Abstract
Aripiprazole (ARI) is a commonly prescribed medication used to treat schizophrenia and bipolar disorder. To date, there have been no studies regarding the molecular pathological and immunotoxicological profiling of aripiprazole. Thus, in the present study, we prepared two different formulas of aripiprazole [Free base crystal of aripiprazole (ARPGCB) and cocrystal of aripiprazole (GCB3004)], and explored their effects on the patterns of survival and apoptosis-regulatory proteins under acute toxicity and cytotoxicity test conditions. Furthermore, we also evaluated the modulatory activity of the different formulations on the immunological responses in macrophages primed by various stimulators such as lipopolysaccharide (LPS), pam3CSK, and poly(I:C) via toll-like receptor 4 (TLR4), TLR2, and TLR3 pathways, respectively. In liver, both ARPGCB and GCB3004 produced similar toxicity profiles. In particular, these two formulas exhibited similar phospho-protein profiling of p65/nuclear factor (NF)-κB, c-Jun/activator protein (AP)-1, ERK, JNK, p38, caspase 3, and bcl-2 in brain. In contrast, the patterns of these phospho-proteins were variable in other tissues. Moreover, these two formulas did not exhibit any cytotoxicity in C6 glioma cells. Finally, the two formulations at available in vivo concentrations did not block nitric oxide (NO) production from activated macrophage-like RAW264.7 cells stimulated with LPS, pam3CSK, or poly(I:C), nor did they alter the morphological changes of the activated macrophages. Taken together, our present work, as a comparative study of two different formulas of aripiprazole, suggests that these two formulas can be used to achieve similar functional activation of brain proteins related to cell survival and apoptosis and immunotoxicological activities of macrophages.
Keywords: Acute toxicity, Aripiprazole, Cocrystal, Immunotoxicology, Survival proteins
INTRODUCTION
Aripiprazole (ARI, 7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1H)-one) is a commonly prescribed atypical antipsychotic drug used to treat psychosis such as schizophrenia, episodes associated with bipolar disorder, delayed sleep phase syndrome, and irritability in children with autism [1,2]. With respect to its mechanism of action, several recent pharmacological studies have revealed that aripiprazole directly modulates monoaminergic systems by acting as both a dopamine agonist and antagonist while stabilizing the dopamine D2 and 5-hydroxytryptamine (5-HT)1A receptor Gαi protein-complex [3,4]. The pharmacological roles of aripiprazole as a free radical scavenger [5] as well as its gastroprotective properties [6] have led to expanded usage in other diseases such as gastritis and reactive oxygen species (ROS)-mediated diseases or symptoms such as cancer and aging. Indeed, many other scientists have studied various disease conditions to test the efficacy of aripiprazole, identifying potential uses as a gastroprotective agent, anti-oxidative drug, and modulator of adipogenic gene expression [6,7,8].
However, aripiprazole has a very low aqueous solubility leading to a slow onset time of approximately 3 to 5 h. This limitation should be overcome for better drug-delivery efficiency and improved systemic exposure in the clinic. The cocrystal formulation of aripiprazole (GCB3004) was found to improve the limitation of free base crystal formula of aripiprazole (ARPGCB) in terms of higher water solubility and pharmacokinetic profiles (data not shown). In addition, it was reported that there is no significant acute toxicity following intraperitoneal injection of aripiprazole at a dose range of 1 to 20 mg/kg [9]. The metabolism of aripiprazole is mainly managed by hepatic cytochrome P450 (CYP) isozymes 3A4 and 2D6, and dehydroaripiprazole is known to be a major metabolite, showing reasonable affinities to the dopamine 2 receptor [10]. Aripiprazole has long half-life, taking 2 weeks to reach steady state in plasma [11].
Based on these findings, the necessity to examine the pathological and immunotoxicological profiles of aripiprazole in the body at the molecular level has greatly increased. Therefore, in the present study, we aimed to investigate the effect of aripiprazole on the activation of death-related proteins in organs from mice orally administered conventionally crystallized aripiprazole (ARPGCB) or cocrystallized aripiprazole (GCB3004), the latter of which is pharmaceutically and pharmacokinetically improved with respect to solubility, chemical stability, oral adsorption, and dissolution properties [12]. In addition, we evaluated the cytotoxic and immunotoxicological activities of ARPGCB and GCB3004 in C6 glioma cells and activated macrophage-like RAW264.7 cells.
METHODS
Mice
Six-week-old male ICR mice were purchased from DAEHAN BIOLINK (Chungbuk, Korea). Mice were given food pellets (Samyang, Daejeon, Korea) and water ad libitum under a 12-h light/dark cycle. Studies were performed in accordance with guidelines established by the Sungkyunkwan University Institutional Animal Care and Use Committee.
