Abstract
Background. The first case of Ebola diagnosed in the United States and subsequent cases among 2 healthcare workers caring for that patient highlighted the importance of hospital preparedness in caring for Ebola patients.
Methods. From October 21, 2014 to November 11, 2014, infectious disease physicians who are part of the Emerging Infections Network (EIN) were surveyed about current Ebola preparedness at their institutions.
Results. Of 1566 EIN physician members, 869 (55.5%) responded to this survey. Almost all institutions represented in this survey showed a substantial degree of preparation for the management of patients with suspected and confirmed Ebola virus disease. Despite concerns regarding shortages of personal protective equipment, approximately two thirds of all respondents reported that their facilities had sufficient and ready availability of hoods, full body coveralls, and fluid-resistant or impermeable aprons. The majority of respondents indicated preference for transfer of Ebola patients to specialized treatment centers rather than caring for them locally. In general, we found that larger hospitals and teaching hospitals reported higher levels of preparedness.
Conclusions. Prior to the Centers for Disease Control and Prevention's plan for a tiered approach that identified specific roles for frontline, assessment, and designated treatment facilities, our query of infectious disease physicians suggested that healthcare facilities across the United States were making preparations for screening, diagnosis, and treatment of Ebola patients. Nevertheless, respondents from some hospitals indicated that they were relatively unprepared.
Keywords: Ebola, healthcare facilities, preparedness
The 2014 Ebola outbreak that first began in Guinea in December 2013 [1] is the largest and most geographically dispersed Ebola outbreak ever reported, affecting multiple countries in West Africa. Unlike previous Ebola outbreaks, which have occurred in rural areas, the majority of transmission has occurred in more populated and urban areas [2]. Indeed, higher population densities have helped spread the disease [3]. Clusters of Ebola transmission have been noted in clinics and hospitals in affected countries [4]. Transmission to healthcare providers has occurred in West Africa [5, 6] and, rarely, in the United States [7] and Spain [8]. These cases highlight the importance of hospital preparedness in countries outside of West Africa. Hospital preparedness includes a wide range of activities including infection-control planning, monitoring healthcare staff, environmental cleaning, waste handling, diagnostics, systematic screening for exposures, and ensuring the availability, training, and appropriate use of personal protective equipment (PPE). We queried the Infectious Disease Society of America's (IDSA) Emerging Infections Network (EIN) to gain a better understanding of hospital preparedness for Ebola in the United States. In this article, we provide a cross-section of Ebola preparedness in October–November 2014.
METHODS
The IDSA EIN is a provider-based network of practicing infectious disease physicians from all 50 states, the District of Columbia, and Canada [9]. An 18-question survey (http://www.int-med.uiowa.edu/Research/EIN/Ebola2014_query.pdf) was conceived, developed, and conducted by EIN staff with technical assistance from the Centers for Disease Control and Prevention (CDC). The EIN sent the electronic query to all network physicians on October 21, 2014 and it remained open until November 11, 2014. We sent 2 reminders to nonresponders at 1-week intervals. Data about region of practice, years of experience, and employer were taken from the EIN database.
Respondents were asked to indicate their facility type (community, nonuniversity teaching, university, Veterans Affairs/Department of Defense hospital, city/county hospital) and its inpatient bed size (<200, 200–350, 351–450, 451–600, >600 beds). The survey included questions regarding Ebola and patient care, screening protocols, personnel, PPE and laboratory testing. Finally, to address the issue of multiple responses from the same institution, we performed a sensitivity analysis to determine whether our results changed when we only considered a single respondent from each institution, specifically the member with the longest duration of EIN membership. We analyzed the data using SAS software, version 9.3 (SAS Institute, Cary, NC). Fisher's exact and χ2 tests were used when appropriate.
RESULTS
We distributed the query to 1566 enrolled EIN members and received 869 (55.5%) responses. Respondents from 47 states and the District of Columbia represented a broad geographic distribution (see Table 1). Sixteen percent of respondents to the survey (n = 143) were excluded from the remainder of these results because they indicated that they either did not see inpatients or were not aware of their hospital's Ebola planning process.
Table 1.
