Abstract
In persons with advanced immunosuppression, Mycobacterium avium complex (MAC) typically causes disseminated disease with systemic symptoms. We report 2 cases in which MAC caused localized osteomyelitis in human immunodeficiency virus (HIV)-infected individuals on antiretroviral therapy with rising CD4 counts. We summarize 17 additional cases of HIV-associated MAC osteomyelitis from the literature and compare CD4 count at presentation for vertebral cases versus nonvertebral cases, which reveals a significantly higher CD4 at presentation for vertebral cases (median 251 cells/µL vs 50 cells/µL; P = .043; Mann–Whitney U test). The literature review demonstrates that the majority of cases of MAC osteomyelitis, especially vertebral, occurs in individuals with CD4 counts that have increased to above 100 cells/µL on antiretroviral therapy. Among HIV-infected individuals with osteomyelitis, MAC should be considered a possible etiology, particularly in the setting of immune reconstitution.
Keywords: HIV, Mycobacterium avium complex, opportunistic infections, osteomyelitis
Mycobacterium avium complex (MAC) remains the most frequent bacterial opportunistic infection in persons with acquired immune deficiency syndrome and typically causes disseminated disease in the setting of advanced immunosuppression [1, 2]. Prophylaxis is indicated when the CD4 count drops below 50 cells/µL and continues until the CD4 count stabilizes above 100 cells/µL on antiretroviral therapy (ART) [2]. Immune reconstitution inflammatory syndrome (IRIS), a frequent complication of MAC disease, most commonly presents with fever and lymphadenitis after the CD4 count increases in response to ART [3].
In this study, we report 2 cases of localized MAC osteomyelitis in patients who were taking ART and had rising CD4 counts above the range in which disseminated MAC infection would be expected or prophylaxis indicated, suggesting a role for IRIS. These cases underscore the importance of considering MAC as a cause of osteomyelitis in individuals with human immunodeficiency virus (HIV), including those with reconstituted CD4 counts. We review the literature of HIV-associated MAC osteomyelitis and summarize previously published cases. In addition, we compare the CD4 count at presentation between vertebral and nonvertebral cases, which reveals a significantly higher CD4 count at presentation in vertebral cases.
Case 1
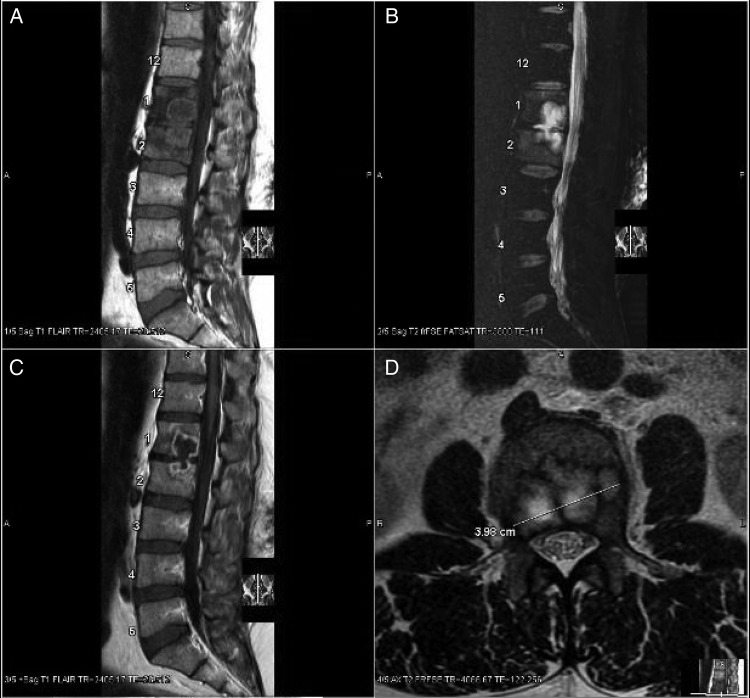
A 50-year-old man, who had been diagnosed with HIV in 2007 with a CD4 nadir of 14 cells/µL and had never taken ART, was found to have stage 1 large B-cell non-Hodgkin's lymphoma of the esophagus in August 2010. He received 1 cycle of R-EPOCH chemotherapy. His CD4 count before chemotherapy was 20 cells/µL. He also started ART in August 2010 and initially struggled with adherence but had confirmed suppression of HIV RNA by April 2011 and HIV RNA remained routinely suppressed thereafter. Computed tomography (CT) scans of the chest, abdomen, and pelvis performed in January, March, and July 2011 for cancer surveillance showed no pathologic lesions of the spine. In January 2012, at which time his CD4 count had increased to 414 cells/µL, he presented to care for back pain. Magnetic resonance imaging (MRI) demonstrated decreased T1 signal and increased T2 signal in L1 and L2 with an enhancing 4.3 × 4 cm mass involving the inferior L1 and superior L2 vertebral bodies (Figure 1). He reported no systemic symptoms. Prophylactic azithromycin had been stopped more than 2 years prior.
