Abstract
We examined the effects of peripherally or centrally administered botulinum neurotoxin type A (BoNT-A) on orofacial inflammatory pain to evaluate the antinociceptive effect of BoNT-A and its underlying mechanisms. The experiments were carried out on male Sprague-Dawley rats. Subcutaneous (3 U/kg) or intracisternal (0.3 or 1 U/kg) administration of BoNT-A significantly inhibited the formalin-induced nociceptive response in the second phase. Both subcutaneous (1 or 3 U/kg) and intracisternal (0.3 or 1 U/kg) injection of BoNT-A increased the latency of head withdrawal response in the complete Freund's adjuvant (CFA)-treated rats. Intracisternal administration of N-methyl-D-aspartate (NMDA) evoked nociceptive behavior via the activation of trigeminal neurons, which was attenuated by the subcutaneous or intracisternal injection of BoNT-A. Intracisternal injection of NMDA up-regulated c-Fos expression in the trigeminal neurons of the medullary dorsal horn. Subcutaneous (3 U/kg) or intracisternal (1 U/kg) administration of BoNT-A significantly reduced the number of c-Fos immunoreactive neurons in the NMDA-treated rats. These results suggest that the central antinociceptive effects the peripherally or centrally administered BoNT-A are mediated by transcytosed BoNT-A or direct inhibition of trigeminal neurons. Our data suggest that central targets of BoNT-A might provide a new therapeutic tool for the treatment of orofacial chronic pain conditions.
Keywords: BoNT-A, NMDA, Pain, Transcytosed, Trigeminal
INTRODUCTION
Botulinum neurotoxins (BoNTs), synthesized by the anaerobic bacterium Clostridium botulinum, consist of a heavy and light chain linked together by a disulfide bond [1]. BoNTs inhibit acetylcholine release from the peripheral nerve terminal by the cleavage of the soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) protein complex, which is required for neurotransmitter release [2,3]. Recent evidence support that botulinum neurotoxin type A (BoNT-A) can be used for the treatment of various pain syndromes. In clinical practice, BoNT-A treatment was effective in alleviating chronic tension-type headache [4], chronic low back pain [5], musculoskeletal pain [6], migraine [7,8], and myofacial pain [9]. In animal studies, peripheral application of BoNT-A into the rat paw pad significantly reduced the enhanced sensitivity to mechanical and thermal stimuli provoked by carrageenan or capsaicin injections [10], and reduced the formalin-induced nociceptive behaviors [11,12].
In addition to its peripheral actions, central administration of BoNT-A also modulates pain processing. Intrathecal administration of BoNT-A significantly decreased the formalin-induced nociceptive responses in mice [12,13] and the hypersensitivity to acidic saline-induced bilateral pain [14]. These results suggest that central administration of BoNT-A induces antinociception through blockade of neurotransmitter release at the central terminals of primary afferent nerves. However, these results did not reveal whether BoNT-A acts on neurons in the spinal cord beyond the primary afferent nerve fibers.
Previous studies also demonstrated that BoNT-A attenuated orofacial nociception. Intradermal injection of BoNT-A in the innervation area of the infraorbital branch of the trigeminal nerve alleviated mechanical allodynia in rats with chronic constriction injury of the infraorbital nerve [15]. In a clinical study, BoNT-A suppressed experimental trigeminal/cervical pain evoked by the intradermal injection of capsaicin into the forehead [16]. Moreover, BoNT-A was effective in patients with chronic facial pain including temporomandibular joint syndrome, postsurgical pain syndromes, essential headache, and idiopathic trigeminal neuralgia [17]. These results suggest that BoNT-A might be beneficial for the modulation of orofacial pain. However, the application of BoNT-A is very limited for the treatment of orofacial nociception.
Here we examined the effects of peripherally and centrally administered BoNT-A on the orofacial formalin response and complete Freund's adjuvant (CFA)-induced thermal hypersensitivity in rats. We also investigated the effect of peripherally and centrally administered BoNT-A on the intracisternal NMDA-induced nociceptive behavior by the direct activation of trigeminal neurons. To confirm participation of trigeminal neurons in BoNT-A-induced antinociception, we evaluated changes in c-Fos expression in the medullary dorsal horn after the subcutaneous or intracisternal injection of BoNT-A.
METHODS
Animals
The experiments were carried out on male Sprague-Dawley rats weighing between 230 and 280 g. The rats were maintained at a constant temperature and under a standard 12 h/12 h light/dark cycle. Food and water were freely available. All procedures including the use of animals were approved by the Institutional Care and Use Committee of the School of Dentistry, Kyungpook National University (Daegu, Korea). Experiments were carried out in accordance with the ethical guidelines for the investigation of experimental pain in conscious animals issued by the International Association for the Study of Pain (1982). All experiments were performed in a blinded manner.
Intracisternal catheterization
For intracisternal injections, the rats were anesthetized with a mixture of ketamine (40 mg/kg) and xylazine (4 mg/kg). The anesthetized rat was mounted onto a stereotaxic frame. A polyethylene tube (PE10; Clay Adams, Parsippany, NJ) was implanted, as described previously [18,19,20]. The polyethylene tube was inserted through a tiny hole made in the atlanto-occipital membrane and dura using a 27-gauge syringe needle. The tip of the cannula was placed at the obex level. The PE10 tube was guided subcutaneously to the top of the skull and secured in place with a stainless steel screw and dental acrylic resin. The drugs were administered intracisternally after a 72-h recovery period following surgery.