Practice Characteristics of EIN Respondents vs Nonrespondents
| Practice Characteristic | Respondents (N = 869) | Nonrespondents (N = 697) |
|---|---|---|
| Practice: Adult ID | 646 (74%) | 547 (78%) |
| Pediatric ID | 198* (23%) | 122 (18%) |
| Both adult and pediatric ID | 25 (3%) | 28 (4%) |
| Region: New England | 66 (8%) | 40 (6%) |
| Mid Atlantic | 128 (15%) | 89 (13%) |
| East North Central | 124 (14%) | 103 (15%) |
| West North Central | 76 (9%) | 74 (11%) |
| South Atlantic | 148 (17%) | 133 (19%) |
| East South Central | 50 (6%) | 31 (4%) |
| West South Central | 58 (7%) | 39 (6%) |
| Mountain | 50 (6%) | 47 (7%) |
| Pacific | 156 (18%) | 130 (19%) |
| Puerto Rico | 1 (0.1%) | 1 (0.1%) |
| Canada | 12 (1%) | 10 (1%) |
| Years experience since ID fellowship | ||
| <5 yr | 183 (21%) | 230 (33%) |
| 5–14 yr | 246 (28%) | 237 (34%) |
| 15–24 yr | 225** (26%) | 109 (16%) |
| ≥25 yr | 214 (25%) | 121 (17%) |
| Employer: Hospital/clinic | 251 (29%) | 202 (29%) |
| Private/group practice | 222 (26%) | 179 (26%) |
| University/medical school | 343 (40%) | 279 (40%) |
| VA and military | 45 (5%) | 34 (5%) |
| State government | 8 (1%) | 3 (0.4%) |
Abbreviations: EIN,Emerging Infections Network; ID, infectious disease; VA, Veterans Affairs.
* P = .02.
** P < .0001.
Ebola Testing and Patient Care
Most respondents (494 of 726, 68%) reported that they would prefer transferring Ebola patients to a regional facility rather than treating them within their own facility. These preferences differed substantially by hospital type and size (see Table 2).
Table 2.
Preferences for Continued Care for Ebola Patients in Their Own Facilities vs Transfer to a Regional Ebola Care Facility, Shown by Facility Type and Inpatient Bed Size
| Facility Type or Bed Size | Continued Care in Your Facility | Transfer to a Regional Ebola Facility |
|---|---|---|
| By facility type* | ||
| Community (n = 220) | 28 (13%) | 192 (87%) |
| Nonuniversity teaching (n = 177) | 42 (24%) | 135 (76%) |
| University (n = 260) | 143 (55%) | 117 (45%) |
| VA or DoD hospital (n = 39) | 8 (21%) | 31 (79%) |
| City/county (n = 30) | 11 (37%) | 19 (63%) |
| By inpatient bed size* | ||
| <200 (n = 106) | 18 (17%) | 88 (83%) |
| 200–350 (n = 207) | 58 (28%) | 149 (72%) |
| 351–450 (n = 113) | 32 (28%) | 81 (72%) |
| 451–600 (n = 122) | 44 (36%) | 78 (64%) |
| >600 (n = 178) | 80 (45%) | 98 (55%) |
| Total | 232 (32%) | 494 (68%) |
Abbreviations: DoD, Department of Defense; VA, Veterans Affairs.
* P < .0001.
Of 726 infectious disease physicians involved in inpatient care or aware of their hospital's Ebola planning, only 94 (13%) respondents reported that a patient in their hospital had been tested for Ebola in the previous 3 months. Respondents at smaller hospitals were significantly less likely to report patient testing for Ebola (P = .0003; see Figure 1), and testing a patient for Ebola also varied by type of hospital (P < .0001). For the 94 (13%) respondents reporting at least 1 patient being tested, we received 68 open-text-field responses for alternative clinical diagnoses that explained their symptoms, and these represented a wide range of conditions. Alternative reported diagnoses included malaria (n = 40), upper respiratory infection (n = 10), gastroenteritis/traveler's diarrhea (5), undifferentiated febrile illness (n = 4), psychiatric illness or erroneous history (n = 3), typhoid (n = 2), and other (n = 4).
Figure 1.
