experiments. The values were evaluated by one-way analysis of variance (ANOVA) with Bonferroni multiple comparison post tests using GraphPad Prism 4.0 software (Graph-Pad Software Inc., San Diego, CA). p-values<0.05 were considered statistically significant.
RESULTS
Zerumbone inhibits HUVEC migration and tube formation
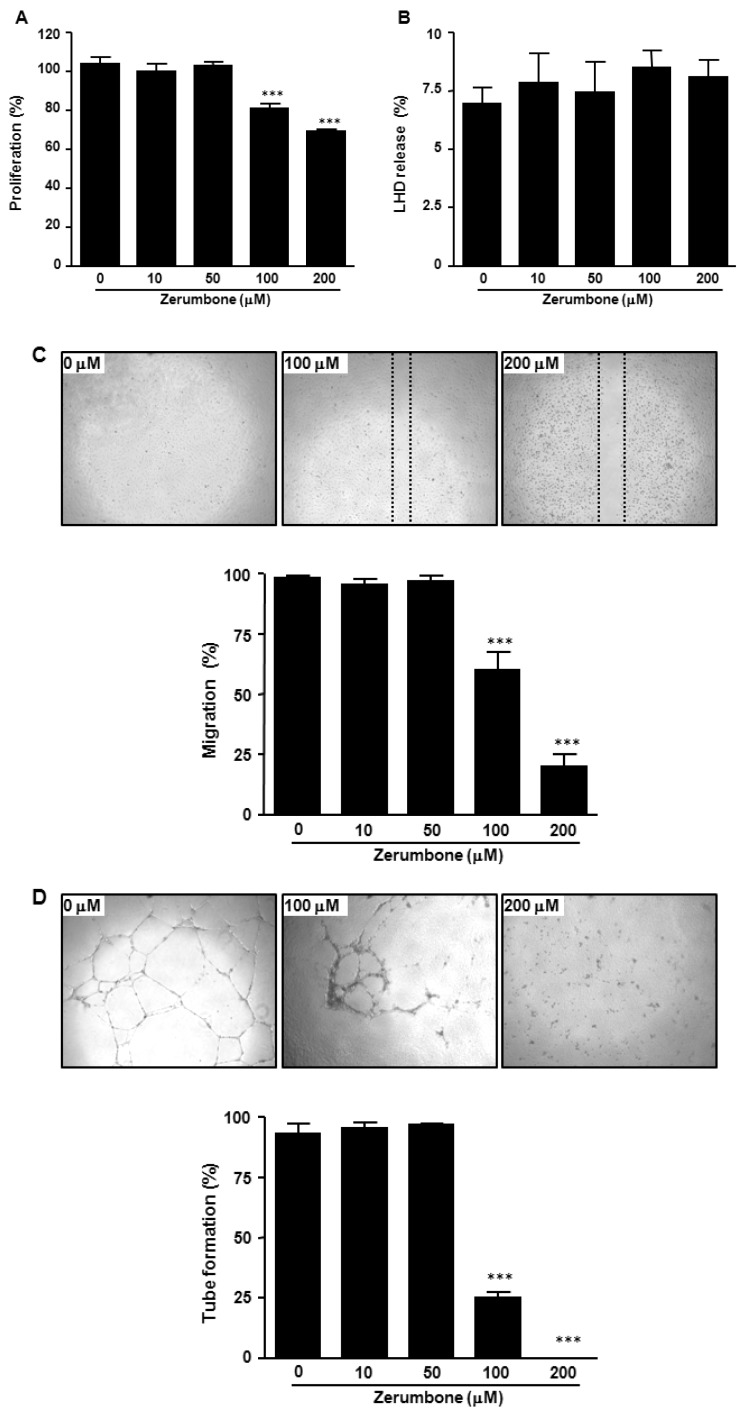
We first assessed whether zerumbone treatment affected HUVECs proliferation using the MTS assay. Treatment with zerumbone induced a dose-dependent anti-proliferative effect in HUVECs (Fig. 2A). Zerumbone treatment at >100 µM inhibited the proliferation of HUVECs. To confirm whether the anti-proliferative effect of zerumbone was due to cytotoxicity, we next measured activity of LDH, a stable enzyme in the cell cytoplasm that is rapidly released into the culture medium upon damage of the plasma membrane. As shown in Fig. 2B, zerumbone had no effect on cytotoxicity in HUVECs suggesting that the anti-proliferative activity of zerumbone on HUVECs is not due to cytotoxicity.