Orofacial pain models
Orofacial formalin response
The formalin test was performed to assess the inflammatory nociceptive response in the orofacial region of rats [21,22,23]. Briefly, 40 µl of 3% formalin was applied subcutaneously into the vibrissa pad. For each animal, the number of noxious behavioral responses, including rubbing of the facial region proximal to the injection site, was recorded over 9 sequential 5-min intervals. The nociceptive responses were recorded for 45 min after the formalin injection. The orofacial formalin responses showed 2 distinct phases; an early, short response (0 to 10 min, first phase) and a continuous, prolonged response (11 to 45 min, second phase) that were separated by an interval of relative inactivity [24,25].
CFA-induced thermal hypersensitivity
CFA (Sigma-Aldrich, St Louis, MO) was used to induce chronic inflammation. A 40 µl of CFA were injected subcutaneously into the left vibrissa pad. A previous study reported that the subcutaneous injection of CFA induced thermal hypersensitivity within 3 days, which peaked on postoperative day 5, and returned to the preoperative levels on postoperative day 14 [26]. To evaluate heat hypersensitivity, each rat was placed in a customized cylindrical acrylic rodent restrainer (height 40~60 mm, length 70~120 mm). The restrainer had a hole on the top to allow thermal stimulation of the head and head withdrawal. Each restrainer was placed in a darkened and noise-free room, and animals were habituated to the room and apparatus for a minimum of 30 min before the experiment. After the application of radiant heat, the latency for head withdrawal was recorded as described previously [27,28,29]. Heat stimulus was applied using an infrared thermal stimulator (Infrared Diode Laser, LVI-808-10, LVI tech, Seoul, Korea) set to 11 W and 18.1 A. When the distance between the heat source and vibrissa pad was 10 cm, we noticed a stable head withdrawal with a latency of approximately 12 s. Thermal hyperalgesia was tested 1, 3, 5, 7, 9, 11, 13, 15, and 18 days after CFA injection. The latency of head withdrawal was recorded three times with 5 min intervals. A cut-off time of 20 sec was used in these experiments to prevent possible tissue damage.
Nociceptive behavior induced by intracisternally injected NMDA
Wilcox (1988) first demonstrated that intrathecal administration of excitatory amino acids including NMDA produced behavior like that following the presentation of some noxious stimuli [32]. NMDA-induced nociceptive behavior was blocked pretreatment with DAMGO, a µ-opioid agonist. Previous studies also demonstrated that intrathecal or intracisternal administration of NMDA induced nociceptive behavior by activating spinal or trigeminal neurons [30,31,33]. In the present study, the orofacial nociceptive behavioral responses were monitored after the intracisternal injection of NMDA. Microinjection of NMDA (0.5 µg/7 µl) through the implanted PE10 tube induced an intense rubbing behavior exclusively in the orofacial region. For each animal, the number of rubbing actions targeting the facial region was recorded in freely moving rats.
Administration of BoNT-A
The present study investigated the peripheral and central effects of BoNT-A using orofacial pain models. We injected BoNT-A (1 or 3 U/kg, 30 µl) subcutaneously into the vibrissa pad 3 days before formalin, CFA, or NMDA injections to determine the peripheral effects of BoNT-A. BoNT-A (0.3 or 1 U/kg, 10 µl) was injected intracisternally 3 days before formalin, CFA, or NMDA injections with a Hamilton syringe connected to the implanted PE10 tube in freely moving rats to determine the central effects of BoNT-A. Hundred units of BoNT-A (Botulax®, Hugel Inc, Chuncheon, Korea) were diluted in 1 ml of saline.
Immunohistochemical staining of c-Fos
To examine whether subcutaneous or intracisternal injection of BoNT-A affects trigeminal neurons, we evaluated the changes in c-Fos expression in the medullary dorsal horn induced by NMDA injection in rats. BoNT-A was injected subcutaneously (3 U/kg) or intracisternally (1 U/kg) 3 days before NMDA injection. Rats (n=5 per group) were anesthetized, and then perfused transcardially with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The caudal medulla was dissected and postfixed in the same fixative at 4℃ for 2 h, and incubated with 30% sucrose in 0.1 M PB overnight. Specimens were frozen and cut into 30 µm thick transverse sections. Free-floating sections were rinsed in phosphate-buffered saline (PBS) and blocked with 2.5% normal horse serum (Vector Laboratories, Burlingame, CA) for 1 h at room temperature. The sections were incubated at 4℃ overnight with the rabbit polyclonal anti-c-Fos antibody (1:1000, Abcam, Cambridge, MA). Sections were then incubated in peroxidase-conjugated anti-rabbit IgG (Vector Laboratories) for 2 h at room temperature. After washing, sections were incubated in a buffer containing diaminobenzidine (DAB) and hydrogen peroxide (pH 7.5) for approximately 1 min (Vector Laboratories). Stained sections were analyzed using a microscope (BX 41 and U-RFL-T, Olympus, Tokyo, Japan). Neurons immunopositive for c-Fos were counted in the superficial laminae (I-II) of the medullary dorsal horn in three sections.
Statistical analysis
Statistical analysis of behavioral data in the CFA model was carried out using repeated measures analysis of variance (RM-ANOVA) followed by Holm-Sidak post hoc test. Behavioral data between the formalin and NMDA-treated groups were evaluated using one-way analysis of variance (ANOVA) followed by Holm-Sidak post hoc test. In all statistical comparisons, p<0.05 was used as criterion for significance. All data are presented as mean±standard error of the mean (SEM).
