Abstract
We present a case of primary disseminated herpes simplex virus type 2 (HSV-2) cutaneous disease in a 22-year-old male. We discuss the immune response to HSV-2 infection as well as the extragenital manifestations of HSV-2 observed in immune-competent and immune-suppressed persons.
Keywords: disseminated herpes, herpes simplex virus, HSV
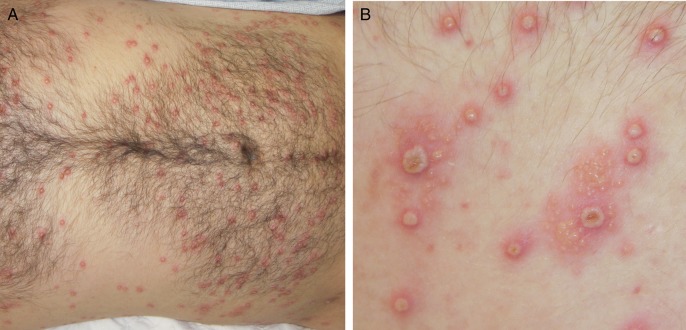
A 22-year-old male with a recent diagnosis of atopic dermatitis presented to our hospital with a rash, fever, and dysuria. Approximately 6 weeks prior, he sought care at an urgent care clinic for a peeling rash on the palmar surface of his hand. At that time, he was diagnosed with atopic dermatitis and treated with systemic corticosteroids. Three weeks later, the patient had unprotected vaginal sex with a new female partner. Approximately 1 week later, he developed a painless but pruritic penile ulcer. He was treated for primary syphilis at a local emergency department. The following day, he developed fever, weakness, mild confusion, and lethargy. The genital rash had become more extensive, painful, and associated with dysuria. He also reported a new disseminated painless, erythematous, papular rash. Physical examination was remarkable for fever (101.1°F) and vesiculopustules on erythematous bases with some clustering on his torso, arms, and legs (Figure 1). Numerous vesicles, ulcers, and crusts were noted on the penile glans and shaft bilaterally. No oral lesions were noted. White blood cell count was 7.6 × 103/cm with 62% neutrophils, 15% bands, 7% lymphocytes, 11% monocytes, and 5% eosinophils, but subsequent differentials rapidly normalized. Alanine aminotransferase and aspartate aminotransferase levels were elevated at 226 and 66 units/L, respectively. Urinalysis showed 1+ protein and trace ketones. The patient reported a history of varicella-zoster virus (VZV) vaccination (confirmed by serology). Rapid plasma reagin was nonreactive, and Treponema pallidum hemagglutination was negative. Nucleic acid amplification for gonorrhea and Chlamydia were negative. Point-of-care human immunodeficiency virus (HIV) antibody and subsequent HIV viral load were negative. Direct fluorescent antibody (DFA) test for VZV was negative from a skin punch biopsy, whereas DFA for herpes simplex virus type 2 (HSV-2) was positive. Culture and polymerase chain reaction from an unroofed vesicle confirmed HSV-2. Intravenous (IV) acyclovir (10 mg/kg) every 8 hours was initiated.
Figure 1.
Disseminated vesicles on erythematous bases at the time of presentation (A). Note clustering of some vesicles (B). The patient provided written consent to use his photographs.
After 3 days of IV acyclovir therapy, the lesions began to crust and liver function tests normalized. The patient was transitioned to oral valacyclovir, 1 gram twice daily for 2 weeks. Lesions had re-epithelized by day 14 of therapy. At that time, type-specific HSV-1 immunoglobulin (Ig)G and HSV-2 IgG serologies were tested; HSV-1 IgG was negative and HSV-2 IgG was positive. Taken together, the patient's presentation and laboratory evaluation were interpreted as indicative of primary HSV-2 infection. The patient opted not to initiate suppressive antiviral therapy. Within 2 weeks, he returned noting 2 crops of painful vesicles, 1 on the right shoulder and 1 on the left thigh. Suppressive therapy for HSV has been shown unsuccessful in preventing recurrent episodes of meningitis. However, the proven benefit of valacyclovir in reducing mucogenital lesions and the patient's prompt relapse of symptoms led to us recommending indefinite suppressive therapy [1]. The patient elected to begin valacyclovir, and the lesions rapidly resolved.