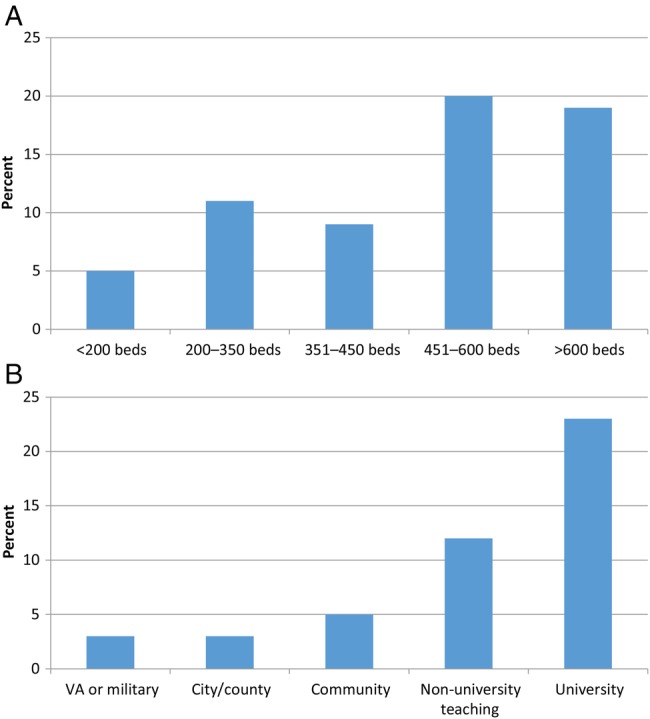
Percentage of facilities that had tested a patient for Ebola by (A) number of beds and (B) type of hospital, October 21–November 11, 2014.
Protocol and Screening
Most respondents (650 of 726, 89%) reported that their hospital had a written protocol for managing and testing suspected Ebola patients (see Table 3). Of these 650 respondents, 616 (95%) reported that this protocol included instructions for screening patients for Ebola, and 515 (79%) reported that there were arrangements for disposal of contaminated items.
Table 3.
Written Protocol Available to Healthcare Providers for Dealing With Suspected Ebola Patients, Shown by Facility Type, Inpatient Bed Size and Week of Response
| Facility Type, Bed Size or Week of Response | Yes | No | Unsure |
|---|---|---|---|
| By facility type* | |||
| Community (n = 220) | 194 (88%) | 16 (7%) | 10 (5%) |
| Nonuniversity teaching (n = 177) | 161 (91%) | 9 (5%) | 7 (4%) |
| University (n = 260) | 241 (93%) | 7 (3%) | 12 (5%) |
| VA or DoD hospital (n = 39) | 27 (69%) | 8 (21%) | 4 (10%) |
| City/county (n = 30) | 27 (90%) | 2 (7%) | 1 (3%) |
| By inpatient bed size** | |||
| <200 (n = 106) | 91 (86%) | 9 (8%) | 6 (6%) |
| 200–350 (n = 207) | 179 (86%) | 19 (9%) | 9 (4%) |
| 351–450 (n = 113) | 102 (90%) | 4 (4%) | 7 (6%) |
| 451–600 (n = 122) | 113 (93%) | 3 (2%) | 6 (5%) |
| >600 (n = 178) | 165 (93%) | 7 (4%) | 6 (3%) |
| By week of response*** | |||
| Week 1 (n = 380) | 332 (87%) | 28 (8%) | 20 (5%) |
| Week 2 (n = 218) | 197 (90%) | 11 (5%) | 10 (5%) |
| Week 3 (n = 128) | 121 (95%) | 3 (2%) | 4 (3%) |
| Total | 650 (90%) | 42 (6%) | 34 (5%) |
Abbreviations: DoD, Department of Defense; VA, Veterans Affairs.
* P = .0015.
** P = .15.
*** P = .20.
Most respondents (646 of 690, 94%) reported that screening would take place at initial intake rather than during provision of patient care (26 of 690, 4%). To trigger a screening, 254 (37%) reported that only a history of travel to endemic areas would be required, 30 (4%) reported that only signs and symptoms (eg, fever) would be required, and 391 (57%) reported that both positive travel history and signs/symptoms would be required.
Healthcare Personnel
When asked whether their hospital had a specific team of healthcare personnel to care for Ebola patients, 505 of 690 (73%) of respondents answered yes. Most respondents (517 of 690, 75%) also had policies that limit the number of healthcare providers who have direct patient contact. Finally, 59% (411 of 690) said their hospital limited the number of trainees who have direct patient contact (19% responded that they did not have trainees in their hospital).