Fig. 2. Inhibition of endothelial cell proliferation, migration, and capillary-like tubule formation by zerumbone. HUVECs were plated in 96-well plates, allowed to attach overnight, and then cultured for 24 h with indicated concentration of zerumbone. (A) The proliferation and (B) LDH releases were determined as described in the Methods section. (C) For cell migration, a monolayer of inactivated HUVECs was wounded by scratching with a 0.1 ml pipette tip, and fresh medium containing indicated concentration of zerumbone was added. After 18 h, migration of HUVECs was measured as described in the Methods section. (D) For capillary-like tubule formation, HUVECs were seeded onto matrigel-coated 48-well plates and incubated with indicated concentration of zerumbone for 18 h. Endothelial tubules were photographed and quantitated. The results are means±SEM for at least three independent experiments. ***p<0.001 versus 0 µM zerumbone-treated cells.
Endothelial migration is the key process in angiogenesis. To determine whether zerumbone could inhibit endothelial cell migration, wound-healing assay was employed to explore the changes in migration. As shown in Fig. 2C, the endothelial cell migration was significantly blocked by 100 and 200 µM zerumbone in a concentration-dependent manner.
We next analyzed the effect of zerumbone on tube formation of HUVECs. Tube formation of endothelial cell recapitulates some angiogenesis steps, such as cell migration, alignment, formation of tubes and tube branching, and anastomosis with adjacent tubes [21]. Presently, when HUVECs were plated on matrigel in a short-term culture, they displayed alignment into networks of tubules (Fig. 2D). Zerumbone at 100 and 200 µM strongly disrupted HUVEC capillary morphogenesis (Fig. 2D). These results suggested that zerumbone inhibits endothelial cell differentiation and indicated its potential angiogenesis inhibition activity.
Zerumbone inhibits microvessel outgrowth from rat aortic ring
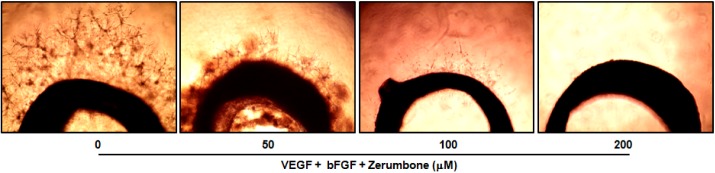
An ex vivo rat thoracic aorta ring assay was employed to evaluate the anti-angiogenic activity of zerumbone. The rat thoracic aorta ring model mimics the in vivo condition compared with the HUVECs tuber formation model in view of the multiple steps (sprouting, proliferation, migration and differentiation) involved in angiogenesis [22]. As predicted, zerumbone appeared to reduce microvessel outgrowth in a concentration-dependent manner, with angiogenesis being nearly abrogated at 100 and 200 µM (Fig. 3).
Fig. 3. Effect of zerumbone on microvessel outgrowth arising from rat aortic rings. Aortic rings isolated from SD rats were embedded in matrigel in 48-well plates, and then fed with medium containing zerumbone for 7 days. Photographs are representative of three independent experiments.
Zerumbone down-regulates signaling molecules
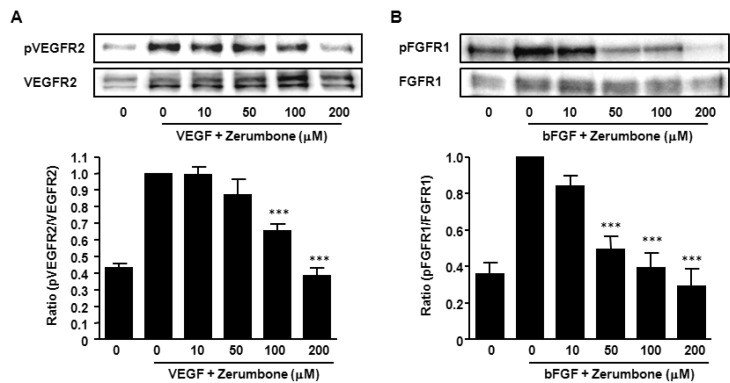
To investigate the molecular mechanism of the anti-angiogenic effect of zerumbone, the essential signal pathways of angiogenesis were explored. VEGFR2 is the primary receptor for VEGF that mediates angiogenic activity, endothelial cell proliferation, migration, differentiation and tube formation [23]. Appropriately we tested whether zerumbone interacted with the VEGF/VEGFR2 signaling pathway. VEGFR2 was phosphorylated by exogenous VEGF in HUVECs and zerumbone blocked this phosphorylation in a concentration-dependent manner (Fig. 4A).