RESULTS
Effects of BoNT-A on the orofacial formalin-induced response
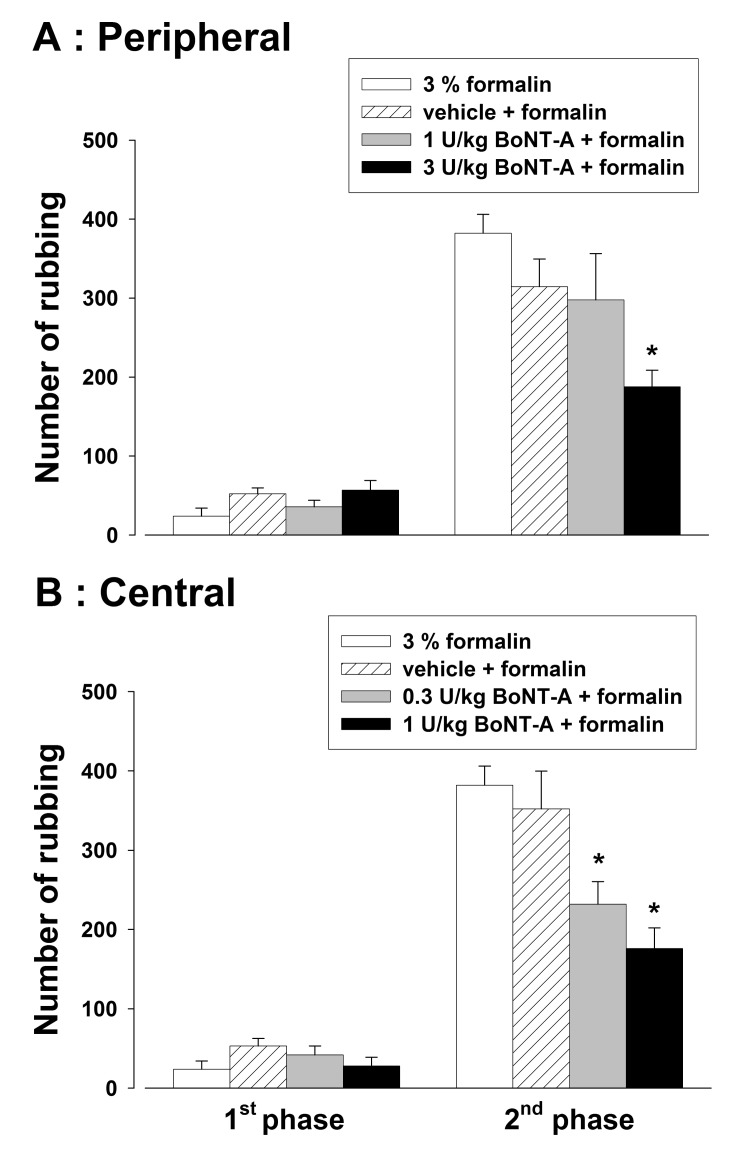
Effects of BoNT-A on the orofacial formalin-induced response are illustrated in Fig. 1. Subcutaneous injection of 40 µl of 3% formalin evoked a nociceptive behavior. Subcutaneous injection of 3 U/kg BoNT-A significantly inhibited the formalin-induced nociceptive behavior in the second phase (p<0.05, Fig. 1A). However, a low dose of BoNT-A (1 U/kg) and vehicle did not affect the formalin-induced nociceptive behavior. Intracisternal administration of vehicle did not affect the orofacial formalin-induced response. However, intracisternal administration of BoNT-A (0.3 and 1 U/kg) significantly reduced the number of rubbing responses in the second phase evoked by the injection of formalin (p<0.05, Fig. 1B).
Fig. 1. Effects of the subcutaneous (A) or intracisternal (B) injection of botulinum neurotoxin type A (BoNT-A) on the formalin-induced nociceptive behavior. Subcutaneous injection (1 or 3 U/kg) and intracisternal (0.3 or 1 U/kg) administration of BoNT-A attenuated the formalin-induced nociceptive behavior in the second phase. *p<0.05 vehicle vs. BoNT-A treated group, n=8 animals per group.
Effects of BoNT-A on CFA-induced thermal hypersensitivity
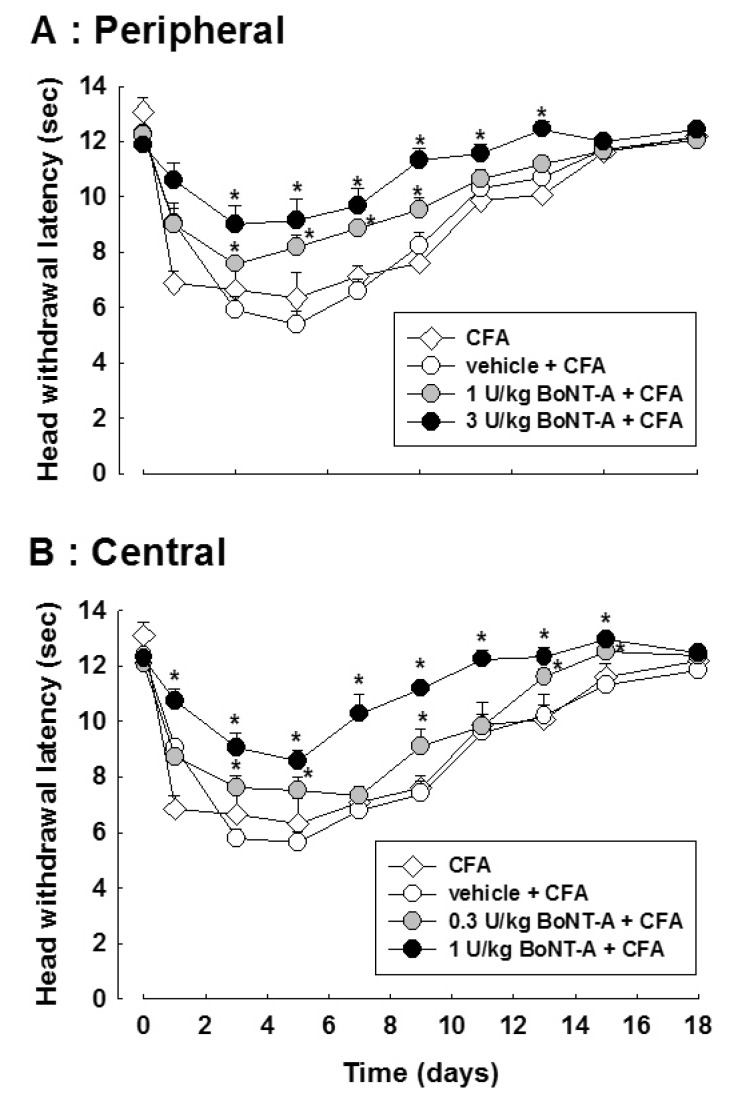
Fig. 2 illustrates the effects of BoNT-A on the CFA-induced thermal hyperalgesia in the orofacial region. Subcutaneous injection of CFA evoked thermal hypersensitivity. Subcutaneous injection of 1 or 3 U/kg BoNT-A increased the latency of the head withdrawal in CFA-treated rats (p<0.05, Fig. 2A). In addition, intracisternal administration of 0.3 or 1 U/kg BoNT-A blocked the thermal hyperalgesia in CFA-treated rats compared to that in vehicle treated rats (p<0.05, Fig. 2B). Both the subcutaneous and intracisternal injections of vehicle did not affect the head withdrawal latency in CFA-treated rats.