The CDC's Interim U.S. Guidance for Monitoring and Movement of Persons with Potential Ebola Virus Exposure [10] was released on October 27, 2014. An excerpt is shown in Box 1. In brief, healthcare workers who provide care to Ebola patients in US facilities while wearing appropriate PPE and with no known breaches in infection control are considered to have low (but not zero) risk of exposure. Healthcare workers taking care of Ebola patients in a US facility where another healthcare worker has been diagnosed with confirmed Ebola without an identified infection control breach are considered to have a higher level of potential exposure (exposure level: high risk). Such individuals would be subject to restrictions, including controlled movement.
Box 1.
Excerpt from the Centers for Disease Control and Prevention's Interim U.S. Guidance for Monitoring and Movement of Persons with Potential Ebola Virus Exposure
| Healthcare workers who provide care to Ebola patients in US facilities while wearing appropriate personal protective equipment and with no known breaches in infection control are considered to have low (but not zero) risk of exposure because of the possibility of unrecognized breaches in infection control and should have direct active monitoring. As long as these healthcare workers have direct active monitoring and are asymptomatic, there is no reason for them not to continue to work in hospitals and other patient care settings. There is also no reason for them to have restrictions on travel or other activities. Review and approval of work, travel, use of public conveyances, and attendance at congregate events are not indicated or recommended for such healthcare workers, except to ensure that direct active monitoring continues uninterrupted. |
| Healthcare workers taking care of Ebola patients in a US facility where another healthcare worker has been diagnosed with confirmed Ebola without an identified breach in infection control are considered to have a higher level of potential exposure (exposure level: high risk). A similar determination would be made if an infection control breach is identified retrospectively during investigation of a confirmed case of Ebola in a healthcare worker. These individuals would be subject to restrictions, including controlled movement and the potential use of public health orders, until 21 days after the last potential unprotected exposure. |
Because the query was conducted from October 21, 2014 to November 11, 2014 (CDC Interim U.S. Guidance was released on October 27, 2014, in the middle of the reporting period), respondents understandably reported varying views about the monitoring and movement of healthcare workers with potential Ebola virus exposure at their institutions. Some respondents (252 of 690, 36%) reported that they would have these individuals self-monitor and report symptoms if they occur, 182 (26%) of respondents were unsure, 129 (19%) reported that there would be daily active contact and institutional monitoring, 82 (12%) reported that both self-monitoring and institutional monitoring would be required, and 45 (7%) chose “other”. In open-text-field comments, numerous respondents indicated confusion regarding what the 21-day restrictions for healthcare providers, considered to have a higher level of potential exposure (exposure level: high risk), entailed. Some respondents also reported concern that the nursing staff might not be available due to the potential risk of being required to take off work for 21 days in the unlikely event they were found to be in a high-risk category.
When asked whether technology (eg, video link/telemedicine) would be used for consultative care to avoid direct patient contact, most respondents (416 of 690, 60%) reported yes, but 138 (20%) were unsure. Finally, most respondents believed that they had adequate staff to treat Ebola patients: 512 of 690 (74%) were not concerned that there would be too few healthcare providers who were willing to care for Ebola patients if the need arose.
Personal Protective Equipment
Respondents were queried about the specific types of PPE available in their hospital and their protocols for training staff for PPE use. Sixty-three percent (435 of 690) of facilities had PPE that covers the head and neck currently available and in sufficient supply (as determined by the respondents), 20% (139) did not, and 17% (116) were unsure. Sixty-four percent (442 of 690) of respondents worked in facilities with sufficient full-body protective suits (19% did not, and 17% were unsure). Sixty-six percent (457 of 690) reported that their facilities had sufficient disposable, fluid-resistant, or impermeable aprons (13% do not, and 21% were unsure).
Most respondents reported that their institutions had implemented specific PPE protocols including in-person training and practice for donning PPE before a case appears (604 of 690, 88%), use of a buddy system for PPE removal (558, 81%), use of a trained observer to manage PPE removal (481, 70%), and full-scale drills with simulated patients (298, 43%). However, 9% of respondents report that their hospital had implemented none of the above protocols.
Laboratory Testing and Other Issues
A variety of plans for clinical laboratory testing (other than Ebola diagnostic testing) were reported by respondents, including point-of-care testing at the patient's bedside (iSTAT, etc; 451 of 690, 65%), additional testing in a BSL3 hood or special laboratory (153, 22%), testing in the hospital's main laboratory with additional safeguards (162, 24%), and testing offsite including arrangements for transit/shipping (262, 38%), whereas 85 (12%) were unsure.