Fig. 4. Effects of zerumbone on VEGFR2- and FGFR1-mediated signaling pathways. (A) HUVECs were treated with various concentrations of zerumbone and stimulated with human VEGF (10 ng/ml) for 15 min. Phosphorylation of VEGFR2 by VEGF was determined using Western blot. (B) HUVECs were treated with zerumbone and stimulated with human bFGF (50 ng/ml) for 15 min. Phosphorylation of of FGFR by bFGF were determined using Western blot. The bands were quantified using image analysis software and their relative intensity was expressed as fold against the image of the VEGF- or bFGF- stimulated cells. ***p<0.001 versus VEGF or bFGF+0 µM zerumbone-treated cells.
In addition to VEGF, bFGF are an important class of angiogenic factors [24,25]. We examined whether zerumbone also inhibited phosphorylation of FGFR1 induced by bFGF stimulation. The observation of the markedly reduced levels of phosphorylated-FGFR1 in the presence of zerumbone (Fig. 4B) indicated that zerumbone-mediated interference with the VEGF and FGF signaling pathway might, at least in part, be the basis of the anti-angiogenic effect.
Zerumbone inhibits angiogenesis in vivo
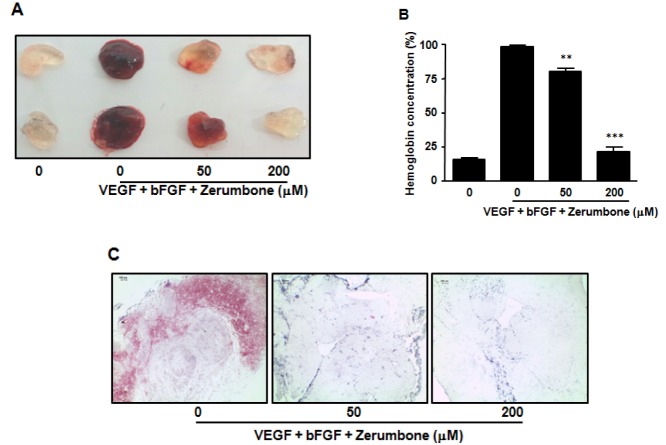
To further examine the anti-angiogenic effects of zerumbone in vivo, we tested its effects using the mouse matrigel plug neovascularization assay. C57BL/6 mice received subcutaneous matrigel containing angiogenic factors (VEGF, bFGF and heparin) in the absence or presence of zerumbone. After 7 days, the plugs were removed and matrigel hemoglobin content was measured. The positive control group (VEGF, bFGF and heparin) displayed extensive neovascularization of the matrigel, as shown by the red color distributed in the whole plug (Figs. 5A, 5B). In contrast, matrigel implants treated with both angiogenic factor and zerumbone (200 µM) displayed strong inhibition of vascular development similar to that of the negative control (matrigel alone). In addition, H&E staining showed that the presence of red blood cells was markedly reduced in the zerumbone-containing plug (Fig. 5C). This observed in vivo anti-angiogenic activity was consistent with the in vitro anti-angiogenic effect of zerumbone.
Fig. 5. Effects of zerumbone in vivo angiogenesis by matrigel plug assay. (A) Matrigel containing mouse VEGF (100 ng/ml), mouse bFGF (100 ng/ml), and zerumbone was subcutaneously injected into C57BL/6J mice. After 7 days, matrigels ware removed and photographed. (B) Paraffin sections of matrigel plugs were stained with hematoxylin and eosin. Original magnification, ×100; Scale bar 100 µm. (C) The quantification of neovascularization on the matrigel was performed by measuring the hemoglobin content. The results are reported as the mean±SEM of three independent experiments. Statistical significance is based on the difference when compared with non-treated animals, **p<0.01, ***p<0.001.
DISCUSSION
Presently, zerumbone inhibited the proliferation, migration and capillary tube formation of HUVECs in a non-cytotoxic fashion (Fig. 2). Coincidentally, zerumbone potently inhibited the outgrowth of new blood vessels in the rat aortic rings in a dose-dependent manner (Fig. 3). Zerumbone treatment also blocked angiogenesis in mice as demonstrating in the matrigel plug assay (Fig. 5). Our data might point toward an anti-angiogenic activity of zerumbone. However, it has to be mentioned that the in vitro data were gained at a dose of 100 and 200 µM. As demonstrated, lower than 100 µM of zerumbone hardly showed anti-angiogenic activity. Thus, 100 µM seems to be (near) the minimum dose to exert anti-angiogenic effect of zerumbone.
