Abstract
Background
Anastomosing hemangioma (AH) of the kidney is a recently described morphological variant of hemangioma. It poses a diagnostic dilemma for clinicians because of its rarity and the overlapping features it shares with other renal vascular tumors. The aim of this paper is to review all the cases of AH of the kidney in the literature.
Methods
The literature was extensively searched for case reports of AH of the kidney and the clinical and pathological characteristics of the tumor were extracted.
Results
A total of 45 cases were reviewed. The mean age of presentation was 50 years (range, 15-83 years) and male sex accounted for 68.8% of the cases reviewed. AH of the kidney was mostly unilateral with only 4 cases of bilateral involvement of the kidney. The average size of the tumor is 1.5 cm (range, 0.1-7 cm). Incidental finding of AH of the kidney accounted for 62% of the cases reviewed. The ultrasound findings demonstrated varying echogenicity and the tumor appeared as solid and well demarcated heterogeneous masses on CT. The average follow up of the patients in this review was 26 months (range, 1-156 months).
Conclusions
AH of the kidney is a rare vascular tumor and a morphological variant of hemangioma. It has a characteristic sinusoidal architecture with a semblance of splenic sinusoids. It has overlapping clinical and imaging features with other vascular tumors of the kidney. Histological review and immunohistochemical studies are essential for accurate diagnosis. AH runs a benign course without evidence of disease recurrence during follow up.
Keywords: Anastomosing hemangioma (AH), kidney, renal hemangioma
Introduction
Primary vascular tumor of the kidney is rare despite the fact that the kidney is well vascularized and receives approximately 25% of the total cardiac output. These tumors have been largely reported as case reports in the literature. A large case series of primary vascular tumors and tumor-like lesions of the kidney by Brown et al. reported 25 cases from four collaborating institutions spanning a period of 15 years (1). Montgomery and Epstein reported a total of 26 renal vascular lesions from the archives of three collaborating institutions spanning a period of 10 years (2). Approximately 220 cases of renal hemangioma and less than 40 cases of renal angiosarcoma have been reported in the literature (1,3).
Hemangiomas are more commonly located in the skin and subcutaneous tissues. Visceral hemangiomas are generally not common and occur mostly in the liver. Histologically, hemangiomas have been broadly classified as cavernous and capillary (1,4).
Montgomery and Epstein described a new variant of capillary renal hemangiomas which they observed, had distinctive overlapping features of both sinusoidal and hobnail hemangioma of the skin and soft tissues. They termed the tumor “anastomosing hemangioma (AH)” because of the unique histological architecture reminiscent of splenic sinusoids (2). AH though believed to be unique to the genitourinary system, with a particular predilection for the kidneys (2,4,5), have been reported in other sites of the body which includes the testes, thigh, abdominal wall (2), ovaries (5), adrenal gland (6), liver and the gastrointestinal tract (7).
New cases of AH have been reported since the description of this new variant of hemangioma. This is a review of the AH of the kidney because of the rarity of this tumor and the overlapping features that it shares with other vascular tumors of the kidney, with concerns for malignancy and the potential implications on the management of the tumor.
Methods
Extensive literature search of PubMed, EMBASE, and Ovid SP databases for case reports of AH of the kidney was carried out. Further search of the literature was carried out by manual search of relevant references of the studies retrieved. The inclusion criteria were all cases of AH of the kidney in the literature.
Pathological and clinical data were retrieved and assessed for all studies. The data retrieved when available included age, sex, clinical features, tumor size in centimeters, and laterality of the tumor, treatment and follow up. The keywords were AH, kidney and renal hemangioma.
Results
Tables 1,2 present a summary of the clinical presentations, pathological features and treatment outcomes of the 45 cases of AH of the kidney in the literature. Table 3 presents the frequency of immunohistochemical markers used in the diagnosis of AH of the kidney which stained positive.
Table 1. Epidemiological and clinical features of anastomosing hemangioma (AH) of the kidney.