Finally, respondents were asked about communications between their hospital and public health officials. Eighty-one percent (560 of 690) of respondents reported that their hospital had a designated individual who was responsible for communicating with public health officials; 8% (54) answered that they did not have such an individual identified, and 11% (76) were unsure. Among those who answered yes to this question, 78% (437 of 560) reported that they could identify the designated person at the hospital.
Sensitivity Analysis
On review of our data, we concluded that 767 of the 869 respondents were from unique facilities, whereas 102 respondents represented at least the second responder from a single institution. We repeated all analyses, ignoring 102 “duplicate responses”, and found that all statistical results remained unchanged (at the P < .05 level). In addition, all response results based on the smaller data set remained within 2 percentage points of the complete data set. Finally, the frequency distribution and relative frequencies for all results were also unchanged.
DISCUSSION
Since the time this query was conducted, the CDC has adopted a tiered approach to US hospital preparedness that identifies specific roles for frontline healthcare facilities, assessment hospitals, and designated Ebola treatment centers. Frontline healthcare facilities should be able to rapidly identify, triage, and isolate any patient with exposure history and signs or symptoms compatible with Ebola. Ebola assessment hospitals should be prepared to receive, isolate, and care for patients under investigation until a diagnosis of Ebola can be confirmed or ruled out, and transfer to a designated Ebola treatment center, if indicated, is completed. [11]. In October–November 2014, almost all institutions represented in this survey showed a substantial degree of preparation for the screening, diagnosis, and management of patients with suspected and confirmed Ebola. The focus on preparation seemed to parallel the public concern regarding this disease and state and local efforts to improve preparedness for Ebola. In general, the larger the hospital, the higher the level of reported preparedness. Although the majority of respondents indicated that they would prefer to transfer Ebola patients to specialized treatment centers rather than care for them locally, the reported preparation efforts indicated recognition of the importance of their ability to effectively screen, diagnose, and initially manage patients locally. Furthermore, in the event of a confirmed case, only a minority of respondents (26%) thought that they would have difficulty finding healthcare providers to take care of Ebola patients. Reasons for healthcare workers' unwillingness to care for Ebola patients included financial issues, eg, payment/compensation during furlough or 21 days of isolation postexposure. Other concerns focused on travel restrictions and being afraid to go home to family after caring for Ebola patients.
Measuring preparedness using a one-time query is difficult because hospitals' reactions to guidelines were evolving throughout October. In an ideal setting, we would have answers for the same questions from the same respondents repeatedly at sequential time intervals. However, this query was not designed as a longitudinal study, and statistical inferences comparing responses from the first week to the third week should not be made. It is interesting to note that, in the comments section, a few respondents volunteered that had they answered the survey earlier, their answers would have reflected a lower degree of preparedness. Our survey was initially distributed on October 20, 2014, the day after the clarified healthcare PPE recommendations, which followed the Ebola virus disease transmission that occurred among healthcare providers caring for the index patient in Dallas [12]. Our questions were designed to address this revised CDC guidance [13]. Thus, it is not surprising that more people indicated better preparedness over time, especially regarding new and more extensive PPE recommendations. Responses from different EIN members over the 3-week period of response suggest that preparedness may have increased over time.
Despite concerns regarding potential shortages, approximately two thirds of respondents reported sufficient availability of hoods, full-body coveralls, and fluid-resistant or impermeable aprons. We did not specifically ask how many days' worth of supplies institutions have onsite or the anticipated rate of use. It should be noted that early reports on the care of patients with Ebola have indicated a prodigious rate of supply usage [14, 15]. When asked in an open-text field to describe any issue that needed to be addressed to enable their facility to safely care for suspected Ebola patients, the most frequently mentioned topic was concern about PPE. A number of respondents reported that their facilities had sufficient supplies for a short period of time, but concern was expressed over availability of ongoing supplies should a suspected patient be admitted. Concern was also expressed regarding the need for additional PPE training of staff and, in particular, donning and doffing protective equipment. Ambulatory care settings were identified as a particular area of concern given needs for training staff in these settings.