Fig. 2. Effects of the subcutaneous (A) or intracisternal (B) injection of botulinum neurotoxin type A (BoNT-A) on the complete Freund's adjuvant (CFA)-induced thermal hypersensitivity. Both subcutaneous and intracisternal injection of BoNT-A attenuated the head withdrawal latency induced by CFA injection. *p<0.05 vehicle vs. BoNT-A treated group, n=8 animals per group.
Effects of BoNT-A on the NMDA-induced nociceptive behavior
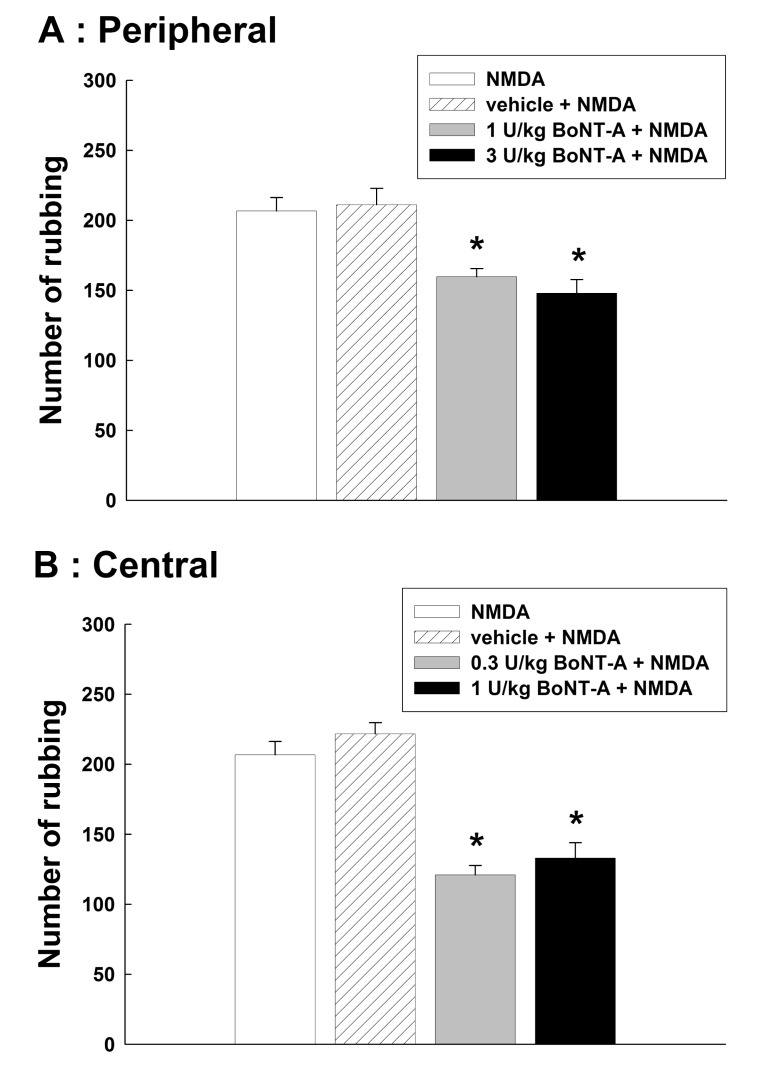
Fig. 3 illustrates the effects of BoNT-A on the nociceptive behavior induced by the intracisternal injection of NMDA. Intracisternal administration of 0.5 µg NMDA produced significant nociceptive behavior via the activation of trigeminal neurons. Subcutaneous injection of 1 or 3 U/kg BoNT-A attenuated the NMDA-induced nociceptive rubbing response (p<0.05, Fig. 3A). Then we examined the NMDA-induced nociceptive behavior after the intracisternal injection of BoNT-A. Intracisternal injection of 0.3 or 1 U/kg BoNT-A significantly reduced the number of rubbing induced by the intracisternal administration of NMDA (p<0.05, Fig. 3B).
