Abstract
Achondroplasia, the most common genetic form of dwarfism, is an autosomal dominant disorder whose underlying mechanism is a defect in the maturation of the cartilage growth plate of long bones. Achondroplasia has recently been shown to result from a Gly to Arg substitution in the transmembrane domain of the fibroblast growth factor receptor 3 (FGFR3), although the molecular consequences of this mutation have not been investigated. By substituting the transmembrane domain of the Neu receptor tyrosine kinase with the transmembrane domains of wild-type and mutant FGFR3, the Arg380 mutation in FGFR3 is shown to activate both the kinase and transforming activities of this chimeric receptor. Residues with side chains capable of participating in hydrogen bond formation, including Glu, Asp, and to a lesser extent, Gln, His and Lys, were able to substitute for the activating Arg380 mutation. The Arg380 point mutation also causes ligand-independent stimulation of the tyrosine kinase activity of FGFR3 itself, and greatly increased constitutive levels of phosphotyrosine on the receptor. These results suggest that the molecular basis of achondroplasia is unregulated signal transduction through FGFR3, which may result in inappropriate cartilage growth plate differentiation and thus abnormal long bone development. Achondroplasia may be one of the number of cogenital disorders where constitutive activation of a member of the FGFR family leads to development abnormalities.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. E., Jr, Hauge M. Congenital generalised bone dysplasias: a clinical, radiological, and epidemiological survey. J Med Genet. 1989 Jan;26(1):37–44. doi: 10.1136/jmg.26.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Increased tyrosine kinase activity associated with the protein encoded by the activated neu oncogene. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5394–5398. doi: 10.1073/pnas.85.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J. 1988 Jul;7(7):2043–2052. doi: 10.1002/j.1460-2075.1988.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellus G. A., Hefferon T. W., Ortiz de Luna R. I., Hecht J. T., Horton W. A., Machado M., Kaitila I., McIntosh I., Francomano C. A. Achondroplasia is defined by recurrent G380R mutations of FGFR3. Am J Hum Genet. 1995 Feb;56(2):368–373. [PMC free article] [PubMed] [Google Scholar]
- Cao H., Bangalore L., Bormann B. J., Stern D. F. A subdomain in the transmembrane domain is necessary for p185neu* activation. EMBO J. 1992 Mar;11(3):923–932. doi: 10.1002/j.1460-2075.1992.tb05131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham B., Shoelson S. E., Yamada K., Goncalves E., Kahn C. R. Substitution of the erbB-2 oncoprotein transmembrane domain activates the insulin receptor and modulates the action of insulin and insulin-receptor substrate 1. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7336–7340. doi: 10.1073/pnas.90.15.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drebin J. A., Link V. C., Stern D. F., Weinberg R. A., Greene M. I. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985 Jul;41(3):697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