Figure 1.
Sagittal views of the lumbar spine reveal a heterogeneous mass demonstrating decreased T1 (A) and increased T2 signal (B and D) involving the L1 and L2 lumbar body and disc. The mass reveals peripheral enhancement in postcontrast T1 flair images (C).
Biopsy of the mass demonstrated a neutrophilic inflammatory reaction with necrosis but no evidence of tumor or granuloma. Acid-fast bacilli were seen by fluorochrome stain and culture grew MAC. Blood mycobacterial cultures were not performed. He started therapy with clarithromycin, ethambutol, and rifabutin. The organism was susceptible to macrolides. Five months later, a repeat MRI, done for worsening back pain, revealed evolution of the nondescript mass to a frank intradiscal and intraosseous abscess with progression of spondylodiscitis, new paraspinal phlegmon, and inflammatory changes in the psoas. He underwent surgical evacuation of the abscess, culture again grew MAC, and thrice-weekly amikacin was added empirically. He eventually underwent L1 and L2 corpectomy with placement of an interbody cage due to spinal instability. He completed a total of 6 months of amikacin and 1 year of clarithromycin, ethambutol, and rifabutin, before transitioning to clarithromycin secondary prophylaxis. He is without signs of relapse 12 months after completing intensive MAC therapy.
Case 2
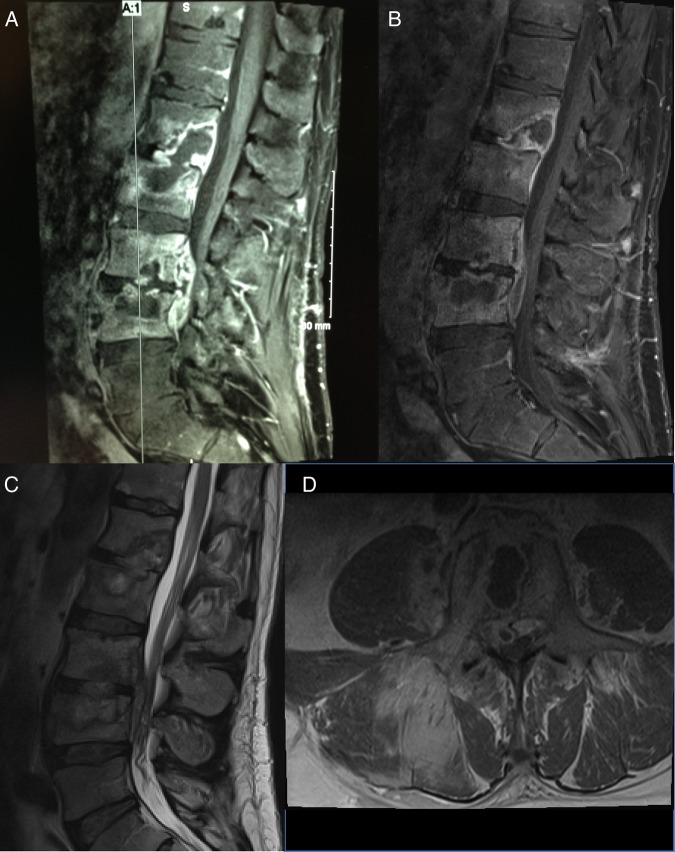
A 52-year-old man was diagnosed with HIV in 2011 with baseline CD4 count of 40 cells/µL. He started ART and 5 months later was instructed to stop prophylactic azithromycin because his CD4 count had remained >100 cells/µL for 3 months. Nine months into ART, his CD4 count had increased to 250 cells/µL, but he developed severe axial low back pain. Plain radiographs indicated only mild lumbar disc degeneration. He returned 3 months later with increased pain. MRI demonstrated changes at L1–L2 and L3–L4, thought to be Schmorl's nodes. Despite conservative therapy, his pain persisted. He did not have any constitutional symptoms. Repeat imaging 4 months after the initial MRI showed what appeared to be increased size of the L1 Schmorl's node extending into L2, destruction of the posterior left lateral aspect of L1, and possible Schmorl's node extending from L3 into L4 (Figure 2). Due to concern for potential opportunistic infection, he underwent CT-guided biopsy of the L1 and L3 vertebral bodies; histopathology revealed changes consistent with chronic osteomyelitis, but cultures for bacteria, fungi, and mycobacteria remained negative.