Herpes simplex virus type 2 is the most common cause of genital ulcers worldwide, and it is typically characterized by local eruptions manifesting as painful genital or perianal vesicles and ulcers [2, 3]. Atypical presentations of genitourinary HSV-2 disease as well as extragenital manifestations including cutaneous, neurologic, visceral, and disseminated forms are well described in immunocompromised patients [4–8]. Herpes simplex virus type 1 is typically less severe than HSV-2; however, there are isolated reports of severe manifestations of HSV-1 [9]. More rarely, such presentations are seen in apparently immunocompetent hosts [10–12]. However, the ever-increasing discovery of complex primary immunodeficiencies, some involving particular susceptibility to herpes viruses, often with mutations in previously unknown or unappreciated genes involved in viral immunity, makes it likely that such apparently healthy individuals have some sort of underlying immune defect. Among immunocompromised and immunocompetent hosts, case reports describe disseminated cutaneous HSV-2 disease as relatively limited in distribution and/or lesion number [11, 13, 14]. This case differs from most previous reports in both its extensive distribution and the large number of cutaneous lesions with some visceral involvement suggested by his increased transaminases.
Herpes simplex virus type 2 infection involves a complex and dynamic cycle of immune control and viral reactivation. The innate immune response to HSV-2 infection includes pathogen-associated molecular patterns through Toll-like receptor-dependent and -independent pathways and a robust Type I interferon response [15]. The adaptive immune response includes both HSV-specific antibody production thought to play a role in neutralization of free HSV-2 virus and the cell-mediated immune response thought to be important in controlling HSV-2 reactivation and replication in the epithelium [16–18].
Although the patient was previously healthy, the severity of his primary HSV-2 infection raises concern for an underlying transient or long-standing immune deficiency, specifically of the innate immune response. Systemic corticosteroid therapy is implicated in HSV hepatitis and colitis [8, 14, 19]. The patient's receipt of glucocorticoids 3 weeks before acquisition may have transiently compromised his innate host defenses resulting in wide cutaneous dissemination of HSV-2. Alternatively, he may suffer an underlying genetic innate immune system defect. Although the patient describes no past medical history concerning for increased susceptibility to viral, bacterial, or fungal pathogens, he was referred to an immunologist to better characterize his immune function. Lymphocyte flow cytometry on a follow-up visit after the patient had recovered showed normal numbers of CD4+ and CD8+ T cells with a normal CD4/CD8 ratio, but the proportion of T cells bearing neither CD4 or CD8 coreceptors was elevated at approximately 32%, suggesting expansion of T-cell receptor γδ T cells. B cells were borderline low at 89/mm3 (5.5%), and natural killer (NK) cells were essentially normal at 103/mm3 (6%). Unfortunately, at the time of this report, the patient has not returned for additional studies.
Among the primary immunodeficiencies with increased susceptibility to HSV, NK cell deficiencies figure prominently [20]. Functional defects in NK cells may be present even though absolute numbers of NK cells are normal as in this patient's case. There may be functional defects in cytotoxic T-cell function as well as NK cell function if the defect lies in secretory granule formation, secretion, or granule contents (eg, perforin) as seen in the hemophagocytic disorders [21]. Dominant-negative forms of hemophagocytic lymphohistiocytosis are being identified in which the patients may have a normal past medical history and present with sudden overwhelming illness (macrophage activation syndrome [MAS]) [22]. It is possible that our patient might fall into this category and that early and effective treatment of his HSV infection interrupted the development of MAS. There is also an ever-widening number of genes identified as causing combined immunodeficiency disorders with increased susceptibility to herpesviruses [23]. Some of the patients presenting with these types of infections are proving to bear hypomorphic mutations in genes that lead to severe combined immune deficiency when fully deficient [24]. Finally, deficient responses to herpesviruses can be due to more broad-based defects in immunity such as the recently identified patients with Toll-like receptor 3 (TLR3) deficiency [25]. However, TLR3-deficient patients so far have proven to be healthy except for increased susceptibility to HSV encephalitis.
In summary, we present a case of primary disseminated HSV-2 cutaneous disease. Follow-up evaluation of this patient's lymphocytes by flow cytometry suggests that he may have an underlying, thus far unidentified, primary immune deficiency. This case highlights the spectrum of disease caused by HSV-2, and it reminds the healthcare provider to consider HSV-2 as a rare but important cause of a disseminated vesicular rash.