| Cases | Age | Sex | Laterality | Size (cm) | Clinical features |
|---|---|---|---|---|---|
| Chou et al. (8) | 50 | F | L | 1 | Incidental |
| Brown et al. (1) | 56 | M | R | 1.3 | NR |
| Montgomery and Epstein (2) | 49 | M | L | 1.3 | NR |
| Montgomery and Epstein (2) | 74 | M | NR | 1.5 | Hematuria |
| Chou et al. (8) | 60 | M | L | 1.8 | Incidental |
| Mehta et al. (9) | 45 | M | NR | 1.9 | NR |
| Mehta et al. (9) | 49 | M | NR | 2 | NR |
| Montgomery and Epstein (2) | 75 | F | NR | 2 | Hematuria |
| Zhao et al. (10) | 48 | M | R | 2.1 | Incidental |
| Brown et al. (1) | 21 | M | R | 2.2 | Incidental |
| Tao et al. (11) | 32 | F | L | 2.6 | Incidental |
| Brown et al. (1) | 33 | F | L | 3.2 | NR |
| Wetherell et al. (4) | 74 | M | R | 5 | LUT symptoms |
| Kryvenko et al. (5) | 39 | M | R | 5 | Incidental |
| Heidegger et al. (12) | 56 | M | R | 7 | Incidental |
| Kryvenko et al. (13) | 40 | M | L | 1 | Incidental |
| Kryvenko et al. (13) | 62 | M | L | 1 | Incidental |
| Kryvenko et al. (13) | 60 | M | L | 1.2 | Incidental |
| Kryvenko et al. (13) | 46 | M | L | 1.6 | Incidental |
| Kryvenko et al. (13) | 29 | M | R, L | 1, 1 | Incidental |
| Kryvenko et al. (13) | 68 | F | R | 0.1, 1.5 | Hematuria |
| Kryvenko et al. (13) | 15 | M | R, L | 0.2, 0.7 | Incidental |
| Kryvenko et al. (13) | 34 | M | R | 0.25-1.3 | Abdominal pain |
| Kryvenko et al. (13) | 40 | M | L | 0.2-2.8 | Incidental |
| Kryvenko et al. (13) | 17 | M | R, L | 0.1, 2.8 | Abd pain, hematuria |
| Kryvenko et al. (13) | 66 | M | R | 3 | Abdominal pain |
| Kryvenko et al. (13) | 49 | M | R | 3.5 | Hematuria |
| Kryvenko et al. (5) | 51 | F | R | 1 | Incidental |
| Kryvenko et al. (5) | 67 | F | L | 1.2 | Incidental |
| Kryvenko et al. (5) | 54 | F | R, L | 1.2, 0.6 | Incidental |
| Pantelides et al. (14) | 57 | F | R | 2.2 | Abdominal pain |
| Tran and Pernicone (15) | 61 | M | R | 2.4 | Abdominal pain |
| Büttner et al. (3) | 43 | F | NR | 0.1 | NR |
| Büttner et al. (3) | 55 | M | NR | 0.2 | NR |
| Büttner et al. (3) | 69 | M | NR | 0.3 | NR |
| Büttner et al. (3) | 42 | M | NR | 0.3 | NR |
| Büttner et al. (3) | 32 | M | NR | 0.6, 0.7 | NR |
| Büttner et al. (3) | 41 | M | NR | 0.15, 0.1 | NR |
| Büttner et al. (3) | 45 | F | NR | 0.1-2.5 | NR |
| Büttner et al. (3) | 32 | M | NR | 0.3-0.6 | NR |
| Tahir and Folwell (16) | 57 | M | L | 3 | Incidental |
| Montgomery and Epstein (2) | 65 | F | NR | 2 | Abdominal pain |
| Mehta et al. (9) | 55 | M | NR | 0.6 | NR |
| Brown et al. (1) | 44 | F | L | 2 | NR |
| Brown et al. (1) | 83 | F | L | 3.5 | NR |
The values in centimeter represent the size of the tumour as unilateral, bilateral and in few instances, as multifocal lesions in one kidney. NR, not reported; L, left; R, right; Abd, abdominal.
Table 2. Treatment modality and outcome of anastomosing hemangioma (AH) of the kidney.
| Cases | Treatment | F/U (months) | Outcome |
|---|---|---|---|
| Chou et al. (8) | Nephrectomy | 14 | NED |
| Brown et al. (1) | Nephrectomy | NR | NR |
| Montgomery and Epstein (2) | Nephrectomy | 12 | NED |
| Montgomery and Epstein (2) | Nephrectomy | 36 | NED |
| Chou et al. (8) | Nephrectomy | 8 | NED |
| Mehta et al. (9) | Nephrectomy | 12 | NED |
| Mehta et al. (9) | Nephrectomy | 3 | NED |
| Montgomery and Epstein (2) | Nephrectomy | NR | NR |
| Zhao et al. (10) | Nephrectomy | 12 | NED |
| Brown et al. (1) | Nephrectomy | 24 | NED |
| Tao et al. (11) | Nephrectomy | 21 | NED |
| Brown et al. (1) | Nephrectomy | NR | NR |
| Wetherell et al. (4) | Nephrectomy | 1 | DFUD |
| Kryvenko et al. (5) | Nephrectomy | 122 | NED |
| Heidegger et al. (12) | Nephrectomy | 156 | NED |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (13) | Nephrectomy | NR | NR |
| Kryvenko et al. (5) | Nephrectomy | 7 | NED |
| Kryvenko et al. (5) | Nephrectomy | 6 | NED |
| Kryvenko et al. (5) | Nephrectomy | 3 | NED |
| Pantelides et al. (14) | Nephrectomy | 6 | NED |
| Tran and Pernicone (15) | Nephrectomy | NR | NR |
| Büttner et al. (3) | Nephrectomy | NR | NR |
| Büttner et al. (3) | Nephrectomy | NR | NR |
| Büttner et al. (3) | Nephrectomy | NR | NR |
| Büttner et al. (3) | Nephrectomy | NR | NR |
| Büttner et al. (3) | Nephrectomy | NR | NR |
| Büttner et al. (3) | Nephrectomy | NR | NR |
| Büttner et al. (3) | Nephrectomy | NR | NR |
| Büttner et al. (3) | Nephrectomy | NR | NR |
| Tahir and Folwell (16) | Nephrectomy | 1 | NED |
| Montgomery and Epstein (2) | Excision | 8 | NED |
| Mehta et al. (9) | Nephrectomy | 3 | NED |
| Brown et al. (1) | Nephrectomy | 72 | NED |
| Brown et al. (1) | Nephrectomy | 24 | NED |
NED, no evidence of disease; NR, not reported; DFUD, died from unrelated disease.