Fig. 3. Effects of the subcutaneous (A) or intracisternal (B) injection of botulinum neurotoxin type A (BoNT-A) on the NMDA-induced nociceptive behavior. Intracisternal administration of 0.5 µg NMDA evoked a significant nociceptive behavior by the activation of trigeminal neurons. Both subcutaneous and intracisternal injections of BoNT-A attenuated the number of rubbing induced by the intracisternal injection of NMDA. *p<0.05 vehicle vs. BoNT-A treated group, n=8 animals per group.
Effects of BoNT-A on c-Fos expression in the NMDA-treated rats
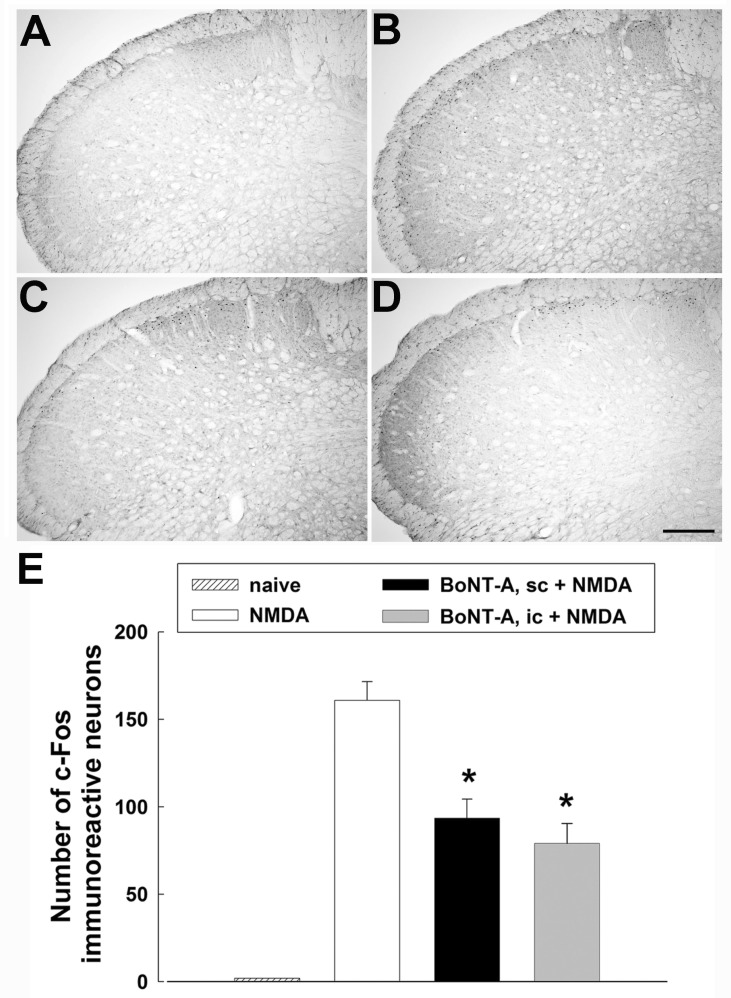
Effects of BoNT-A on c-Fos expression are illustrated in Fig. 4. Intracisternal injection of NMDA evoked intense rubbing. In addition to behavioral responses, intracisternal injection of 0.5 µg NMDA up-regulated c-Fos expression in the trigeminal neurons of the medullary dorsal horn. Most c-Fos-positive neurons were localized in the superficial layer (lamina I-II). Subcutaneous injection of BoNT-A (3 U/kg) significantly attenuated the number of c-Fos immunoreactive neurons induced by the intracisternal injection of NMDA (p<0.05, Fig. 4C). Additionally, intracisternal administration of BoNT-A (1 U/kg) significantly decreased the number of c-Fos immunoreactive neurons in the medullary dorsal horn (p<0.05, Fig. 4D).
Fig. 4. Effects of the subcutaneous or intracisternal injection of botulinum neurotoxin type A (BoNT-A) on c-Fos expression in the medullary dorsal horn. (A) c-Fos immunoreactive neurons in a naïve animal. (B) Intracisternal administration of 0.5 µg NMDA increased the number of c-Fos immunoreactive neurons in the superficial lamina I and II in the medullary dorsal horn. (C) Subcutaneous administration of BoNT-A (3 U/kg) decreased the number of c-Fos immunoreactive neurons. (D) Intracisternal injection of BoNT-A (1 U/kg) decreased the number of c-Fos immunoreactive neurons. (E) The histogram shows the number of c-Fos immunoreactive neurons in the ipsilateral medullary dorsal horn. *p<0.05 vehicle vs. BoNT-A treated group, n=5 animals per group. Scale bar, 100 µm.
DISCUSSION
This is the first study demonstrating that peripheral injection of BoNT-A has an antinociceptive effect on the trigeminal neurons via transcytosed BoNT-A. We showed that peripheral and central administration of BoNT-A attenuated the formalin-induced nociceptive behavior and CFA-induced thermal hyperalgesia in the orofacial region. The trigeminal NMDA receptor-mediated nociceptive behavior was also attenuated by the peripheral and central application of BoNT-A. Furthermore, immunohistochemical stainings revealed that subcutaneous or intracisternal administration of BoNT-A downregulated c-Fos expression in the medullary dorsal horn. These results suggest that the central antinociceptive effects of BoNT-A are mediated by transcytosed BoNT-A or direct inhibition of the trigeminal neurons when the peripheral or central administration of BoNT-A.