Materials
Two forms of aripiprazole [free base crystal form of aripiprazole (ARPGCB) or cocrystallized form of aripiprazole (GCB3004)] were supplied from GCB Company (Suwon, Korea). For preparing cocrystallized aripiprazole by solvent method, free base crystal form of this drug was mixed with acid-pyridine under heating conditions and then obtained co-crystal form of aripiprazone under cooling conditions. The tetrazole (3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), pam3CSK, peptidoglycan (PGN), and lipopolysaccharide (LPS, Escherichia coli 0111:B4) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Fetal bovine serum (FBS) and RPMI1640 were obtained from GIBCO (Grand Island, NY, USA). RAW264.7 cells were purchased from American Type Culture Collection (Manassas, VA, USA). All other chemicals were of reagent grade. Antibodies to total forms or phospho-forms of p65, c-Jun, extracellular signal-related kinase (ERK), c-Jun N-terminal kinase (JNK), p38, caspase 3, bcl-2, and β-actin were obtained from Cell Signaling Technology (Danvers, MA, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Single dose treatment and measurement of serum parameters
Five mice were orally administered with a single dose of ARPGCB or GCB3004 (500 mg/kg). Mortality and changes in body weights were then monitored over the next 24 h. After monitoring, all remaining animals from each group were sacrificed and the weights of key organs were determined. Serum samples were obtained by centrifugation of blood at 4,000 rpm for 15 min at 4℃ and then divided into Eppendorf tubes. Isolated sera were stored at -20℃ until used for analyses. The levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured with a Roche Modular spectrophotometric autoanalyzer as reported previously [13].
Cell culture
RAW264.7 cells, a murine macrophage cell line, were maintained in RPMI1640 media supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% FBS. Cells were grown at 37oC and 5% CO2 in a humidified atmosphere.
Cell viability assay
C6 glioma and RAW264.7 cells (1×106 cells/ml) were plated and grown for 18 h, after which ARPGCB or GCB3004 (0 to 100 µM) were added to the cell suspensions and incubated for 24 h. Cytotoxic effects of testing compounds were evaluated by a conventional MTT assay as reported previously [14]. At 3 h prior to culture termination, 10 µl of MTT solution (10 mg/ml in phosphate buffered saline, pH 7.4) was added and cells were continuously cultured until assay termination. The incubation was halted by the addition of 15% sodium dodecyl sulphate to each well to solubilize the formazan crystals [15], and the absorbance at 570~630 nm (OD570-630) was measured using a Spectramax 250 microplate reader.
Morphological Analysis
C6 glioma and RAW264.7 cells were pretreated with ARPGCB or GCB3004 for 30 min and then incubated with LPS for the indicated time points. Images of the cells in culture at the indicated time points were obtained using an inverted phase contrast microscope that was interfaced with a video camera using Image J software [16,17].
Determination of NO production
After pre-incubation of RAW264.7 cells (1×106 cells/ml) for 18 h, cells were treated with ARPGCB or GCB3004 (0 to 10 µM) for 30 min and then further incubated with LPS (1 µg/ml) for 24 h. The inhibitory effects of ARPGCB or GCB3004 on NO production were determined by analyzing NO levels with Griess reagents as described previously [18].
Preparation of tissue lysates and immunoblotting analysis
Tissues were washed three times in cold PBS with 1 mM sodium orthovanadate and lysed by a sonicator or a Tissuemizer in lysis buffer (20 mM Tris-HCl, pH 7.4, 2 mM EDTA, 2 mM ethyleneglycotetraacetic acid, 50 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1% Triton X-100, 10% glycerol, 10 µg/ml aprotinin, 10 µg/ml pepstatin, 1 mM benzimide, and 2 mM PMSF) for 30 min with rotation at 4℃. The lysates were clarified by centrifugation at 16,000×g for 10 min at 4℃ and stored at -20℃ until needed. Soluble tissue lysates were immunoblotted and protein levels were visualized as previously reported [19].
Statistical analysis
All data in this paper are presented as the mean±SD experiments performed with five (Fig. 1) or six (Fig. 3 and 4) samples. For statistical comparisons, these results were analyzed using ANOVA/Scheffe's post hoc test and Kruskal-Wallis/Mann-Whitney test. p-values<0.05 were considered statistically significant. All statistical tests were carried out using the computer program SPSS (SPSS Inc., Chicago, IL, USA). Similar experimental data were also observed by an additional independent set of in vitro and in vivo experiments performed with the same numbers of samples or mice.
Fig. 1. Acute toxicity of ARPGCB or GCB3004 in mice. (A) The levels of serum parameters (AST and ALT) from mice orally administered ARPGCB or GCB3004 (each 500 mg/kg). (B) Organs from mice orally administered with ARPGCB or GCB3004 (each 500 mg/kg). Data represent the mean±SD of an experiment performed with five mice.