Table 3. Frequency of positive immunostains in the diagnosis of anastomosing hemangioma of the kidney.
| Stains | No. of cases | Percentage (%) |
|---|---|---|
| CD31 | 41 | 91 |
| CD34 | 32 | 71 |
| Factor VIII related protein | 10 | 22 |
| FLI-1 | 7 | 16 |
| Actin | 2 | 4 |
| ERG | 2 | 4 |
Percentage use of the immunohistochemical markers out of the 45 cases reported in the literature. CD 31 and CD34 were mostly diffusely positive.
Clinical findings
The mean age of presentation is 50 years (range, 15-83 years) and male sex accounted for 68.8% of the cases reported in the literature. The patients were mostly asymptomatic with 62% of the cases documented as incidental findings. Clinical features include hematuria, abdominal pain (2,13,14), and LUTs (4). Abdominal pain and hematuria accounted for 21% and 17% of the cases reported respectively. A case of incidental finding on CT for the diagnostic workup for chronic polycythemia reportedly resolved after nephrectomy (5). The tumors were typically unilateral with only four patients with bilateral involvement of the kidney (5,13).
Imaging findings
Ultrasound imaging demonstrated varying echogenicity (4,17). Non-enhanced CT imaging demonstrated lobulated hypo-attenuating to iso-attenuating soft tissue masses in the kidney while enhanced CT imaging appeared as solid, well demarcated heterogeneous masses (Figure 1) with intense enhancement observed in the arterial phase which persisted to the venous phase (11,13).
Figure 1.
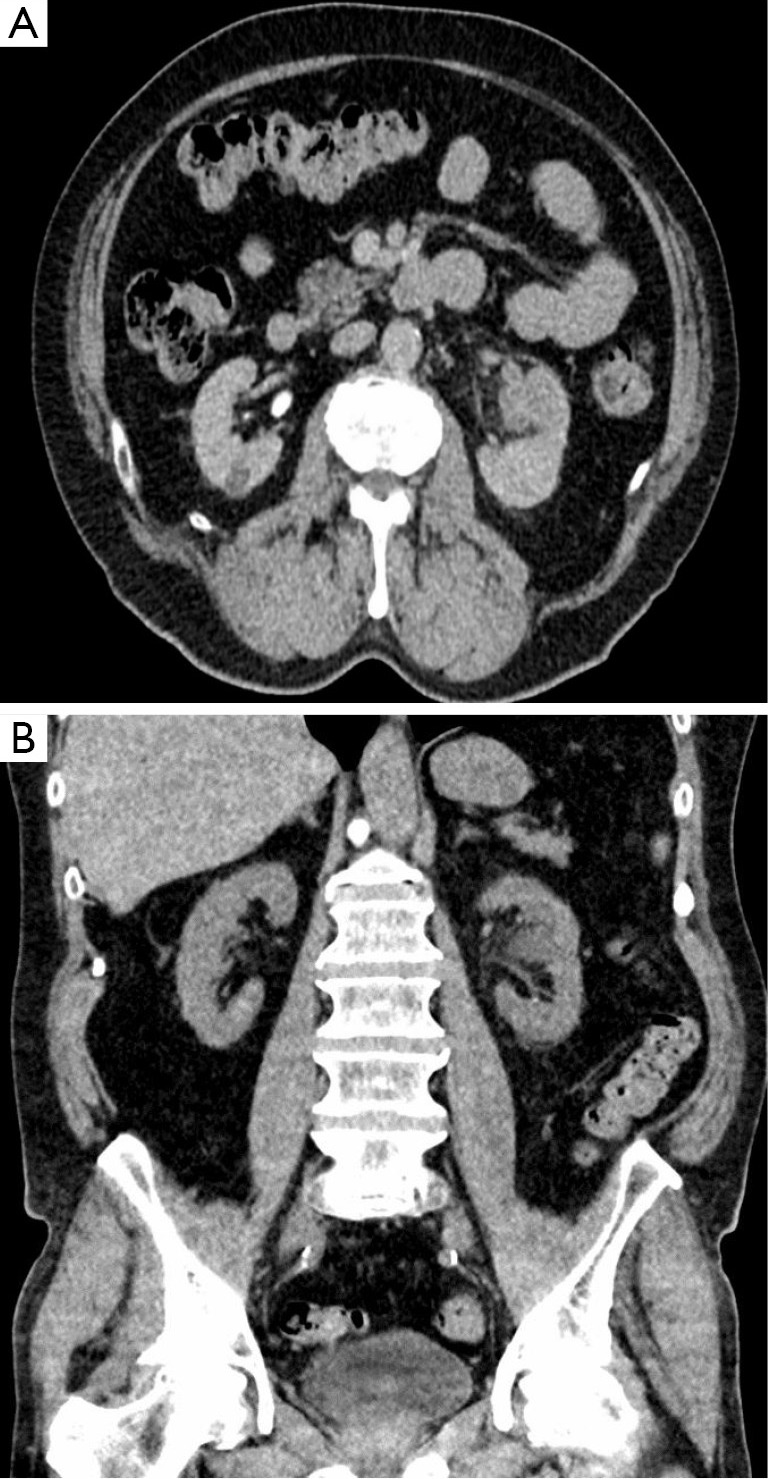
CT image venous phase (A) and non-contrast phase (B) of a solid mass (anastomosing hemangioma) of the left kidney.
Macroscopic findings
The mean size of the tumor in this review is 1.5 cm (range, 0.1-7 cm). The largest tumor size of 7 cm was an incidental finding in a 56-year-old patient admitted for febrile prostatitis (12). Tumors were mostly round, red-brown (1,3) to hemorrhagic mahogany lesions with a spongy consistency (2,5) and well-defined margins with (2,15) or without encapsulation (1,3-5,9,10,12,13).