It has been well known that BoNT-A blocks acetylcholine release into the synaptic cleft [34,35] by the cleavage of SNARE protein complexes resulting in an incomplete formation of the SNARE complex [3,36]. This specialized action in the nerve terminals made BoNT-A a useful tool both in basic and clinical sciences for treating a variety of neuromuscular disorders including strabismus, blepharospasm, hemifacial spasm, and torticollis [37,38]. The present study demonstrated that subcutaneous injection of BoNT-A was antinociceptive in rats with orofacial formalin-induced behavioral responses and CFA-induced thermal hyperalgesia. The antinociceptive effects of peripherally administered BoNT-A are consistent with several previous studies [11,12,39,40]. The proposed underlying mechanism of the antinociceptive action after peripheral injection of BoNT-A is the retrograde axonal transport of BoNT-A from the periphery to the central terminals of the primary afferent fibers [41]. These results suggest that peripheral injection of BoNT-A evokes antinociceptive effects via the modulation of neurotransmitter release from the central terminals of the primary afferent fibers. These results are supported by several previous studies. Subcutaneous administration of BoNT-A, which reduced the formalin-induced nociceptive behaviors, inhibited glutamate release from the primary afferent terminals [11]. In addition, application of BoNT-A inhibited substance P secretion in embryonic rat dorsal root ganglia primary neuronal cultures [42]. Therefore, these previous studies suggest a possible underlying mechanism for the antinociceptive effect of peripherally injected BoNT-A.
Interestingly, the present study demonstrated that peripherally administered BoNT-A reduced the NMDA-induced nociceptive behavioral response. This suggests that peripheral injection of BoNT-A may affect trigeminal neurons beyond the synapses of primary afferent fibers in the medullary dorsal horn. This behavioral evidence is consistent with previous studies. Following a BoNT-A injection into the rat eye, catalytical active BoNT-A is transported from the eye to the superior colliculus via transcytosis in the tectal synapses [43]. After the unilateral delivery of BoNT-A into the hippocampus, SNAP-25 cleavage by BoNT-A was observed at the contralateral side [44]. However, there are no behavioral evidence for the antinociceptive effects of transcytosed BoNT-A after the peripheral injection of BoNT-A. Our results are the first to provide behavioral evidence that transcytosed BoNT-A evoked antinociception in the NMDA-treated rats after peripheral injection of BoNT-A. Our c-Fos data confirmed the behavioral effects of peripherally injected and transcytosed BoNT-A. Subcutaneous injection of BoNT-A attenuated the c-Fos expression evoked by the activation of trigeminal neurons in the medullary dorsal horn.
In addition to its peripheral action, intracisternally administered BoNT-A inhibited the formalin-induced nociceptive behavior and CFA-induced thermal hyperalgesia in our study. The central antinociceptive effects of BoNT-A are consistent with previous studies. Intrathecal administration of BoNT-A reduced the mechanical allodynia and thermal hyperalgesia induced by sciatic nerve ligation [45] and diabetic hyperalgesia [46] in rats. These results, taken together with the present data, suggest that BoNT-A exerts its antinociceptive effects by inhibiting neurotransmitter release from the central terminals of the primary afferent fibers. Here we showed that intracisternal administration of BoNT-A significantly inhibited the NMDA-induced nociceptive response. This result suggests that central administration of BoNT-A induces a strong antinociceptive effect, and BoNT-A-induced antinociception is mediated by the direct inhibition of trigeminal neurons. This central action is further supported by our immunohistochemical data, which revealed that intracisternal injection of BoNT-A attenuated the up-regulation of c-Fos expression in the medullary dorsal horn evoked by the intracisternal NMDA injection. Moreover, we showed that the dose required for the antinociceptive action of centrally injected BoNT-A is lower than that of peripherally injected BoNT-A. These results suggest that central administration of BoNT-A might be beneficial for treating chronic pain conditions in new therapeutic targets. However, the underlying mechanisms for the central antinociceptive effects of BoNT-A are not clear.
A recent study demonstrated that after the intraplantar injection of BoNT-A, neurons immunopositive for cl-SNAP-25 colocalized with astrocytes, which were clearly detected in mice with a chronic constriction injury of the sciatic nerve [41]. This suggests that glial cells are engaged in mediating the antinociceptive effects of BoNT. However, the cellular mechanisms of participation of glial cells in antinociceptive effects of BoNT require further investigations.
In summary, peripheral treatment with BoNT-A attenuated the formalin-induced nociceptive behavior and CFA-induced thermal hyperalgesia in the orofacial region. The trigeminal NMDA receptor-mediated nociceptive behavior and c-Fos expression was attenuated by the peripheral injection of BoNT-A. These results suggest that transcytosed BoNT-A induces antinociception when peripheral injection of BoNT-A. Intracisternal administration of BoNT-A also attenuated the NMDA-evoked nociceptive response and c-Fos expression. These results suggest that the central antinociceptive effect of BoNT-A is mediated by the transcytosed BoNT-A or inhibition of trigeminal neurons. Our data demonstrated that centrally targeted BoNT-A might serve as a new therapeutic tool for the treatment of orofacial chronic pain conditions.
ACKNOWLEDGEMENTS
This research was supported by the National Research Foundation of Korea (NRF) and funded by the Ministry of Science, ICT & Future Planning (2008-0062282 and 2012M-3A9B6055414) and by Hugel Inc. We are grateful to Hugel Inc. (Chuncheon, Republic of Korea) for providing Botulax®.
Footnotes
CONFLICT OF INTEREST: The authors report no conflict of interest.