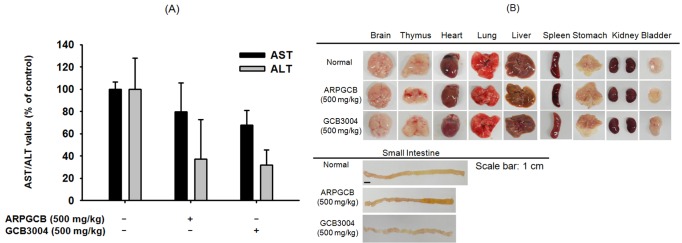
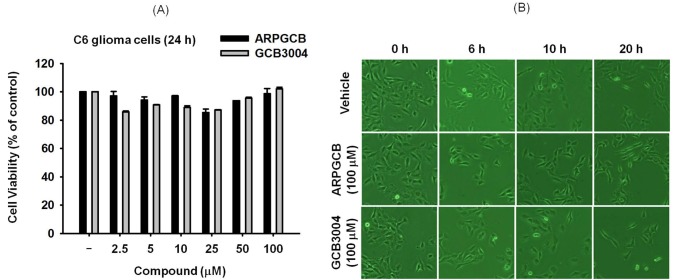
Fig. 3. The effect of ARPGCB or GCB3004 on the viability of C6 glioma cells. (A) C6 glioma cells (2×106 cells/ml) were incubated with ARPGCB or GCB3004 for 24 h. Cell viability was evaluated by conventional MTT assay. (B) Images of the cells in culture at 12 h were obtained with an inverted phase contrast microscope that was interfaced with a video camera using Image J software.
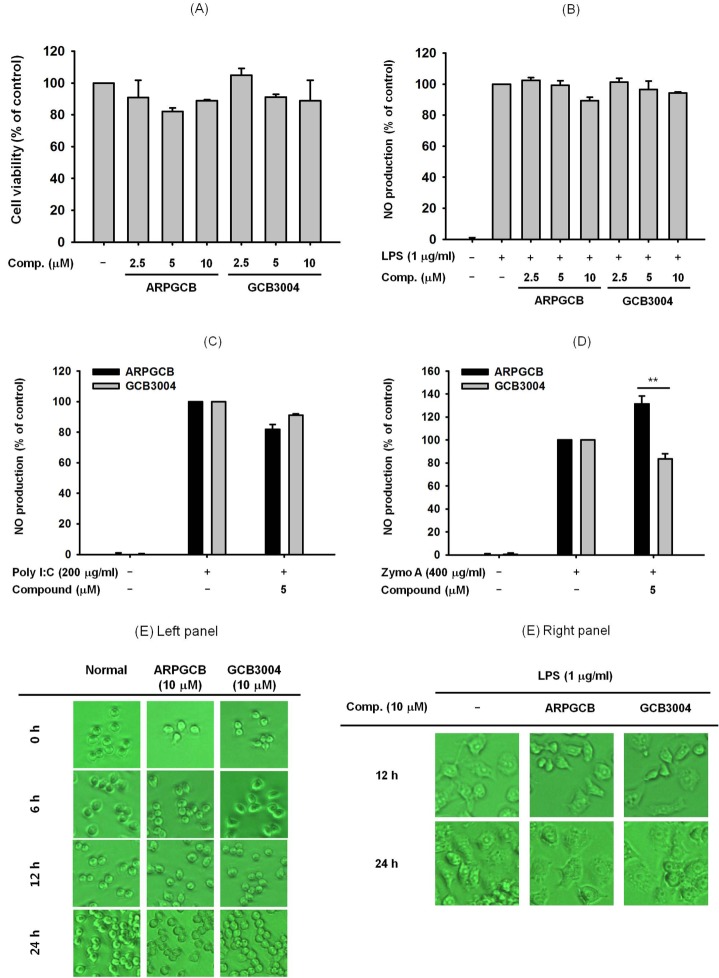
Fig. 4. The effect of ARPGCB or GCB3004 on the immunological responses of RAW264.7 cells. (A) RAW264.7 cells (2×106 cells/ml) were incubated with ARPGCB or GCB3004 for 24 h. Cell viability was evaluated by conventional MTT assay. (B~D) Regulatory activity of ARPGCB or GCB3004 on the production of NO from activated macrophages stimulated by LPS (1 µg/ml), poly(I:C) (200 µg/ml), or Zymosan A (Zymo A, 400 µg/ml) was determined by Griess assay. (E) Images of cells in culture at the indicated time points were obtained with an inverted phase contrast microscope that was interfaced with a video camera using Image J software. **p<0.01 compared to each control group.