Tumors were frequently solitary lesions (4) which accounted for 76% of the cases however few cases were multifocal (3,8,13). Büttner et al. in a retrospective review of 90 nephrectomy specimens reported eight cases of AH. Four out of the cases were multifocal lesions with a maximum of four discrete lesions (3). Chou et al. and Kryvenko et al. identified a maximum of seven and eight circumscribed lesions in one kidney respectively (8,13). The multifocal lesions occurred mostly in the setting of end stage renal disease (ESRD).
AH of the kidney were typically hilar in location (2,4,16), however, other locations in the kidney have been documented. These include the medulla (3,13), cortex (3,8), perinephric adipose tissue (2,5) renal sinus (8) and tumors abutting the renal capsule without invasion (4). The cases of AH reported by Kryvenko et al. were mostly located in the medulla with portions of the tumor abutting and protruding into the renal sinus fat (13). The tumors in most cases, were without vascular invasion or gross evidence of necrosis however in rare instances, gross tumor extension into tributaries of the renal vein have been described (8,13,15).
Microscopic findings
AH of the kidney often demonstrated a vaguely lobulated architecture at low magnification with alternating paucicellular zones and cellular areas (4,11,13). At higher magnification, the cellular areas were composed of anastomosing sinusoidal capillary sized vessels or oval shaped vascular channels with scattered endothelial cells. The endothelial cells with hobnail appearance (Figure 2A) were seen within a framework of non-endothelial supporting cells. This architectural pattern bears a huge semblance of splenic sinusoids (2-5,8,11,13).
Figure 2.