Figure 2.
Sagittal views of the lumbar spine demonstrate a heterogeneous mass with diffuse signal alterations in the L1 vertebra extending into L2 and in L3 extending into L4 with enhancement of the vertebral bodies (A). Repeat images again reveal destruction of L1–L4 with small epidural fluid collection communicating between superior aspect of L3 through inferior aspect of L4 and extending into right L3–L4 neural foramen (B–D).
Repeat CT-guided biopsy 4 months later, this time of the L2 and L4 vertebral bodies, demonstrated chronic osteomyelitis with possible granulomatous inflammation. Stains for bacteria and fungi were negative, but Ziehl-Neelsen stain revealed acid-fast organisms and polymerase chain reaction (PCR) detected MAC DNA (by heat shock protein 65 [hsp65] amplified probe); result of the PCR assay returned 9 days after the biopsy. Based on the PCR result, he started therapy with azithromycin, ethambutol, and ciprofloxacin. Rifamycins were avoided in initial MAC therapy because his ART included a ritonavir-boosted protease inhibitor. Cultures of bone grew MAC after 22 days while blood culture for mycobacteria remained negative. The organism was susceptible to macrolides.
Repeat MRI 2 months after initiation of therapy demonstrated an interval increase in L4 vertebral body destruction and a small abscess extending through the anterior cortex of L4 into prevertebral soft tissues, as well as unchanged discitis and osteomyelitis of L1–L3 (Figure 2). Thrice-weekly rifabutin and thrice-weekly amikacin were added; amikacin was stopped after approximately 6 months due to tinnitus. After 14 months of antimycobacterial treatment, the patient is steadily improving.
Literature Review and Statistical Analysis
We reviewed the published English literature for cases of MAC osteomyelitis in HIV-infected individuals. We searched PubMed/MEDLINE and EMBASE for reports that included the terms “mycobacterium” or “mycobacteria” or “mycobacterial” or “MAC” or “MAI” plus “osteomyelitis” plus “HIV.” We also performed a second, broader search for “osteomyelitis” and “HIV.” We reviewed all results to determine whether they fit the qualifications of HIV-infected individuals with MAC osteomyelitis, and we communicated with authors of 1 report to confirm and supplement published information.