Angiogenesis is vital for the growth and development of an organism, as well as in wound repair. The human body regulates angiogenesis by balancing stimulatory and inhibitory factors. Because this process is essential for healing, growth, development and maintenance, angiogenesis is a therapeutic target in various diseases. Among these diseases, cancer is associated with angiogenesis [26]. Angiogenesis is important for multiple steps in the progression of cancer pathophysiology, extending from the initial development of the tumor to the growth in the local environment invasion and extravasation into the vasculature. Anti-angiogenic agents are attractive for the treatment of several cancers [12]. The anti-angiogenic activity of zerumbone may be at least a partial basis of the compound's anti-cancer activity.
A variety of different growth factors can promote angiogenesis. One of the most important is VEGF. VEGF is a soluble protein secreted by most types of cells, but not endothelial cells [5]. Endothelial cells express receptors for this growth factor [27]. VEGF binding normally triggers the phosphorylation of its receptor, which in turn initiates an intracellular cascade of signaling events leading to physiological pro-angiogenic responses. Among their receptors, VEGFR2 phosphorylation may represent an important mechanism for the control of angiogenesis [7,27]. Inhibition of VEGFR2 phosphorylation by zerumbone might be a potential strategy to regulate angiogenesis. Therefore, we examined the effects of zerumbone on VEGFR2 expression and the VEGFR2 phosphorylation in HUVECs. Zerumbone at concentrations of 100 and 200 µM inhibited VEGF-induced VEGFR2 phosphorylation in HUVECs without any change the total VEGFR2 expression (Fig. 4A). These results implicate interference with VEGFR phosphorylation in the anti-angiogenic effect of zerumbone.
While the exact role of bFGF in tumor angiogenesis remains elusive, this potent mitogen of vascular endothelial cells and fibroblasts is believed to cooperate with VEGF during blood vessel development [28]. bFGF mediates its activity by primarily binding with FGFR1 and recruitment of vascular sprouting [24,25]. Since VEGFR2 and FGFR1 directly influence tumor angiogenesis, simultaneous inhibition of these two receptor tyrosine kinases is postulated to enhance antitumor activity. To this end, small molecule inhibitors of these receptors that inhibit VEGFR2 and FGFR1 have been described [29,30,31]. Furthermore, since anti-angiogenic therapies are directed toward highly regulated endothelial cells of the host, rather than genetically unstable tumor cells, it is anticipated that selective VEGFR2 and FGFR1 inhibition will lead to cancer therapeutics that have a lower risk of developing classical drug resistance compared to traditional chemotherapy. In this regard, zerumbone might be an ideal agent to regulate angiogenesis because it blocks the VEGFR2 and FGFR1 signaling pathways that are essential for angiogenesis.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2013R1A1A2057742).
ABBREVIATIONS
- HUVECs
human umbilical vein endothelial cells
- FGF
fibroblast growth factor
- VEGF
vascular endothelial growth factor
References
- 1.Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4:384S–392S. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Key TJ, Appleby PN, Crowe FL, Bradbury KE, Schmidt JA, Travis RC. Cancer in British vegetarians: updated analyses of 4998 incident cancers in a cohort of 32,491 meat eaters, 8612 fish eaters, 18,298 vegetarians, and 2246 vegans. Am J Clin Nutr. 2014;100(Suppl 1):378S–385S. doi: 10.3945/ajcn.113.071266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 4.Canter PH, Thomas H, Ernst E. Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol. 2005;23:180–185. doi: 10.1016/j.tibtech.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 6.Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986;83:7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 9.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 10.Spano D, Zollo M. Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastasis. 2012;29:381–395. doi: 10.1007/s10585-012-9457-5. [DOI] [PubMed] [Google Scholar]