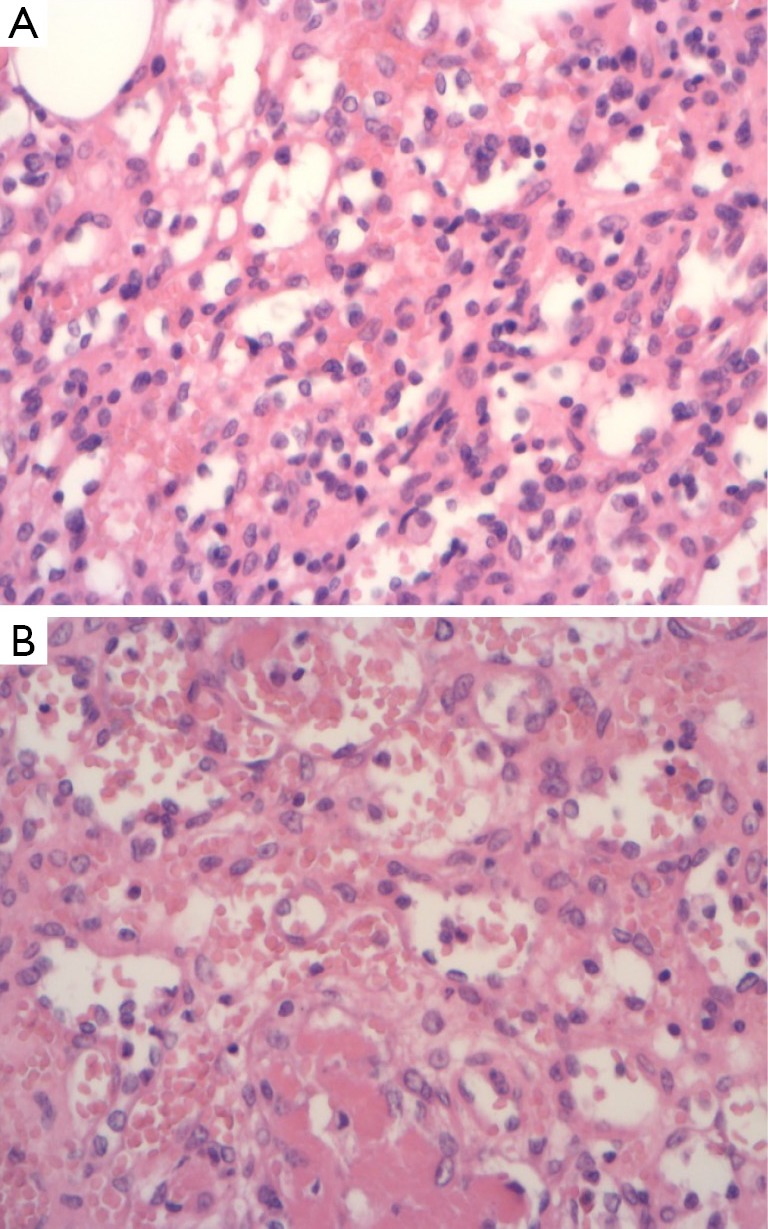
Demonstrates hobnail endothelial cells (A) and fibrin thrombi in some vascular spaces (B). (HE, ×20).
The hypocellular areas were composed of loose stromal edema or hyalinised stroma with collagen deposits and non-endothelial supporting cells between the proliferating elastic thin-walled vessels. Some cases had stromal hemorrhage (2,4,9) and typically, intraluminal fibrin thrombi (Figure 2B) of varying sizes and infiltration of large renal veins have been documented (2,4,5,8). Endothelial cells were plump with vaguely eosinophilic cytoplasm and ill-defined cell membrane. Most cases reported extramedullary haemopoiesis (2,5,8,9,13,15), however, Wetherell et al. reported no evidence of extramedullary haemopoiesis (4). Various cells within the vascular space such as histiocytic cells, eosinophils, lymphocytes, immature appearing granulocytes, normoblasts and isolated megakaryocytes consistent with extramedullary hematopoiesis were observed (8). There was evidence of minimal lymphocytic infiltration without plasma cells or predominant acute inflammatory cells (2,4,5).
Some cases demonstrated prominent sclerosis, hyalinization and occasional hemorrhage (3). There was no evidence of nuclear atypia, endothelial tufting, multilayering, apoptotic figures, mitotic activity and other features suggestive of malignancy (3,4,12). Although a rare instance with mild cytological atypia has been reported (2).
Immunohistochemistry
AH of the kidney demonstrated evidence of endothelial differentiation with positive staining for endothelial markers CD31, CD34 (Figure 3) and factor VIII-related protein (2,4,5,15). Positivity to smooth muscle actin by stromal cells was reported in some cases (11,15). CD8, GLUT-1 and D2-40 staining was negative suggesting that the tumor was not related to juvenile hemangioma (GLUT-1 positive), splenic sinusoids (CD8 positive) and was not lymphatic in origin or differentiation (D2-40 positive) (1,3,12). The eosinophilic globules were diffusely and strongly PAS positive in cases with globules (5). The tumor lacked immunoreactivity for HHV-8, a marker of Kaposi sarcoma, epithelial membrane antigen (EMA), HMB45, S100 protein, desmin, AFP, human chorionic gonadotropin and cytokeratin staining (2,5,8,12). Ki-67 expression in the tumor ranged from <1-3% demonstrating a low proliferative index (3,11,12).
Figure 3.