We eliminated reports involving other mycobacterial organisms, joint infection (such as septic arthritis) without osteomyelitis, epidural abscess without osteomyelitis, or HIV-uninfected persons. We eliminated 1 report of MAC osteomyelitis that does not include specific information about the patient so it could not be included for analysis; however, we do refer to this case in the discussion. We considered CD4 count at presentation to be the documented CD4 count closest to the time that the patient presented to care with symptoms that led to a diagnosis of MAC osteomyelitis. To evaluate differences in the distributions of CD4 count at presentation between vertebral and nonvertebral osteomyelitis cases, we performed a nonparametric Mann–Whitney U test [4].
RESULTS
In total, we summarize 19 cases of MAC osteomyelitis, including the 2 reported here (Table 1). The most common site of infection is the spine, with vertebral bodies involved in 10 of 19 cases (53%). The median CD4 nadir is 24 cells/µL.
Table 1.
Summary of MAC Osteomyelitis Cases, Organized by Site of Infection (Vertebral vs Nonvertebral)
| Vertebral Cases | Age/Sex | CD4 Nadir (cells/mm3) | CD4 at Pres (cells/mm3) | Osteomyelitis Site | Time From ART Start to Presa | MAC Prophylaxisb | AFB Blood Culture | Surgical Debridement? | MAC Therapyc |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 50/M | 14 | 414 | L1–L2 | 18 mo | None | Not done | Yes | Clarithro, ethambutol, rifabutin; amikacin later added |
| Case 2 | 52/M | 40 | 250 | L1–L4 | 9 mo | Azithro stopped 4 mo before pres | Negative | No | Azithro, ethambutol, cipro; amikacin and rifabutin later added |
| Matt 2013 et al [15]d | 50/F | 24 | 68 | L1 | 3 mo | Azithro started 5 wk before pres | Negative 4 mo before pres | Yes | Isoniazid, ethambutol, rifampin; cipro also for first 6 weeks |
| Corrales-Medina 2006 et al [18] | 35/M | 24 | 320 | L1–L3 | 12 mo | NR | NRe | CT-guided drainage | Clarithro, ethambutol, rifabutin, isoniazid, pyrazinamide; dexamethasone later added |
| Phillips 2005 et al [22]f | 37/M | 60 | 180 | T10 | 27 mo | NR | NR | CT-guided drainage | Clarithro, ethambutol |
| Aberg 2002 et al [7] #1g | 49/M | 16 | 465 | T9–T10 | 13 mo | Stopped 15 mo before pres | Negative | Yes | Clarithro, ethambutol, rifabutin, cipro |
| Aberg 2002 et al [7] #2 | 49/M | 23 | 118 | T6–T7 | NR | Stopped 3 mo before pres | NR | Yes | Clarithromycin and ethambutol initially; later changed to azithro and cipro |
| Fraser 2002 et al [13] | 56/M | 24 | 27 | T9–T11 | 15 mo | Azithro | Negative | Yes | Clarithro, ethambutol, rifabutin |
| Erard 1999 et al [8] | 36/F | 44 | 423 | T8–T10 | 9 mo | None | Negative | Yes | Clarithro, ethambutol, rifabutin |
| Libraty 1998 et al [21] | 30/M | 55 | 251 | T5–T6, T10–T11 | 15 mo | None | NR | Yes | NR |
| Nonvertebral Cases | Age/Sex | CD4 Nadir (cells/mm3) | CD4 at Pres (cells/mm3) | Site | Time to Presa | MAC prophylaxisb | AFB blood culture | Surgical debride-ment? | MAC therapyc |
| Kadzielski 2009 et al [16]h | 51/F | 20 | 34 | Tibia | 2 mo | Azithromycin | Negative | Yes | Clarithro, ethambutol, rifabutin, pyrazinamide and isoniazid initially; later narrowed to clarithro, ethambutol, rifabutin |
| Kahlon 2008 et al [14]i | 58/M | 15 | 47 | Calcaneus, cuboid | 5.5 mo | Azithro | Negative | Yes | Clarithro, ethambutol, rifabutin |
| Aberg 2002 et al [7] #3 | 40/M | 10 | 188 | 6th rib | >12 mo | Secondary prophylaxis stopped 16 mo prior | Positive 4 yr prior | No (lesion spontaneously drained through chest wall) | Clarithro, ethambutol, rifabutin, cipro initially; later narrowed to clarithro and ethambutol |