In general, larger hospitals and teaching hospitals were significantly more prepared than other types of hospitals, which may be related to infection-prevention physician and nurse staffing, dedicated isolation units, and other resources [16, 17]. It is interesting that our respondents at military/Veterans' Affairs hospitals reported the lowest availability of an Ebola patient management protocol.
Although responses to this query indicated substantial preparedness, challenges remain. For example, although some physicians reported their hospital had a detailed plan in place, only 43% had practiced full-scale drills including simulated patients. Nine percent had not practiced donning PPE, used a buddy system/trained observer for PPE donning and doffing, or had a site manager oversee PPE use, or full-scale drills. In addition, our results indicated that communication and messaging could be improved in hospitals. For example, although 81% were aware that their institution had an individual responsible for communicating with public health officials, 22% of these respondents did not know who this person was. Finally, a number of respondents indicated a desire for more detailed communications from public health officials at all levels. In the comments section of our query, respondents focused on the lack of clear guidance about a variety of issues including where monitoring of exposed healthcare workers should occur and whether exposed healthcare workers could enter patient rooms while still asymptomatic. More specific guidance was also requested on (1) ambulatory care and (2) ethical guidelines for cardiopulmonary resuscitation.
There are several limitations to our study. First, as noted above, our query was not specifically designed to address time-related changes because we did not ask the same respondents the same questions in a serial fashion. Thus, the later respondents may have reported greater levels of preparedness for a variety of reasons. Second, we did not specifically ask what role the respondents played in Ebola planning at their institution. We did ask whether members were unsure of the level of preparedness at their institutions, but only 10%–20% of members indicated that they were unsure about PPE supplies, and these respondents were significantly more likely to answer “unsure” to the healthcare, personnel plans, and training questions. Thus, the majority of respondents seem well prepared to answer questions about Ebola preparedness. Third, there was potential for bias in our sample. The EIN is not a random sample of providers, and clinicians who participate in the EIN may not necessarily represent the opinions of clinicians who do not participate. In addition, EIN physicians involved in Ebola preparation might have been more likely to respond to this survey than others, leading to upwardly biased results. However, the response rate for this survey was high relative to previous EIN queries (56%), and respondents from all sizes and types of hospitals as well as all US Census Bureau divisions were represented. Because some of the questions were focused at the institutional level, an additional potential limitation is the issue of multiple responders from a single institution. However, our sensitivity analysis showed that ignoring multiple responses did not change our results.
CONCLUSIONS
In general, infectious disease physicians practice at larger hospitals that can support subspecialists; it follows that our results may not reflect the smallest frontline hospitals, and this is another limitation for our study. However, our respondents are used by a wide range of hospitals including university hospitals, nonuniversity teaching hospitals, community hospitals, veterans' hospitals, and city/county public hospitals, and our respondents represented a wide range of hospital bed sizes from <200 to greater than 600 beds. Of concern, we found that respondents from smaller hospitals (<200 beds) reported that these facilities were, in general, less prepared. To address issues about preparedness in smaller hospitals, states are (1) developing Ebola response plans that include specific roles for frontline small hospitals to rapidly identify and isolate persons with a travel or exposure history and signs and symptoms of Ebola and (2) identifying other hospitals in their jurisdiction that can receive transferred patients with suspected or confirmed Ebola [18]. In addition, persons currently in the United States with potential Ebola exposure are actively monitored by public health officials on a daily basis during the 21 days after their last exposure [19]. Our results provide a cross-section of Ebola preparedness in October–November 2014, before the CDC's plan for a tiered approach identifying specific roles for frontline, assessment, and designated treatment [20] facilities. Our query of infectious disease physicians suggested that healthcare facilities across the United States were making preparations for screening, diagnosis and treatment of Ebola patients. Nevertheless, respondents from some small hospitals indicated that they were relatively unprepared.