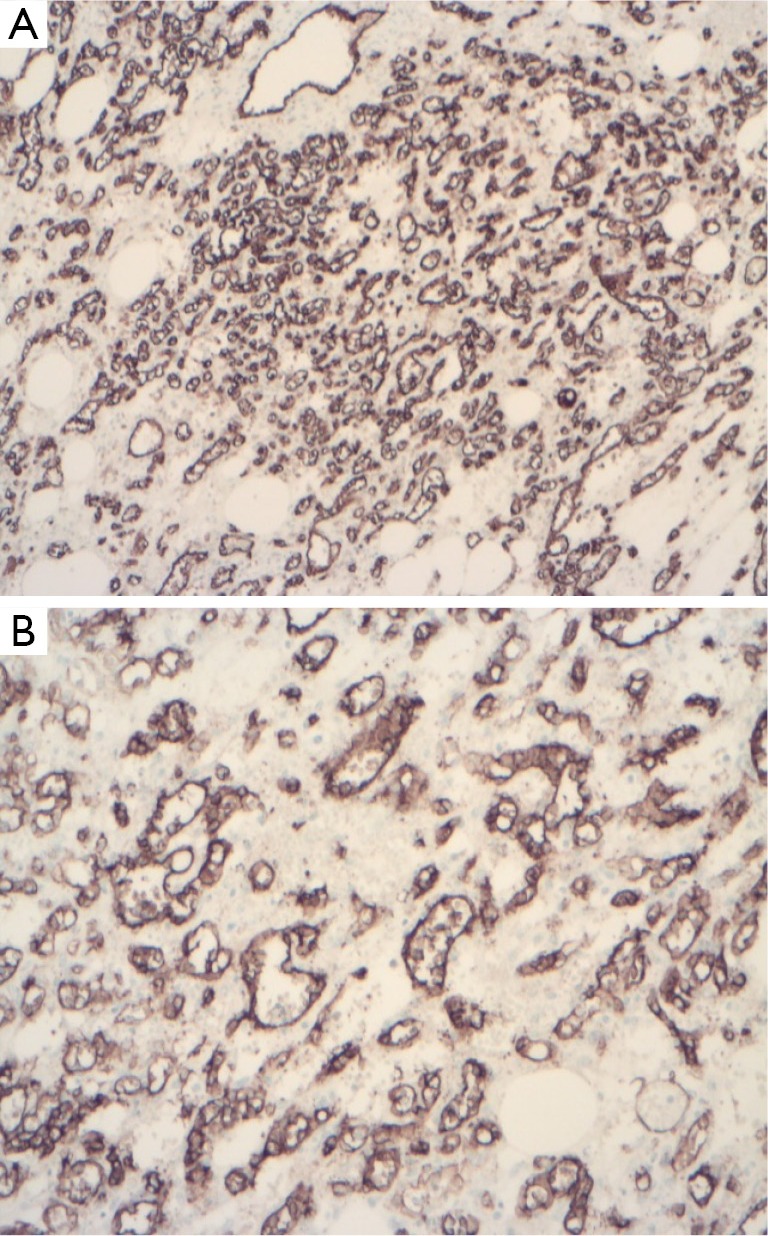
Immunohistochemical expression of the tumor positive for CD31 (A) and CD34 (B). (×4).
Treatment and follow up
The mainstay of treatment was nephrectomy. Follow up data were available for 21 cases with a mean follow up of 26 months (range, 1-156 months) and there was no evidence of disease recurrence. One patient died of unrelated disease (cerebrovascular accident) (4). Evidence so far in the literature suggests a benign course with the longest duration of follow up for 156 months in this review (12).
Discussion
Vascular tumors of the kidney are rare. Brown et al. in a clinicopathological analysis of primary vascular tumors of the kidney documented 5 cases of AH out of the reported 25 cases of vascular renal tumors (1). Previous reports have documented a slight male predilection in renal hemangioma with a male to female ratio of 1.8:1 (1) and this is consistent with the findings of this review which demonstrated that AH of the kidney occurred predominantly in the male sex (68.8%).
The clinical presentation of the tumor is non-specific although hemangioma of the bladder has been associated with tuberous sclerosis, Sturge-Weber syndrome and Klippel-Trenaunay syndrome (18), there is no evidence to suggest the involvement of AH of the kidney with any systemic or syndromic condition (1,9). The association between ESRD and malignant renal epithelial neoplasms is well documented (13). Two recent papers have described a novel clinicopathological association between AH and ESRD. There was a wide spectrum in the cause of the ESRD in the cases reported which precludes the possibility of ESRD-associated AH arising from a specific underlying cause. Coexisting renal epithelial neoplasms like papillary RCC, clear cell RCC, papillary adenoma and benign mesenchymal lesions like angiomyolipoma were also described (3,13). The pathogenesis of ESRD associated AH is unknown but what is known, is the propensity of kidneys damaged by chronic disease to develop not only epithelial renal tumors but also benign mesenchymal tumors (3). It is important to note that AH is not unique to ESRD as observed in this review, AH also developed in kidneys without chronic disease as well.