| Hospenthal 2001 et al [10] | 41/M | NR | 23 | Proximal tibia | NR | Rifabutin | Negative | Bone biopsy only | Clarithro, ethambutol |
| MGH case records 2000 [9] | 49/M | NR | 81 | Proximal tibia | 3 yr | None | NRe | No | NR |
| Sheppard 1997 et al [19] | 46/M | 8 | 53j | Tibial plateau | 11 mo | None | Negative | Yes | Clarithro, ethambutol, rifabutin |
| Valdez 1997 et al [23]k | 32/F | NR | 450 | Ileum | Not on ART | None | Negative | Yes | Initially rifampin, isoniazid, pyrazinamide, ethambutol, and amikacin; later narrowed to clarithro, ethambutol, and cipro |
| Weingardt 1996 et al [24] | 51/M | NR | 13 | Distal femur, proximal tibia | NR | NR | NR | CT-guided bone biopsy | NR |
| Blumenthal 1990 et al [20] | 30/M | NR | NR | Wrists, ankle | Not on ART | NR | Positive | No | Ansamycin, cycloserine, clofazamine, ethionamide |
Abbreviations: AFB, acid-fast bacilli; ART, antiretroviral therapy; azithro, azithromycin; cipro, ciprofloxacin; clarithro, clarithromycin; CT, computed tomography; Dx, diagnosis; L, lumbar vertebra; MAC, Mycobacterium avium complex; MGH, Massachusetts General Hospital; MTB, Mycobacterium tuberculosis; NR, not reported; pres, presentation; RIPE, rifampin, isoniazid, pyrazinamide, and ethambutol; T, thoracic vertebra.
a Estimated time to presentation of illness (MAC osteomyelitis) from start or restart of antiretroviral therapy (if multiple prior regimens, time from start of most recent regimen used).
b Prophylaxis being prescribed at the time of MAC diagnosis.
c Several patients initially started 4-drug therapy for presumed MTB before changing to MAC-targeted therapy; in these cases, only MAC-targeted therapy is listed (secondary MAC prophylaxis not included).
d Also had epidural abscess and surgical cultures also grew Staphylococcus epidermidis and Streptococcus viridans.
e Report states that blood cultures were negative but does not specify whether this was bacterial or mycobacterial or both.
f Presented 31 months after ART start with T10 pathological fracture and posterior mediastinal mass; treated as MTB for 12 months based on granulomas and AFB smear (no culture done); infection relapsed 27 months after ART restart, at which point MAC identified (information for relapsed infection included for this analysis because cause of initial infection not confirmed as MTB vs MAC). Developed epidural abscess after initiation of RIPE for MTB and required 2nd surgical drainage before MAC diagnosis and therapy.
g Also history of prior T6–T7 decompression for osteomyelitis 19 months before pres and T6 aspiration 17 months before pres with negative histopathology and cultures both times; time since initial ART was 30 months, although time since most recent ART regimen not reported.
h Presented 2 months earlier, at time of HIV/AIDS diagnosis, with CD4 20 and imaging evidence of tibial osteomyelitis (MAC not identified at that time); MAC identified after patient presented with recurrent symptoms after 2 months on ART so information for time of MAC identification used for this analysis.
i On prednisone for rheumatoid arthritis.
j CD4 count at presentation not reported; CD4 count 2 months before presentation was 8 cells/mm3 and 2 months after presentation was 98 cells/mm3, so 53 cells/mm3 represents the mean of these 2 values and is used as an estimate of the CD4 count at presentation.
k Prior corticosteroid use for sarcoidosis.