RESULTS
Effect of singly treated aripiprazole on acute toxicity in mice
To understand the toxicological features of aripiprazole, we first examined whether a single dose treatment (500 mg/kg) of aripiprazole induces acute toxicity in mice. Two pharmaceutical formulations of aripiprazole [free base crystallized formulation (ARPGCB) and cocrystallized formulation (GCB3004)] were chosen for this study. As shown in Fig. 1, these two formulas did not strongly exhibit toxic activity. Specifically, the serum levels of AST and ALT were not different between the two treatment groups, although the levels of each were decreased following treatment (Fig. 1A). While there was no striking difference in the shape and size of most organs treated with the aripiprazole compared with control animals, we did notice a slight variation in the length of the small intestine upon aripiprazole treatment (Fig. 1B). Thus, more detailed toxicological studies by exploring the levels of apoptotic and cell survival proteins were deemed necessary to evaluate the toxicological activities of the aripiprazole formulations.
Effect of aripiprazole on the phospho-protein levels of survival- and apoptosis-regulatory proteins in organs
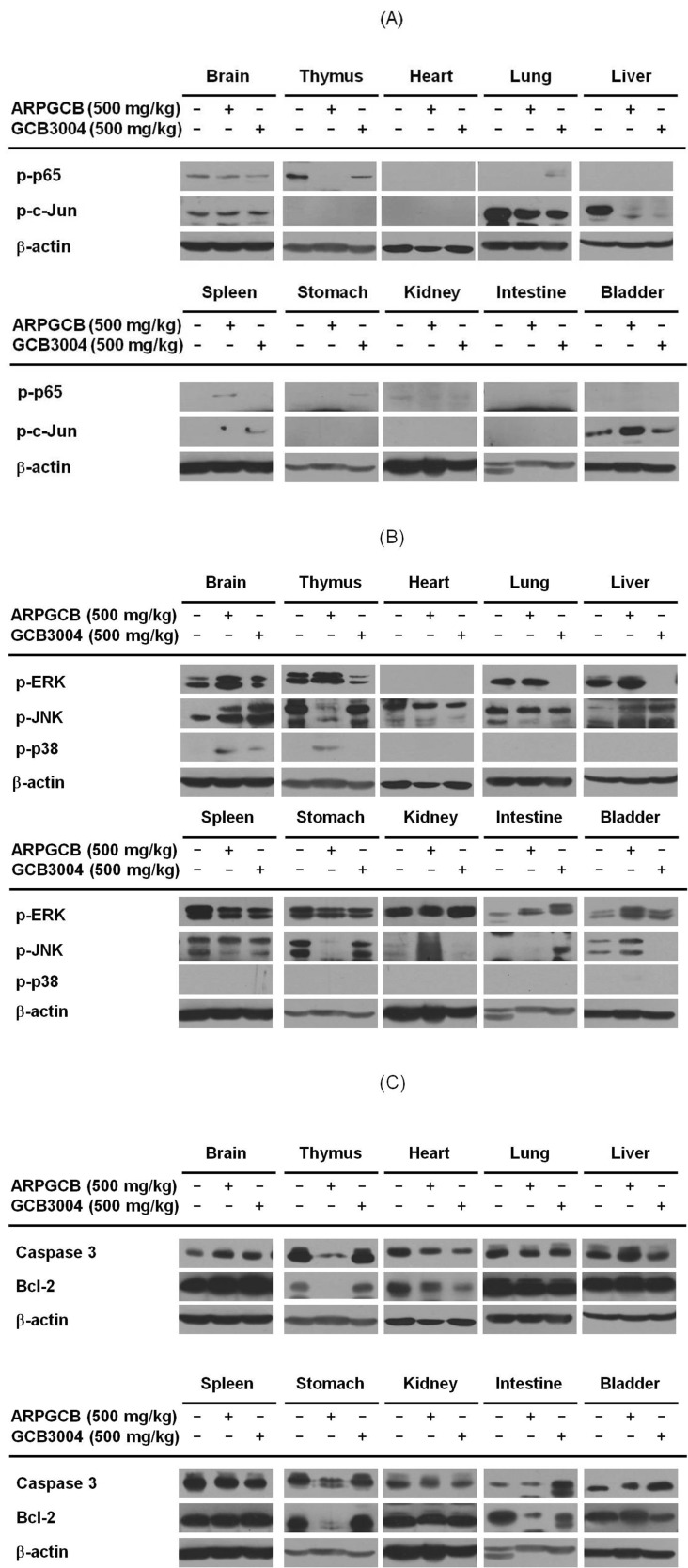
To evaluate the toxicological properties of aripiprazole in each organ, we next investigated whether aripiprazole was able to modulate the levels of activated survival/death-regulatory proteins such as p65/NF-κB, c-Jun/AP-1, and MAPKs as well as apoptosis-regulatory proteins such as bcl-2 and caspase-3 which are involved in controlling cell growth, proliferation, and apoptosis [20,21]. This analysis was performed by preparing whole tissue lysates from mice treated with a single dose of one of the two aripiprazole formulations and evaluating the phosphorylated levels of p65, c-Jun, ERK, JNK, caspase 3, and bcl-2 in immunoblotting analysis. As shown in Fig. 2, the phospho- and total protein patterns of these proteins were variable between organs in each group. First of all, phospho-protein levels of ERK, JNK, and p38 and total protein levels of caspase 3 and bcl-2 were increased by the two formulations in the brain, which is the known target organ of aripiprazole [5], while p-p65 but not p-c-Jun was decreased by aripiprazole treatment (Fig. 2). With respect to other organs (thymus, heart, lung, liver, spleen, stomach, kidney, intestine, and bladder), the two formulations exhibited opposite effects on protein expression in tissues for p-p65, p-JNK, caspase-3, and bcl-2 levels, but similar patterns for p-c-Jun level in mice (Fig. 2).