Imaging findings in AH of the kidney are not specific and often mimic other renal tumors as observed in this review. Microscopically, the typical demonstration of interlacing network of vascular channels with endothelial cells which has hobnail appearance is a diagnostically helpful feature of the tumor. Hyaline globules within endothelial cells which represents secondary lysosomes (thanatosomes) (2,4,8,13) has been reported. Hyaline globules are also present in cutaneous and soft tissue vascular lesions such as Kaposi sarcoma (19,20), angiosarcoma (21), pyogenic granulomas (19) and littoral cell angioma of the spleen (22).
The differential diagnosis of AH of the kidney includes angiosarcoma, intravascular papillary endothelial hyperplasia (IPEH), Kaposi sarcoma and angiomyolipoma.
Angiosarcoma remains the main differential diagnosis because it is an aggressive malignant tumor that often presents in the 6th to 7th decade of life. Angiosarcoma often metastasize to the liver, lungs and bones and may have metastasized at the time of diagnosis (1,23). AH of the kidney and angiosarcoma of the kidney both have overlapping clinicopathological features which include hematuria, flank pain, presence of hyaline globules and immunopositivity for endothelial cell markers (1,2,23). However AH of the kidney are usually small sized lesions with loosely lobulated anastomosing architecture on histology, hobnail appearances of the endothelial cells and without features suggestive of malignancy. In contrast to AH, angiosarcoma of the kidney often present as a large, mostly necrotic renal lesion with parenchymal invasion. They demonstrate highly cellular infiltrative patterns, mitotic activity, necrosis, multilayering and cytological atypia. Angiosarcoma tend to recur rapidly and aggressively (1,2,8,13).
IPEH is a vascular lesion that was initially recognized as a malignant lesion by Masson but subsequently it was described as a benign lesion, which is thought to represent a form of organizing thrombus. IPEH is very rare and has been described in varying locations of the body with six cases of renal involvement in the literature (24). There are three broad classification of IPEH which includes; a primary type unrelated to trauma and which arises from vessels; a secondary type which arises from pre-existing vascular lesions such as hemangioma and a third type which arises from hematomas (9). IPEH and AH of the kidney share similar clinical and pathological features which include non-specific presentation and positivity to vascular markers such as CD31 and CD34. However, the primary histopathological feature of IPEH is the formation of papillary structures lined by hyperplastic endothelial cells in the vascular lumen without the splenic sinusoidal architectural pattern seen in AH of the kidney (25).
Kaposi sarcoma notably demonstrates hyaline globules reportedly seen in some cases of AH which could possibly present a diagnostic challenge, however, Kaposi sarcoma stains positive to HHV-8 which is negative in AH of the kidney (12).
Angiomyolipoma may demonstrate a classic (triphasic or mixed) appearance or a lipoma-like type which is mostly composed of fat or myoid (leiomyoma-like) cells (3). The blood vessels in angiomyolipoma are characteristically poorly formed with smooth muscle cells originating and radiating from the media with the obliteration of the adventitia. The cells are diffusely positive to smooth muscle and melanocytic markers (13). Although the stromal cells of AH may be positive to smooth muscle markers (11,15), they are often negative to melanocytic markers such as HMB-45 or Melan-A (1-3,9,13).
In conclusion, this is a clinicopathological review of AH of kidney in the literature which is a rare vascular tumor and a morphological variant of capillary hemangioma. It has a typical histological architecture which bears a huge semblance of splenic sinusoids. AH of the kidney shares clinical and imaging features with other vascular tumors of the kidney, with concerns for malignancy, hence the need for histopathological review and immunohistochemical studies for accurate diagnosis. It appears to run a benign course and it is amenable to surgery without evidence of disease recurrence during follow up.
Acknowledgements
Many thanks to Dr Kalyanasundaram and Dr Mann for the kind provision of the slides.
Disclosure: The author declares no conflict of interest.