The median CD4 count at presentation for all cases is 149 cells/µL (range, 13–465). Patients presenting with vertebral osteomyelitis had significantly higher CD4 counts (median, 251; range, 27–465 cells/µL) compared with those presenting with nonvertebral infection (median, 50; range, 13–450 cells/µL) (Mann–Whitney U test; P = .043).
DISCUSSION
Mycobacterium avium complex infection of persons with HIV generally presents as disseminated disease with systemic symptoms in the setting of advanced immunosuppression [1, 2]. We report 2 cases of localized MAC osteomyelitis and review 17 additional cases from the literature. The majority of cases involve the spine, which is similar to tuberculous osteomyelitis, in which at least 50% of cases are vertebral [5]. Presumably, MAC osteomyelitis occurs after inhalation or ingestion of organisms followed by mycobacteremia and secondary spread to bone, as occurs in spinal tuberculosis [5, 6]. However, most cases lacked constitutional symptoms, and out of 10 case reports that included the result of mycobacterial blood cultures near the time of MAC osteomyelitis diagnosis, only 1 was positive. Therefore, as others have suggested, MAC osteomyelitis may be the result of transient, often asymptomatic, mycobacteremia that inoculates bone [7–10]. Alternatively, osteomyelitis may occur secondary to contiguous spread from infected pulmonary, gastrointestinal, or regional lymph nodes that erode into adjacent bony structures.
Although the median CD4 nadir in this series was below 50 cells/µL, most cases of MAC osteomyelitis presented months to years later (as much as 3 years) after CD4 reconstitution on ART. Cases of spinal infection generally presented at higher CD4 count than cases of nonspinal infection. The reason for this difference is not known but may be due to a difference in pathogenesis; we hypothesize that spinal infection may be the result of a robust immune response to cryptic infection, whereas nonspinal infection may occur secondary to uncontrolled mycobacterial replication with a more meager immune response. Two cases of nonspinal infection occurred in individuals with advanced HIV disease who were not taking ART, whereas all cases of spinal infection occurred in individuals taking ART, supporting the hypothesis that spinal infection occurs as the result of a more robust immune response. Alternatively, individuals with vertebral infection may present to care later, and thus at higher CD4, because symptoms such as back pain are more likely to be attributed to noninfectious etiologies initially; however, even nonvertebral cases took as much as 1 to 3 years to present for symptoms, so this is unlikely to be the only explanation. In addition, some individuals may be genetically predisposed to spinal infection, as has been proposed for vertebral tuberculosis [6, 11]. We identified 1 report of concomitant vertebral (T7) and nonvertebral (5th metatarsal) MAC osteomyelitis; however, this report does not include enough clinical data to be included in the analysis [12].
Several patients had been prescribed MAC prophylaxis before presentation, demonstrating that MAC prophylaxis is not perfectly effective or, as others have suggested, may have limited infection before immune reconstitution [7, 10, 13–15]. Alternatively, MAC infection may have been acquired before initiation of prophylaxis or adherence to prophylaxis may have been imperfect. It is notable that several cases (vertebral and nonvertebral) included a history of previous trauma at the site of MAC infection, suggesting that locus minoris resistentiae may have predisposed bony sites to MAC infection [7, 9, 16, 17].
Diagnosis of MAC osteomyelitis was made by bone biopsy in all cases, and often empiric treatment for bacterial infection or tuberculosis had been initiated before MAC was identified. In several patients, multiple diagnostic procedures were necessary. Acid-fast bacilli stain and/or culture eventually revealed the diagnosis in all cases, but in 1 patient (Case 2), mycobacterial PCR allowed for earlier diagnosis and initiation of MAC-directed therapy. In this case, PCR was performed at the University of Washington (UW) using MAC-specific probes in a nested PCR protocol that targets hsp65. This assay has been validated by comparison with culture and has an analytical sensitivity of 5 genomes per PCR reaction (from UW Department of Laboratory Medicine, personal communication). In case 2, results of the PCR assay were available 13 days before the culture result, which permitted earlier initiation of MAC-targeted therapy.