- 11.Gao F, Liang B, Reddy ST, Farias-Eisner R, Su X. Role of inflammation-associated microenvironment in tumorigenesis and metastasis. Curr Cancer Drug Targets. 2014;14:30–45. doi: 10.2174/15680096113136660107. [DOI] [PubMed] [Google Scholar]
- 12.Bellou S, Pentheroudakis G, Murphy C, Fotsis T. Anti-angiogenesis in cancer therapy: Hercules and hydra. Cancer Lett. 2013;338:219–228. doi: 10.1016/j.canlet.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Dev S. Studies in sesquiterpenes-XVI: zerumbone, a monocyclic sesquiterpene ketone. Tetrahedron. 1960;8:171–180. [Google Scholar]
- 14.Murakami A, Takahashi M, Jiwajinda S, Koshimizu K, Ohigashi H. Identification of zerumbone in Zingiber zerumbet Smith as a potent inhibitor of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation. Biosci Biotechnol Biochem. 1999;63:1811–1812. doi: 10.1271/bbb.63.1811. [DOI] [PubMed] [Google Scholar]
- 15.Kirana C, McIntosh GH, Record IR, Jones GP. Antitumor activity of extract of Zingiber aromaticum and its bioactive sesquiterpenoid zerumbone. Nutr Cancer. 2003;45:218–225. doi: 10.1207/S15327914NC4502_12. [DOI] [PubMed] [Google Scholar]
- 16.Huang GC, Chien TY, Chen LG, Wang CC. Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo. Planta Med. 2005;71:219–224. doi: 10.1055/s-2005-837820. [DOI] [PubMed] [Google Scholar]
- 17.Szabolcs A, Tiszlavicz L, Kaszaki J, Pósa A, Berkó A, Varga IS, Boros I, Szüts V, Lonovics J, Takács T. Zerumbone exerts a beneficial effect on inflammatory parameters of cholecystokinin octapeptide-induced experimental pancreatitis but fails to improve histology. Pancreas. 2007;35:249–255. doi: 10.1097/mpa.0b013e318070d791. [DOI] [PubMed] [Google Scholar]
- 18.Kitayama T. Attractive reactivity of a natural product, zerumbone. Biosci Biotechnol Biochem. 2011;75:199–207. doi: 10.1271/bbb.100532. [DOI] [PubMed] [Google Scholar]
- 19.Shamoto T, Matsuo Y, Shibata T, Tsuboi K, Nagasaki T, Takahashi H, Funahashi H, Okada Y, Takeyama H. Zerumbone inhibits angiogenesis by blocking NF-κB activity in pancreatic cancer. Pancreas. 2014;43:396–404. doi: 10.1097/MPA.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 20.Tsuboi K, Matsuo Y, Shamoto T, Shibata T, Koide S, Morimoto M, Guha S, Sung B, Aggarwal BB, Takahashi H, Takeyama H. Zerumbone inhibits tumor angiogenesis via NF-κB in gastric cancer. Oncol Rep. 2014;31:57–64. doi: 10.3892/or.2013.2842. [DOI] [PubMed] [Google Scholar]
- 21.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12:267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 22.Go RS, Owen WG. The rat aortic ring assay for in vitro study of angiogenesis. Methods Mol Med. 2003;85:59–64. doi: 10.1385/1-59259-380-1:59. [DOI] [PubMed] [Google Scholar]
- 23.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woad KJ, Hunter MG, Mann GE, Laird M, Hammond AJ, Robinson RS. Fibroblast growth factor 2 is a key determinant of vascular sprouting during bovine luteal angiogenesis. Reproduction. 2012;143:35–43. doi: 10.1530/REP-11-0277. [DOI] [PubMed] [Google Scholar]
- 26.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 27.Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol. 2014;41:235–251. doi: 10.1053/j.seminoncol.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 29.Ayers M, Fargnoli J, Lewin A, Wu Q, Platero JS. Discovery and validation of biomarkers that respond to treatment with brivanib alaninate, a small-molecule VEGFR-2/FGFR-1 antagonist. Cancer Res. 2007;67:6899–6906. doi: 10.1158/0008-5472.CAN-06-4555. [DOI] [PubMed] [Google Scholar]
- 30.Renhowe PA, Pecchi S, Shafer CM, Machajewski TD, Jazan EM, Taylor C, Antonios-McCrea W, McBride CM, Frazier K, Wiesmann M, Lapointe GR, Feucht PH, Warne RL, Heise CC, Menezes D, Aardalen K, Ye H, He M, Le V, Vora J, Jansen JM, Wernette-Hammond ME, Harris AL. Design, structure-activity relationships and in vivo characterization of 4-amino-3-benzimidazol-2-ylhydroquinolin-2-ones: a novel class of receptor tyrosine kinase inhibitors. J Med Chem. 2009;52:278–292. doi: 10.1021/jm800790t. [DOI] [PubMed] [Google Scholar]
- 31.Bello E, Colella G, Scarlato V, Oliva P, Berndt A, Valbusa G, Serra SC, D'Incalci M, Cavalletti E, Giavazzi R, Damia G, Camboni G. E-3810 is a potent dual inhibitor of VEGFR and FGFR that exerts antitumor activity in multiple preclinical models. Cancer Res. 2011;71:1396–1405. doi: 10.1158/0008-5472.CAN-10-2700. [DOI] [PubMed] [Google Scholar]