Fig. 2. Effect of ARPGCB or GCB3004 on the level of cell death-related molecules in organs. (A, B, and C) The levels of cell death-related proteins such as (p65/NF-κB, c-Jun/AP-1, ERK/MAPK, JNK/MAPK, p38/MAPK, caspase 3, bcl-2, and β-actin) were identified by immunoblotting from tissue lysates prepared from mice orally administered with ARPGCB or GCB3004 (each of 500 mg/kg). The results shown are representative of three independent experiments.
Cytotoxic effects of aripiprazole
The cytotoxicity of the two aripiprazole formulas was examined by determining their effects on cell viability. Specifically, we determined the anti-proliferative activity of ARPGCB and GCB3004 using C6 glioma cells. As shown in Fig. 3A, there was no significant decrease of C6 glioma cell viability by the two aripiprazole formulas up to 100 µM, indicating that both forms of aripiprazole can be considered non-toxic. Furthermore, neither of the two formulas induced any morphological changes, a strong marker of cell death [22,23], in C6 glioma cells up to 20 h compared with control treated cells (Fig. 3B).
Effect of aripiprazole on the induction of macrophage activation
The immunomodulatory activity of aripiprazole at in vivo available concentrations was explored by treating macrophage-like RAW264.7 cells with the two formulations to determine their influence on immune cell survival and function. As shown in Fig. 4A, neither formulation (0 to 10 µM) suppressed RAW264.7 cell viability. Similarly, NO production in LPS-stimulated RAW264.7 cells was not significantly suppressed by the two formulations (Fig. 4B), suggesting that aripiprazole does not induce a blockade of the immunological response of macrophages. Moreover, NO release stimulated by Poly(I:C) and Zym A was not decreased (Fig. 4C and 4D). Finally, the morphology of RAW264.7 cells under LPS exposure was not strikingly different between the aripiprazole treated cells and control cells (Fig. 4E).
DISCUSSION
In this study, therefore, we intended to evaluate the pathological and immunotoxicological activity of these two formulas at the molecular levels using a single, high dose (500 mg/kg) oral administration of two different formulations of aripiprazole. As shown in Fig. 1, there was no significant difference between free base crystallized aripiprazole and cocrystal formulation with respect to serum parameters such as AST and ALT, which are two major marker proteins that can be used to indicate liver toxicity [24], although both formulations lowered ALT levels (Fig. 1A). Considering that the LD50 values of the conventional formulation of aripiprazole ranges from 700 to 950 mg/kg, we considered the possibility that the dose used in the present study (500 mg/kg) could induce cellular and organ damage [25] without having a significant effect on liver parameters. Therefore, we next performed molecular profiling of survival and apoptosis-regulatory proteins in tissues of mice treated with the different aripiprazole formulations compared with control. As shown in Fig. 2, the molecular activation patterns of survival-regulatory proteins, p65/NF-κB and c-Jun/AP-1, and apoptosis-regulatory proteins, caspase-3 and bcl-2 were not significantly varied between the two different formulations, although differences between their effects in individual organs in mice were noted. Importantly, the fact that these two formulations exhibited very similar molecular profiles for p65, c-Jun, ERK, JNK, p38, caspase-3, and bcl-2 in the brain (Fig. 2), which is the primary target organ of aripiprazole [26], suggested that the pharmacological activity of the cocrystal form of aripiprazole (GCB3004) possesses comparable activity to the conventional form of the drug (ARPGCB) in addition to improved drug solubility (data not shown). Interestingly, these two formulations induced different phospho-protein patterns in other organs (Fig. 2). Furthermore, ARPGCB and GCB3004 did not induce any significant cytotoxicity and morphological changes in C6 glioma cells (Fig. 3A and 3B). Taken together, these results strongly suggest that there is a possibility that these formulations may induce additional pharmacological actions in an organ-specific manner without inducing cellular cytotoxicity. In fact, immune cell rich organs such as thymus, lung, intestine, and spleen exhibit opposite patterns between these two formulas (Fig. 2), implying that different formulations with aripiprazole could induce distinct responses in the body. Although it is not clear why these two formulas show variable effects, two potential possibilities could be explained. First, different pharmacokinetic profiles between these formulas could be prime reason of this. Since blood concentrations are dependent of pharmacokinetic feature of orally administered drug and subsequently affect cellular concentrations, difference of molecular profiling in various organs and tissues between these formulas could be due to their pharmacokinetic characteristics. Indeed, under our experience, we have reported that the phosphorylation of cellular proteins is variable according to treatment time of inducers and drugs [27,28]. Thus, it is supposed that highly increased levels of co-crystallized aripiprazole seem to induce different profiling of phosphorylation patterns compared to free base crystalized aripiprazole. As the other point, different three dimensional structures between cocrystal and free base crystal forms of aripiprazole can be considered. It has been reported that salt form and cocrystal from of adenine display distinctive three dimensional structures [29]. Namely, different crystal formulas could alter molecular interaction between target proteins and aripiprazone. To exactly explain the possibilities, however, additional experiments will be continued.