Treatment, in the majority of cases, included both surgical debridement and antimycobacterial therapy. In most spinal cases, MAC therapy was followed by the development of a paraspinal abscess. Therefore, like spinal tuberculosis, vertebral MAC infection may be associated with “cold abscess” development and surgical intervention is often necessary [5–7]. Treatment of HIV-associated IRIS sometimes requires corticosteroids, but in this series, corticosteroids were used in only 1 case, suggesting that anti-inflammatories are seldom necessary for IRIS-induced MAC osteomyelitis or, alternatively, that they are underused in this type of infection [18]. As with other IRIS syndromes, ART was not stopped in these cases and most patients had a favorable outcome.
This review is limited by its retrospective nature, small sample size, incomplete clinical data in some case reports, and variable timing of the reported CD4 counts. For the analysis, we used CD4 count closest to presentation to care for illness confirmed to be MAC osteomyelitis; this was the most consistently reported CD4 value. In 1 case, the CD4 count at presentation was not reported, although CD4 counts 2 months before and 2 months after presentation were reported, so the mean was used as an estimate [19]. In addition, several of the reports of nonvertebral osteomyelitis did not include ART information. There may be underreporting of MAC osteomyelitis in the literature, and management strategies are variable. Despite these limitations, this is the largest report of MAC osteomyelitis cases to date, and the data help to inform diagnosis and treatment decisions for future cases.
CONCLUSIONS
In summary, the majority of MAC osteomyelitis cases affect the spine and occur in patients taking ART. Cases affecting the spine generally present at higher CD4 counts than nonspinal cases, although both seem to present as a result of IRIS. Patients generally respond to treatment with antimycobacterials, ART, and surgical debridement, and in this series anti-inflammatory medications were rarely required.
Acknowledgments
We thank Drs. Dhruba SenGupta and Brad Cookson (University of Washington Department of Laboratory of Medicine) for input regarding the mycobacterial PCR assay.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Karakousis PC, Moore RD, Chaisson RE. Mycobacterium avium complex in patients with HIV infection in the era of highly active antiretroviral therapy. Lancet Infect Dis 2004; 4:557–65. [DOI] [PubMed] [Google Scholar]
- 2.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf Accessed 25 September 2014. Pages G1-G6.
- 3.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis 2005; 5:361–73. [DOI] [PubMed] [Google Scholar]
- 4.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat 1947; 18:50–60. [Google Scholar]
- 5.Shikare SN, Singh DR, Shimpi TR, Peh WC. Tuberculous osteomyelitis and spondylodiscitis. Semin Musculoskeletal Radiol 2011; 15:446–58. [DOI] [PubMed] [Google Scholar]
- 6.Garg RK, Somvanshi DS. Spinal tuberculosis: a review. J Spinal Cord Med 2011; 34:440–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aberg JA, Chin-Hong PV, McCutchan A et al. . Localized osteomyelitis due to Mycobacterium avium complex in patients with human immunodeficiency virus receiving highly active antiretroviral therapy. Clin Infect Dis 2002; 35:E8–13. [DOI] [PubMed] [Google Scholar]
- 8.Erard P, Robert-Grandpierre F, Kaeser P. Vertebral osteomyelitis caused by Mycobacterium avium-intracellulare in a patient with AIDS. Clin Microbiol Infect 1999; 5:643–4. [DOI] [PubMed] [Google Scholar]