Acknowledgments
Financial support. This work was funded by National Institutes of Health (grant 1K2323A1097267; to N. V. W.).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Aurelius E, Franzen-Rohl E, Glimaker M et al. Long-term valacyclovir suppressive treatment after herpes simplex virus type 2 meningitis: a double-blind, randomized controlled trial. Clin Infect Dis 2012; 54:1304–13. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Warren T, Wald A. Genital herpes. Lancet 2007; 370:2127–37. [DOI] [PubMed] [Google Scholar]
- 3.Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 7th ed Philadelphia, PA: Churchill Livingstone/Elsevier, 2010. [Google Scholar]
- 4.Mommeja-Marin H, Lafaurie M, Scieux C et al. Herpes simplex virus type 2 as a cause of severe meningitis in immunocompromised adults. Clin Infect Dis 2003; 37:1527–33. [DOI] [PubMed] [Google Scholar]
- 5.Abbo L, Alcaide ML, Pano JR et al. Fulminant hepatitis from herpes simplex virus type 2 in an immunocompetent adult. Transpl Infect Dis 2007; 9:323–6. [DOI] [PubMed] [Google Scholar]
- 6.Brown TS, Callen JP. Atypical presentation of herpes simplex virus in a patient with chronic lymphocytic leukemia. Cutis 1999; 64:123–5. [PubMed] [Google Scholar]
- 7.Patel AB, Rosen T. Herpes vegetans as a sign of HIV infection. Dermatol Online J 2008; 14:6. [PubMed] [Google Scholar]
- 8.Schunter MO, Walles T, Fritz P et al. Herpes simplex virus colitis complicating ulcerative colitis: A case report and brief review on superinfections. J Crohn's Colitis 2007; 1:41–6. [DOI] [PubMed] [Google Scholar]
- 9.Glas M, Smola S, Pfuhl T et al. Fatal multiorgan failure associated with disseminated herpes simplex virus-1 infection: a case report. Case Rep Crit Care 2012; 2012:359–60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farr RW, Short S, Weissman D. Fulminant hepatitis during herpes simplex virus infection in apparently immunocompetent adults: report of two cases and review of the literature. Clin Infect Dis 1997; 24:1191–4. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe D, Kuhara T, Ishida N et al. Disseminated mucocutaneous herpes simplex virus infection in an immunocompetent woman. Int J STD AIDS 2010; 21:213–4. [DOI] [PubMed] [Google Scholar]
- 12.Zahariadis G, Jerome KR, Corey L. Herpes simplex virus-associated sepsis in a previously infected immunocompetent adult. Ann Intern Med 2003; 139:153–4. [DOI] [PubMed] [Google Scholar]
- 13.Maalouf E, Moutran R, Maatouk I. Disseminated primary HSV-2 infection of the face. Dermatol Online J 2012; 18:15. [PubMed] [Google Scholar]
- 14.Santos-Antunes J, Abreu C, Magro F et al. Disseminated cutaneous herpes simplex infection in a patient with Crohn's disease under azathioprine and steroids: First case report and literature review. J Crohn's Colitis 2014; 8:326–30. [DOI] [PubMed] [Google Scholar]
- 15.Unterholzner L. The interferon response to intracellular DNA: why so many receptors? Immunobiology 2013; 218:1312–21. [DOI] [PubMed] [Google Scholar]
- 16.Cairns TM, Fontana J, Huang ZY et al. Mechanism of neutralization of herpes simplex virus by antibodies directed at the fusion domain of glycoprotein B. J Virol 2014; 88:2677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston C, Zhu J, Jing L et al. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J Virol 2014; 88:4921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Peng T, Johnston C et al. Immune surveillance by CD8alphaalpha+ skin-resident T cells in human herpes virus infection. Nature 2013; 497:494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seksik P, Gozlan J, Guitton C et al. Fatal herpetic hepatitis in adult following short corticotherapy: a case report. Intensive Care Med 1999; 25:415–7. [DOI] [PubMed] [Google Scholar]
- 20.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol 2013; 132:515–25; quiz 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filipovich AH. The expanding spectrum of hemophagocytic lymphohistiocytosis. Curr Opin Allergy Clin Immunol 2011; 11:512–6. [DOI] [PubMed] [Google Scholar]
- 22.Spessott WA, Sanmillan ML, McCormick ME et al. Hemophagocytic lymphohistiocytosis caused by dominant-negative mutations in STXBP2 that inhibit SNARE-mediated membrane fusion. Blood 2015; 125:1566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parvaneh N, Filipovich AH, Borkhardt A. Primary immunodeficiencies predisposed to Epstein-Barr virus-driven haematological diseases. Br J Haematol 2013; 162:573–86. [DOI] [PubMed] [Google Scholar]
- 24.Abolhassani H, Wang N, Aghamohammadi A et al. A hypomorphic recombination-activating gene 1 (RAG1) mutation resulting in a phenotype resembling common variable immunodeficiency. J Allergy Clin Immunol 2014; 134:1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang SY, Herman M, Ciancanelli MJ et al. TLR3 immunity to infection in mice and humans. Curr Opin Immunol 2013; 25:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]