Acknowledgments
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Financial support. This publication was supported by the Grant or Cooperative Agreement FOA CK11-1102, funded by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Del Rio C, Mehta AK, Lyon GM 3rd, Guarner J. Ebola hemorrhagic fever in 2014: the tale of an evolving epidemic. Ann Intern Med 2014; 161:746–8. [DOI] [PubMed] [Google Scholar]
- 2.WHO Ebola Response Team. Ebola virus disease in West Africa--the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamin D, Gertler S, Ndeffo-Mbah ML et al. . Effect of Ebola progression on transmission and control in Liberia. Ann Intern Med 2015; 162:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer WA 2nd, Hynes NA, Perl TM. Protecting health care workers from Ebola: personal protective equipment is critical but is not enough. Ann Intern Med 2014; 161:753–4. [DOI] [PubMed] [Google Scholar]
- 5.Matanock A, Arwady MA, Ayscue P et al. . Ebola virus disease cases among health care workers not working in Ebola treatment units - Liberia, June-August, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:1077–81. [PMC free article] [PubMed] [Google Scholar]
- 6.Forrester JD, Hunter JC, Pillai SK et al. . Cluster of Ebola cases among Liberian and U.S. health care workers at an Ebola treatment unite and adjacent hospital - Liberia, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:925–9. [PMC free article] [PubMed] [Google Scholar]
- 7.McCarty CL, Basler C, Karwowski M et al. . Response to importation of a case of Ebola virus disease – Ohio, October 2014. MMWR Morb Mortal Wkly Rep 2014; 63:1089–91. [PMC free article] [PubMed] [Google Scholar]
- 8.Parra JM, Salmerón OJ, Velasco M. The first case of Ebola virus disease acquired outside Africa. N Engl J Med 2014; 371:2439–40. [DOI] [PubMed] [Google Scholar]
- 9.Pillai SK, Beekmann SE, Santibanez S, Polgreen PM. The Infectious Diseases Society of America Emerging Infections Network – bridging the gap between clinical infectious diseases and public health. Clin Infect Dis 2014; 58:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Interim U.S. guidance for monitoring and movement of persons with potential Ebola virus exposure. Available at: http://www.cdc.gov/vhf/ebola/pdf/monitoring-and-movement.pdf Accessed 22 December 2014. [PMC free article] [PubMed]
- 11.Centers for Disease Control and Prevention. Interim guidance for U.S. hospital preparedness for patients with possible or confirmed Ebola virus disease: a framework for a tiered approach. Available at: http://www.cdc.gov/vhf/ebola/hcp/us-hospital-preparedness.html Accessed 4 May 4, 2015.
- 12.Chavalier MS, Chung W, Smith J et al. . Ebola virus disease cluster in the United States — Dallas County, Texas, 2014. MMWR Weekly Report. 2014; 63:1087–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Guidance on personal protective equipment to be used by healthcare workers during management of patients with Ebola virus disease in U.S. hospitals, including procedures for putting on (donning) and removing (doffing). Available at: http://www.cdc.gov/vhf/Ebola/hcp/procedures-for-ppe.html Accessed 1 December 2014.
- 14.Decker BK, Sevransky JE, Barrett K et al. . Preparing for critical care services to patients with Ebola. Ann Intern Med 2014; 161:831–2. [DOI] [PubMed] [Google Scholar]
- 15.Murray BE, Calderwood SB, Neill MA. Ebola: lessons learned, from IDWeek 2014. Available at: http://www.idweek.org/Ebola_idweek_2014/ Accessed 1 December 2014.
- 16.Klompas M, Diekema DJ, Fishman NO, Yokoe DS. Ebola fever: reconciling planning with risk in US hospitals. Ann Intern Med 2014; 161:751–2. [DOI] [PubMed] [Google Scholar]
- 17.Herwaldt LA, Appelgate D, Kuntz J et al. . Infection control resources in Iowa. Am J Infect Control 2007; 35:662–5. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Interim guidance for preparing frontline healthcare facilities for patients with possible Ebola virus disease. Available at: http://www.cdc.gov/vhf/ebola/hcp/preparing-frontline-healthcare-facilities.html Accessed 3 December 2014.
- 19.Centers for Disease Control and Prevention. Interim U.S. guidance for monitoring and movement of persons with potential Ebola virus exposure. Available at: http://www.cdc.gov/vhf/Ebola/exposure/monitoring-and-movement-of-persons-with-exposure.html Accessed 1 December 2014. [PMC free article] [PubMed]
- 20.Centers for Disease Control and Prevention. Interim guidance for U.S. hospital preparedness for patients under investigation (PUIs) or with confirmed Ebola virus disease (EVD): a framework for a tiered approach. Available at: http://www.cdc.gov/vhf/ebola/healthcare-us/preparing/hospitals.html Accessed 7 May 2015.