- 9.Case Records of the Massachusetts General Hospital (Case 23–2000). N Engl J Med 2000; 343:281–7. [DOI] [PubMed] [Google Scholar]
- 10.Hospenthal DR, Miller RS, Thomas SJ, Oster CN. Focal Mycobacterium avium complex osteomyelitis in patients with HIV infection. Infect Dis Clin Pract 2001; 10:17–20. [Google Scholar]
- 11.Zhang HQ, Deng A, Guo CF et al. . Association between FokI polymorphism in vitamin D receptor gene and susceptibility to spinal tuberculosis in Chinese Han population. Arch Med Res 2010; 41:46–9. [DOI] [PubMed] [Google Scholar]
- 12.Nalaboff KM, Rozenshtein A, Kaplan MH. Imaging of Mycobacterium avium-intracellulare infection in AIDS patients on highly active antiretroviral therapy: reversal syndrome. AJR Am J Roentgenol 2000; 175:387–90. [DOI] [PubMed] [Google Scholar]
- 13.Fraser TG, Till M. Vertebral osteomyelitis due to Mycobacterium avium complex in a patient with AIDS. Infect Dis Clin Pract 2002; 11:385–9. [Google Scholar]
- 14.Kahlon SS, East JW, Sarria JC. Mycobacterium-avium-intracellulare complex immune reconstitution inflammatory syndrome in HIV/AIDS presenting as osteomyelitis. AIDS Read 2008; 18:515–8. [PubMed] [Google Scholar]
- 15.Matt L, Calvey C, Muakkassa K et al. . A rare case of atypical Mycobacterium-associated spinal cord compression in an HIV-AIDS patient. Infect Dis Clin Pract 2013; 21:196–200. [Google Scholar]
- 16.Kadzielski J, Smith M, Baran JL et al. . Nontuberculous mycobacterial osteomyelitis: a case report of Mycobacterium avium intracellulare complex tibial osteomyelitis in the setting of HIV/AIDS. Orthop J Harv Med Sch. 2009; 11:108–11. Available at: http://www.orthojournalhms.org/volume11/manuscripts/PDF/V11_om_12.pdf Accessed September 25, 2014. [Google Scholar]
- 17.Chan ED, Kong PM, Fennelly K, Dwyer AP, Iseman MD. Vertebral osteomyelitis due to infection with nontuberculous Mycobacterium species after blunt trauma to the back: 3 examples of the principle of locus minoris resistentiae. Clin Infect Dis 2001; 32:1506–10. [DOI] [PubMed] [Google Scholar]
- 18.Corrales-Medina V, Symes S, Valdivia-Arenas M, Boulanger C. Localized Mycobacterium avium complex infection of vertebral and paravertebral structures in an HIV patient on highly active antiretroviral therapy. South Med J 2006; 99:174–7. [DOI] [PubMed] [Google Scholar]
- 19.Sheppard DC, Sullam PM. Primary septic arthritis and osteomyelitis due to Mycobacterium avium complex infection in a patient with AIDS. Clin Infect Dis 1997; 25:925–6. [DOI] [PubMed] [Google Scholar]
- 20.Blumenthal DR, Zucker JR, Hawkins CC. Mycobacterium avium complex-induced septic arthritis and osteomyelitis in a patient with the acquired immunodeficiency syndrome. Arthritis Rheum 1990; 33:757–8. [DOI] [PubMed] [Google Scholar]
- 21.Libraty DH. Vertebral osteomyelitis with paraspinous abscess due to Mycobacterium avium complex in a patient with AIDS. Infect Dis Clin Pract 1998; 7:294. [Google Scholar]
- 22.Phillips P, Bonner S, Gataric N et al. . Nontuberculous mycobacterial immune reconstitution syndrome in HIV-infected patients: spectrum of disease and long-term follow-up. Clin Infect Dis 2005; 41:1483–97. [DOI] [PubMed] [Google Scholar]
- 23.Valdez H, Gripshover BM, Stover MD, Salata RA. Mycobacterium avium complex osteomyelitis in HIV-infected patients: case report and review. Infect Dis Clin Pract 1997; 6:351–6. [Google Scholar]
- 24.Weingardt JP, Kilcoyne RF, Russ PD et al. . Disseminated Mycobacterium avium complex presenting with osteomyelitis of the distal femur and proximal tibia. Skeletal Radiol 1996; 25:193–6. [DOI] [PubMed] [Google Scholar]