Because ARPGCB and GCB3004 exhibited different patterns in immune organs, we next examined whether these two formulations have distinct immunotoxicological profiles. In order to evaluate this possibility, we evaluated the effect of the two formulations on cytotoxic NO release [30] in macrophage-like RAW264.7 cells stimulated by either the TLR4 ligand LPS, the TLR2/dectin1 ligand zymosan A, or the TLR3 ligand poly (I:C). As reported previously, we obtained similar NO production levels (10 to 45 µM of nitrate) with each stimulus group [16,31,32]. As expected, there was no significant inhibition of NO production by the aripiprazole formulations in cells stimulated by the different TLR ligands (Fig. 4) under non-cytotoxic and serum available conditions. Furthermore, the aripiprazole formulations did not alter the morphological changes of RAW264.7 cells triggered by LPS and pam3CSK (Fig. 4E), which can be seen in the activation stages [33]. Moreover, there was no striking difference in NO production or morphological changes in the ARPGCB- and GCB3004-treated groups, indicating that neither of the two formulations affected the immunological responses of macrophages. There is clear evidence indicating that aripiprazole can affect various immunological phenomena managed by B cells, T cells, and macrophages. Furthermore, a few studies have suggested that aripiprazole can directly modulate the production of superoxide, NO, and TNF-α from phorbal-12-myristate-13-acetate- and interferon-γ-stimulated microglical cells [8], which are known to be related to the pathophysiology of schizophrenia [34]. We will continue to search for an explanation for the pharmacological discrepancy between glioma cells and RAW264.7 cells treated with aripiprazole, and are also planning to further investigate the immunotoxicological activity of aripiprazole by utilizing primary immune cells.
In conclusion, we performed comparative study of two different formulas (free base crystal or cocrystal formular) of aripiprazole, of which the cocrystal form exhibited higher solubility and improved pharmacokinetics. The acute toxicity test induced by single oral administration (500 mg/kg) indicated that there was no significant difference of phospho-proteins related to survival and apoptosis in the brain. However, the levels of these proteins were found to be variable in other organs. In addition, neither of the two formulations were cytotoxic or immunotoxic in C6 glioma cells and activated macrophages. Therefore, our data strongly suggest that there was no difference in immunotoxicological profiles between these two formulas.
ACKNOWLEDGEMENTS
This work was supported by a grant (C1224; 2012-2014) from Gyeonggi Technology Development Program funded by Gyeonggi Province, Korea.