References
- 1.de Paiva A, Poulain B, Lawrence GW, Shone CC, Tauc L, Dolly JO. A role for the interchain disulfide or its participating thiols in the internalization of botulinum neurotoxin A revealed by a toxin derivative that binds to ecto-acceptors and inhibits transmitter release intracellularly. J Biol Chem. 1993;268:20838–20844. [PubMed] [Google Scholar]
- 2.Rizo J, Südhof TC. Mechanics of membrane fusion. Nat Struct Biol. 1998;5:839–842. doi: 10.1038/2280. [DOI] [PubMed] [Google Scholar]
- 3.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 4.Rollnik JD, Tanneberger O, Schubert M, Schneider U, Dengler R. Treatment of tension-type headache with botulinum toxin type A: a double-blind, placebo-controlled study. Headache. 2000;40:300–305. doi: 10.1046/j.1526-4610.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 5.Foster L, Clapp L, Erickson M, Jabbari B. Botulinum toxin A and chronic low back pain: a randomized, double-blind study. Neurology. 2001;56:1290–1293. doi: 10.1212/wnl.56.10.1290. [DOI] [PubMed] [Google Scholar]
- 6.Sheean G. Botulinum toxin for the treatment of musculoskeletal pain and spasm. Curr Pain Headache Rep. 2002;6:460–469. doi: 10.1007/s11916-002-0065-y. [DOI] [PubMed] [Google Scholar]
- 7.Silberstein S, Mathew N, Saper J, Jenkins S For the BOTOX Migraine Clinical Research Group. Botulinum toxin type A as a migraine preventive treatment. Headache. 2000;40:445–450. doi: 10.1046/j.1526-4610.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 8.Smuts JA, Schultz D, Barnard A. Mechanism of action of botulinum toxin type A in migraine prevention: a pilot study. Headache. 2004;44:801–805. doi: 10.1111/j.1526-4610.2004.04148.x. [DOI] [PubMed] [Google Scholar]
- 9.Göbel H, Heinze A, Heinze-Kuhn K, Austermann K. Botulinum toxin A in the treatment of headache syndromes and pericranial pain syndromes. Pain. 2001;91:195–199. doi: 10.1016/S0304-3959(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 10.Bach-Rojecky L, Lacković Z. Antinociceptive effect of botulinum toxin type a in rat model of carrageenan and capsaicin induced pain. Croat Med J. 2005;46:201–208. [PubMed] [Google Scholar]
- 11.Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004;107:125–133. doi: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Luvisetto S, Marinelli S, Lucchetti F, Marchi F, Cobianchi S, Rossetto O, Montecucco C, Pavone F. Botulinum neurotoxins and formalin-induced pain: central vs. peripheral effects in mice. Brain Res. 2006;1082:124–131. doi: 10.1016/j.brainres.2006.01.117. [DOI] [PubMed] [Google Scholar]
- 13.Lee WH, Shin TJ, Kim HJ, Lee JK, Suh HW, Lee SC, Seo K. Intrathecal administration of botulinum neurotoxin type A attenuates formalin-induced nociceptive responses in mice. Anesth Analg. 2011;112:228–235. doi: 10.1213/ANE.0b013e3181ffa1d7. [DOI] [PubMed] [Google Scholar]
- 14.Bach-Rojecky L, Lacković Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav. 2009;94:234–238. doi: 10.1016/j.pbb.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura Y, Matsuka Y, Spigelman I, Ishihara Y, Yamamoto Y, Sonoyama W, Kamioka H, Yamashiro T, Kuboki T, Oguma K. Botulinum toxin type a (150 kDa) decreases exaggerated neurotransmitter release from trigeminal ganglion neurons and relieves neuropathy behaviors induced by infraorbital nerve constriction. Neuroscience. 2009;159:1422–1429. doi: 10.1016/j.neuroscience.2009.01.066. [DOI] [PubMed] [Google Scholar]
- 16.Gazerani P, Staahl C, Drewes AM, Arendt-Nielsen L. The effects of Botulinum Toxin type A on capsaicin-evoked pain, flare, and secondary hyperalgesia in an experimental human model of trigeminal sensitization. Pain. 2006;122:315–325. doi: 10.1016/j.pain.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Borodic GE, Acquadro MA. The use of botulinum toxin for the treatment of chronic facial pain. J Pain. 2002;3:21–27. doi: 10.1054/jpai.2002.27142. [DOI] [PubMed] [Google Scholar]
- 18.Ahn DK, Chae JM, Choi HS, Kyung HM, Kwon OW, Park HS, Youn DH, Bae YC. Central cyclooxygenase inhibitors reduced IL-1beta-induced hyperalgesia in temporomandibular joint of freely moving rats. Pain. 2005;117:204–213. doi: 10.1016/j.pain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Wang XM, Zhang ZJ, Bains R, Mokha SS. Effect of antisense knock-down of alpha(2a)- and alpha(2c)-adrenoceptors on the antinociceptive action of clonidine on trigeminal nociception in the rat. Pain. 2002;98:27–35. doi: 10.1016/s0304-3959(01)00464-x. [DOI] [PubMed] [Google Scholar]
- 20.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 21.Choi HS, Ju JS, Lee HJ, Jung CY, Kim BC, Park JS, Ahn DK. Effects of TNF-alpha injected intracisternally on the nociceptive jaw-opening reflex and orofacial formalin test in freely moving rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:613–618. doi: 10.1016/S0278-5846(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 22.Chang KH, Bai SJ, Lee H, Lee BH. Effects of acupuncture stimulation at different acupoints on formalin-induced pain in rats. Korean J Physiol Pharmacol. 2014;18:121–127. doi: 10.4196/kjpp.2014.18.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clavelou P, Pajot J, Dallel R, Raboisson P. Application of the formalin test to the study of orofacial pain in the rat. Neurosci Lett. 1989;103:349–353. doi: 10.1016/0304-3940(89)90125-0. [DOI] [PubMed] [Google Scholar]