References
- 1.Brown JG, Folpe AL, Rao P, et al. Primary vascular tumors and tumor-like lesions of the kidney: a clinicopathologic analysis of 25 cases. Am J Surg Pathol 2010;34:942-9. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: a lesion mimicking angiosarcoma. Am J Surg Pathol 2009;33:1364-9. [DOI] [PubMed] [Google Scholar]
- 3.Büttner M, Kufer V, Brunner K, et al. Benign mesenchymal tumors and tumor like lesions in end-stage renal disease. Histopathology 2013;62:229-36. [DOI] [PubMed] [Google Scholar]
- 4.Wetherell DR, Skene A, Manya K, et al. Anastomosing haemangioma of the kidney: a rare morphological variant of haemangioma characteristic of genitourinary tract location. Pathology 2013;45:193-6. [DOI] [PubMed] [Google Scholar]
- 5.Kryvenko ON, Gupta NS, Meier FA, et al. Anastomosing hemangioma of the genitourinary system: eight cases in the kidney and ovary with immunohistochemical and ultrastructural analysis. Am J Clin Pathol 2011;136:450-7. [DOI] [PubMed] [Google Scholar]
- 6.Ross M, Polcari A, Picken M, et al. Anastomosing hemangioma arising from the adrenal gland. Urology 2012;80:e27-8. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Bigge J, Ulbright TM, et al. Anastomosing haemangioma of the liver and gastrointestinal tract: an unusual variant histologically mimicking angiosarcoma. Am J Surg Pathol 2013;37:1761-5. [DOI] [PubMed] [Google Scholar]
- 8.Chou S, Subramanian V, Lau HM, et al. Renal anastomosing hemangioma with a diverse morphologic spectrum: report of two cases and review of the literature. Int J Surg Pathol 2013;22:369-73. [DOI] [PubMed] [Google Scholar]
- 9.Mehta V, Ananthanarayanan V, Antic T, et al. Primary benign vascular tumors and tumorlike lesions of the kidney: a clinicopathologic analysis of 15 cases. Virchows Arch 2012;461:669-76. [DOI] [PubMed] [Google Scholar]
- 10.Zhao M, Li C, Zheng J, et al. Anastomosing hemangioma of the kidney: a case report of a rare subtype of hemangioma mimicking angiosarcoma and review of the literature. Int J Clin Exp Pathol 2013;6:757-65. [PMC free article] [PubMed] [Google Scholar]
- 11.Tao LL, Dai Y, Yin W, et al. A case report of a renal anastomosing hemangioma and a literature review: an unusual variant histologically mimicking angiosarcoma. Diagn Pathol 2014;9:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidegger I, Pichler R, Schafer G, et al. Long term follow up of renal anastomosing hemangioma mimicking renal angiosarcoma. Int J Urol 2014;21:836-8. [DOI] [PubMed] [Google Scholar]
- 13.Kryvenko ON, Haley SL, Smith SC, et al. Hemangiomas in kidneys with end-stage renal disease: a novel clinicopathological association. Histopathology 2014;65:309-18. [DOI] [PubMed] [Google Scholar]
- 14.Pantelides NM, Agrawal S, Mawson I, et al. An Anastomosing Haemangioma: A Rare Vascular Tumour Presenting as a Solid Renal Mass. Br J Med Surg Urol 2012;5:84-6. [Google Scholar]
- 15.Tran TA, Pernicone P. Anastomosing hemangioma with fatty changes of the genitourinary tract: a lesion mimicking angiomyolipoma Cent European J Urol 2012;65:40-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahir M, Folwell A. Anastomosing hemangioma of the kidney: a rare subtype of vascular tumor of the kidney mimicking angiosarcoma. ANZ J Surg 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Lee HS, Koh BH, Kim JW, et al. Radiologic findings of renal hemangioma: report of three cases. Korean J Radiol 2000;1:60-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavora F, Montgomery E, Epstein JI. A series of vascular tumors and tumor like lesions of the bladder. Am J Surg Pathol 2008;32:1213-9. [DOI] [PubMed] [Google Scholar]
- 19.Fukunaga M, Silverberg SG. Hyaline globules in Kaposi’s sarcoma: a light microscopic and immunohistochemical study. Mod Pathol 1991;4:187-90. [PubMed] [Google Scholar]
- 20.Kao GF, Johnson FB, Sulica VI. The nature of hyaline (eosinophilic) globules and vascular slits of Kaposi’s sarcoma. Am J Dermatopathol 1990;12:256-67. [DOI] [PubMed] [Google Scholar]
- 21.Vuletin JC, Wajsbort RR, Ghali V. Primary retroperitoneal angiosarcoma with eosinophilic globules: a combined light-microscopic, immunohistochemical, and ultrastructural study. Arch Pathol Lab Med 1990;114:618-22. [PubMed] [Google Scholar]
- 22.Michal M, Skalova A, Fakan F, et al. Littoral cell angioma of the spleen: a case report with ultrastructural and immunohistochemical observations. Zentralbl Pathol 1993;139:361-5. [PubMed] [Google Scholar]
- 23.Leggio L, Addolorato G, Abenavoli L, et al. Primary renal Angiosarcoma: a rare malignancy. A case report and review of the literature Urol Oncol 2006;24:307-12. [DOI] [PubMed] [Google Scholar]
- 24.Akhtar M, Aslam M, Al-Mana H, et al. Intravascular papillary endothelial hyperplasia of renal vein: report of 2 cases. Arch Pathol Lab Med 2005;129:516-9. [DOI] [PubMed] [Google Scholar]
- 25.Akdur NC, Donmez M, Gozel S, et al. Intravascular papillary endothelial hyperplasia: histomorphological and immunohistochemical features. Diagn Pathol 2013;8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]