ABBREVIATIONS
- ARI
aripiprazole
- LPS
lipopolysaccharide
- TLR4
toll-like receptor 4
- NF-κB
nuclear factor-κB
- AP-1
activator protein-1
- NO
nitric oxide
- 5-HT
5-hydroxytryptamine
- CYP
cytochrome P450
References
- 1.Moore TJ, Glenmullen J, Mattison DR. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern Med. 2014;174:1930–1933. doi: 10.1001/jamainternmed.2014.5262. [DOI] [PubMed] [Google Scholar]
- 2.Takaki M, Ujike H. Aripiprazole is effective for treatment of delayed sleep phase syndrome. Clin Neuropharmacol. 2014;37:123–124. doi: 10.1097/WNF.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 3.Kling RC, Tschammer N, Lanig H, Clark T, Gmeiner P. Active-state model of a dopamine D2 receptor-Gαi complex stabilized by aripiprazole-type partial agonists. PLoS One. 2014;9:e100069. doi: 10.1371/journal.pone.0100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Berardis D, Fornaro M, Serroni N, Marini S, Piersanti M, Cavuto M, Valchera A, Mazza M, Girinelli G, Iasevoli F, Perna G, Martinotti G, Di Giannantonio M. Treatment of antipsychotic-induced hyperprolactinemia: an update on the role of the dopaminergic receptors D2 partial agonist aripiprazole. Recent Pat Endocr Metab Immune Drug Discov. 2014;8:30–37. doi: 10.2174/1872214807666131229125700. [DOI] [PubMed] [Google Scholar]
- 5.Arunagiri P, Rajeshwaran K, Shanthakumar J, Tamilselvan T, Balamurugan E. Combination of omega-3 Fatty acids, lithium, and aripiprazole reduces oxidative stress in brain of mice with mania. Biol Trace Elem Res. 2014;160:409–417. doi: 10.1007/s12011-014-0067-8. [DOI] [PubMed] [Google Scholar]
- 6.Asmari AA, Arshaduddin M, Elfaki I, Kadasah S, Robayan AA, Asmary SA. Aripiprazole an atypical antipsychotic protects against ethanol induced gastric ulcers in rats. Int J Clin Exp Med. 2014;7:2031–2044. [PMC free article] [PubMed] [Google Scholar]
- 7.Sárvári AK, Veréb Z, Uray IP, Fésüs L, Balajthy Z. Atypical antipsychotics induce both proinflammatory and adipogenic gene expression in human adipocytes in vitro. Biochem Biophys Res Commun. 2014;450:1383–1389. doi: 10.1016/j.bbrc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Kato TA, Monji A, Yasukawa K, Mizoguchi Y, Horikawa H, Seki Y, Hashioka S, Han YH, Kasai M, Sonoda N, Hirata E, Maeda Y, Inoguchi T, Utsumi H, Kanba S. Aripiprazole inhibits superoxide generation from phorbol-myristate-acetate (PMA)-stimulated microglia in vitro: implication for antioxidative psychotropic actions via microglia. Schizophr Res. 2011;129:172–182. doi: 10.1016/j.schres.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Picada JN, Dos Santos Bde J, Celso F, Monteiro JD, Da Rosa KM, Camacho LR, Vieira LR, Freitas TM, Da Silva TG, Pontes VM, Pereira P. Neurobehavioral and genotoxic parameters of antipsychotic agent aripiprazole in mice. Acta Pharmacol Sin. 2011;32:1225–1232. doi: 10.1038/aps.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorado P, de Andrés F, Naranjo ME, Peñas-Lledó EM, González I, González AP, de la Rubia A, Llerena A. High-performance liquid chromatography method using ultraviolet detection for the quantification of aripiprazole and dehydroaripiprazole in psychiatric patients. Drug Metabol Drug Interact. 2012;27:165–170. doi: 10.1515/dmdi-2012-0016. [DOI] [PubMed] [Google Scholar]
- 11.Kim JR, Seo HB, Cho JY, Kang DH, Kim YK, Bahk WM, Yu KS, Shin SG, Kwon JS, Jang IJ. Population pharmacokinetic modelling of aripiprazole and its active metabolite, dehydroaripiprazole, in psychiatric patients. Br J Clin Pharmacol. 2008;66:802–810. doi: 10.1111/j.1365-2125.2008.03223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thakuria R, Delori A, Jones W, Lipert MP, Roy L, Rodríguez-Hornedo N. Pharmaceutical cocrystals and poorly soluble drugs. Int J Pharm. 2013;453:101–125. doi: 10.1016/j.ijpharm.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 13.Seo SJ, Cho JY, Jeong YH, Choi YS. Effect of Korean red ginseng extract on liver damage induced by short-term and long-term ethanol treatment in rats. J Ginseng Res. 2013;37:194–200. doi: 10.5142/jgr.2013.37.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim BJ. Involvement of melastatin type transient receptor potential 7 channels in ginsenoside Rd-induced apoptosis in gastric and breast cancer cells. J Ginseng Res. 2013;37:201–209. doi: 10.5142/jgr.2013.37.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang YJ, Chung ML, Sohn UD, Im C. Cytotoxicity and structure-activity relationships of naphthyridine derivatives in human cervical cancer, leukemia, and prostate cancer. Korean J Physiol Pharmacol. 2013;17:517–523. doi: 10.4196/kjpp.2013.17.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MY, Cho JY. 20S-dihydroprotopanaxatriol modulates functional activation of monocytes and macrophages. J Ginseng Res. 2013;37:300–307. doi: 10.5142/jgr.2013.37.