- 24.Yang GY, Woo YW, Park MK, Bae YC, Ahn DK, Bonfa E. Intracisternal administration of NR2 antagonists attenuates facial formalin-induced nociceptive behavior in rats. J Orofac Pain. 2010;24:203–211. [PubMed] [Google Scholar]
- 25.Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 26.Yang KY, Mun JH, Park KD, Kim MJ, Ju JS, Kim ST, Bae YC, Ahn DK. Blockade of spinal glutamate recycling produces paradoxical antinociception in rats with orofacial inflammatory pain. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:100–109. doi: 10.1016/j.pnpbp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Ahn DK, Lee SY, Han SR, Ju JS, Yang GY, Lee MK, Youn DH, Bae YC. Intratrigeminal ganglionic injection of LPA causes neuropathic pain-like behavior and demyelination in rats. Pain. 2009;146:114–120. doi: 10.1016/j.pain.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Kim MJ, Lee SY, Yang KY, Nam SH, Kim HJ, Kim YJ, Bae YC, Ahn DK. Differential regulation of peripheral IL-1β-induced mechanical allodynia and thermal hyperalgesia in rats. Pain. 2014;155:723–732. doi: 10.1016/j.pain.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Park CK, Kim K, Jung SJ, Kim MJ, Ahn DK, Hong SD, Kim JS, Oh SB. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain. 2009;144:84–94. doi: 10.1016/j.pain.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Kim HD, Lee HJ, Choi HS, Ju JS, Jung CY, Bae YC, Ahn DK. Interleukin-1 beta injected intracisternally inhibited NMDA-evoked behavioral response in the orofacial area of freely moving rats. Neurosci Lett. 2004;360:37–40. doi: 10.1016/j.neulet.2004.01.059. [DOI] [PubMed] [Google Scholar]
- 31.Lee HJ, Choi HS, Jung CY, Ju JS, Kim SK, Bae YC, Ahn DK. Intracisternal NMDA produces analgesia in the orofacial formalin test of freely moving rats. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:497–503. doi: 10.1016/j.pnpbp.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox GL. Pharmacological studies of grooming and scratching behavior elicited by spinal substance P and excitatory amino acids. Ann N Y Acad Sci. 1988;525:228–236. doi: 10.1111/j.1749-6632.1988.tb38608.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang KM, Wang XM, Peterson AM, Chen WY, Mokha SS. alpha2-adrenoceptors modulate NMDA-evoked responses of neurons in superficial and deeper dorsal horn of the medulla. J Neurophysiol. 1998;80:2210–2214. doi: 10.1152/jn.1998.80.4.2210. [DOI] [PubMed] [Google Scholar]
- 34.Dolly JO, Aoki KR. The structure and mode of action of different botulinum toxins. Eur J Neurol. 2006;13(Suppl 4):1–9. doi: 10.1111/j.1468-1331.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 35.Simpson LL. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1980;212:16–21. [PubMed] [Google Scholar]
- 36.Hua Y, Scheller RH. Three SNARE complexes cooperate to mediate membrane fusion. Proc Natl Acad Sci U S A. 2001;98:8065–8070. doi: 10.1073/pnas.131214798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahnert-Hilger G, Bigalke H. Molecular aspects of tetanus and botulinum neurotoxin poisoning. Prog Neurobiol. 1995;46:83–96. doi: 10.1016/0301-0082(95)00003-e. [DOI] [PubMed] [Google Scholar]
- 38.Dutton JJ, Buckley EG. Botulinum toxin in the management of blepharospasm. Arch Neurol. 1986;43:380–382. doi: 10.1001/archneur.1986.00520040060020. [DOI] [PubMed] [Google Scholar]
- 39.Matak I, Stracenski I, Lacković Z. Comparison of analgesic effects of single versus repeated injection of botulinum toxin in orofacial formalin test in rats. J Neural Transm. 2013;120:141–144. doi: 10.1007/s00702-012-0846-3. [DOI] [PubMed] [Google Scholar]
- 40.Yoo KY, Lee HS, Cho YK, Lim YS, Kim YS, Koo JH, Yoon SJ, Lee JH, Jang KH, Song SH. Anti-inflammatory effects of botulinum toxin type a in a complete Freund's adjuvant-induced arthritic knee joint of hind leg on rat model. Neurotox Res. 2014;26:32–39. doi: 10.1007/s12640-013-9447-7. [DOI] [PubMed] [Google Scholar]
- 41.Marinelli S, Vacca V, Ricordy R, Uggenti C, Tata AM, Luvisetto S, Pavone F. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS One. 2012;7:e47977. doi: 10.1371/journal.pone.0047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon. 2000;38:245–258. doi: 10.1016/s0041-0101(99)00153-1. [DOI] [PubMed] [Google Scholar]
- 43.Restani L, Antonucci F, Gianfranceschi L, Rossi C, Rossetto O, Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A) J Neurosci. 2011;31:15650–15659. doi: 10.1523/JNEUROSCI.2618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28:3689–3696. doi: 10.1523/JNEUROSCI.0375-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marinelli S, Luvisetto S, Cobianchi S, Makuch W, Obara I, Mezzaroma E, Caruso M, Straface E, Przewlocka B, Pavone F. Botulinum neurotoxin type A counteracts neuropathic pain and facilitates functional recovery after peripheral nerve injury in animal models. Neuroscience. 2010;171:316–328. doi: 10.1016/j.neuroscience.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 46.Bach-Rojecky L, Salković-Petrisić M, Lacković Z. Botulinum toxin type A reduces pain supersensitivity in experimental diabetic neuropathy: bilateral effect after unilateral injection. Eur J Pharmacol. 2010;633:10–14. doi: 10.1016/j.ejphar.2010.01.020. [DOI] [PubMed] [Google Scholar]