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MY, Cho JY. 20S-dihydroprotopanaxadiol, a ginsenoside derivative, boosts innate immune responses of monocytes and macrophages. J Ginseng Res. 2013;37:293–299. doi: 10.5142/jgr.2013.37.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youn CK, Park SJ, Lee MY, Cha MJ, Kim OH, You HJ, Chang IY, Yoon SP, Jeon YJ. Silibinin inhibits LPS-Induced macrophage activation by blocking p38 MAPK in RAW 264.7 cells. Biomol Ther (Seoul) 2013;21:258–263. doi: 10.4062/biomolther.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JW, Kim NH, Kim JY, Park JH, Shin SY, Kwon YS, Lee HJ, Kim SS, Chun W. Aromadendrin inhibits lipopolysaccharide-Induced nuclear translocation of NF-κB and phosphorylation of JNK in RAW 264.7 macrophage cells. Biomol Ther (Seoul) 2013;21:216–221. doi: 10.4062/biomolther.2013.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eo SH, Cho H, Kim SJ. Resveratrol inhibits nitric oxideinduced apoptosis via the NF-Kappa B pathway in rabbit articular chondrocytes. Biomol Ther (Seoul) 2013;21:364–370. doi: 10.4062/biomolther.2013.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho KS, Kwon KJ, Jeon SJ, Joo SH, Kim KC, Cheong JH, Bahn GH, Kim HY, Han SH, Shin CY, Yang SI. Transcriptional upregulation of plasminogen activator inhibitor-1 in rat primary astrocytes by a proteasomal inhibitor MG132. Biomol Ther (Seoul) 2013;21:107–113. doi: 10.4062/biomolther.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng L, Gao Y, Xue YN, Huang SW, Zhuo RX. Cytotoxicity and in vivo tissue compatibility of poly(amidoamine) with pendant aminobutyl group as a gene delivery vector. Biomaterials. 2010;31:4467–4476. doi: 10.1016/j.biomaterials.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Lee YG, Yoo S, Oh J, Jeong D, Song WK, Yoo BC, Rhee MH, Park J, Cha SH, Hong S, Cho JY. Involvement of Src and the actin cytoskeleton in the antitumorigenic action of adenosine dialdehyde. Biochem Pharmacol. 2013;85:1042–1056. doi: 10.1016/j.bcp.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Lin WL, Wang CJ, Tsai YY, Liu CL, Hwang JM, Tseng TH. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch Toxicol. 2000;74:467–472. doi: 10.1007/s002040000148. [DOI] [PubMed] [Google Scholar]
- 25.Aripiprazole: new drug. Just another neuroleptic. Prescrire Int. 2005;14:163–167. [PubMed] [Google Scholar]
- 26.Chu CS, Tzeng NS, Chang HA, Chang CC, Chen TY. Killing two birds with one stone: the potential role of aripiprazole for patients with comorbid major depressive disorder and nicotine dependence via altering brain activity in the anterior cingulate cortex. Med Hypotheses. 2014;83:407–409. doi: 10.1016/j.mehy.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Kim MY, Yoo BC, Cho JY. Ginsenoside-Rp1-induced apolipoprotein A-1 expression in the LoVo human colon cancer cell line. J Ginseng Res. 2014;38:251–255. doi: 10.1016/j.jgr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YG, Chain BM, Cho JY. Distinct role of spleen tyrosine kinase in the early phosphorylation of inhibitor of kappaB alpha via activation of the phosphoinositide-3-kinase and Akt pathways. Int J Biochem Cell Biol. 2009;41:811–821. doi: 10.1016/j.biocel.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Padiyar GS, Seshadri TP. Preference for syn conformation: crystal structures of free acid and ammonium salt of adenosine 2'-monophosphate: an inhibitor of RNase T1. J Biomol Struct Dyn. 1998;15:793–802. doi: 10.1080/07391102.1998.10508993. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Yu T, Lian YJ, Ma R, Yang S, Cho JY. Nitric oxide synthase inhibitors: a review of patents from 2011 to the present. Expert Opin Ther Pat. 2015;25:49–68. doi: 10.1517/13543776.2014.979154. [DOI] [PubMed] [Google Scholar]
- 31.Kim YM, Kim JH, Kwon HM, Lee DH, Won MH, Kwon YG, Kim YM. Korean Red Ginseng protects endothelial cells from serum-deprived apoptosis by regulating Bcl-2 family protein dynamics and caspase S-nitrosylation. J Ginseng Res. 2013;37:413–424. doi: 10.5142/jgr.2013.37.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim EH, Kim IH, Ha JA, Choi KT, Pyo S, Rhee DK. Antistress effect of red ginseng in brain cells is mediated by TACE repression via PADI4. J Ginseng Res. 2013;37:315–323. doi: 10.5142/jgr.2013.37.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JY, Lee YG, Kim MY, Byeon SE, Rhee MH, Park J, Katz DR, Chain BM, Cho JY. Src-mediated regulation of inflammatory responses by actin polymerization. Biochem Pharmacol. 2010;79:431–443. doi: 10.1016/j.bcp.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Kato TA, Monji A, Mizoguchi Y, Hashioka S, Horikawa H, Seki Y, Kasai M, Utsumi H, Kanba S. Anti-Inflammatory properties of antipsychotics via microglia modulations: are antipsychotics a 'fire extinguisher' in the brain of schizophrenia? Mini Rev Med Chem. 2011;11:565–574. doi: 10.2174/138955711795906941. [DOI] [PubMed] [Google Scholar]