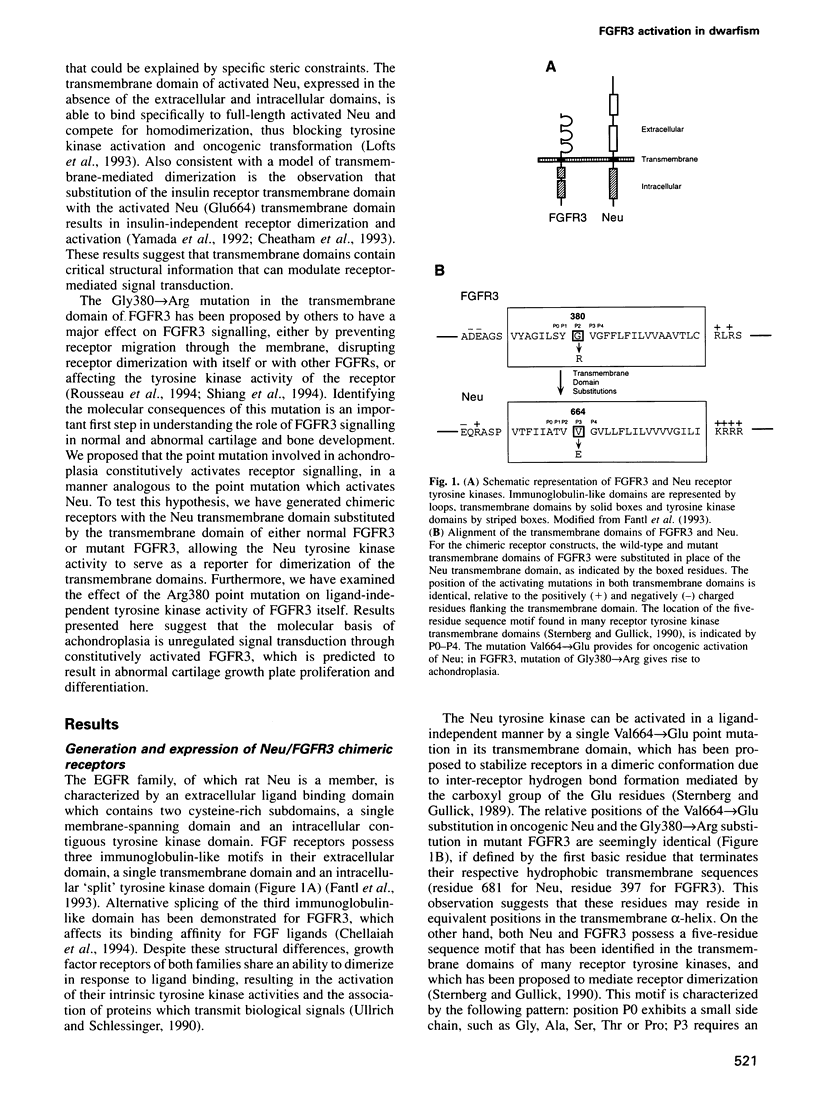
- Fantl W. J., Johnson D. E., Williams L. T. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- Francomano C. A., Ortiz de Luna R. I., Hefferon T. W., Bellus G. A., Turner C. E., Taylor E., Meyers D. A., Blanton S. H., Murray J. C., McIntosh I. Localization of the achondroplasia gene to the distal 2.5 Mb of human chromosome 4p. Hum Mol Genet. 1994 May;3(5):787–792. doi: 10.1093/hmg/3.5.787. [DOI] [PubMed] [Google Scholar]
- Gandelman K. Y., Gibson L., Meyn M. S., Yang-Feng T. L. Molecular definition of the smallest region of deletion overlap in the Wolf-Hirschhorn syndrome. Am J Hum Genet. 1992 Sep;51(3):571–578. [PMC free article] [PubMed] [Google Scholar]
- Gullick W. J., Bottomley A. C., Lofts F. J., Doak D. G., Mulvey D., Newman R., Crumpton M. J., Sternberg M. J., Campbell I. D. Three dimensional structure of the transmembrane region of the proto-oncogenic and oncogenic forms of the neu protein. EMBO J. 1992 Jan;11(1):43–48. doi: 10.1002/j.1460-2075.1992.tb05025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M., Shimazu A., Nakashima K., Suzuki F., Kato Y. Reduction of basic fibroblasts growth factor receptor is coupled with terminal differentiation of chondrocytes. J Biol Chem. 1991 Jan 5;266(1):461–467. [PubMed] [Google Scholar]
- Jabs E. W., Li X., Scott A. F., Meyers G., Chen W., Eccles M., Mao J. I., Charnas L. R., Jackson C. E., Jaye M. Jackson-Weiss and Crouzon syndromes are allelic with mutations in fibroblast growth factor receptor 2. Nat Genet. 1994 Nov;8(3):275–279. doi: 10.1038/ng1194-275. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Williams L. T. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Kato Y., Iwamoto M. Fibroblast growth factor is an inhibitor of chondrocyte terminal differentiation. J Biol Chem. 1990 Apr 5;265(10):5903–5909. [PubMed] [Google Scholar]
- Keegan K., Johnson D. E., Williams L. T., Hayman M. J. Isolation of an additional member of the fibroblast growth factor receptor family, FGFR-3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1095–1099. doi: 10.1073/pnas.88.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan K., Meyer S., Hayman M. J. Structural and biosynthetic characterization of the fibroblast growth factor receptor 3 (FGFR-3) protein. Oncogene. 1991 Dec;6(12):2229–2236. [PubMed] [Google Scholar]
- Lee B. A., Donoghue D. J. Intracellular retention of membrane-anchored v-sis protein abrogates autocrine signal transduction. J Cell Biol. 1992 Sep;118(5):1057–1070. doi: 10.1083/jcb.118.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofts F. J., Hurst H. C., Sternberg M. J., Gullick W. J. Specific short transmembrane sequences can inhibit transformation by the mutant neu growth factor receptor in vitro and in vivo. Oncogene. 1993 Oct;8(10):2813–2820. [PubMed] [Google Scholar]
- Maynard J. A., Ippolito E. G., Ponseti I. V., Mickelson M. R. Histochemistry and ultrastructure of the growth plate in achondroplasia. J Bone Joint Surg Am. 1981 Jul;63(6):969–979. [PubMed] [Google Scholar]
- Meyer A. N., Xu Y. F., Webster M. K., Smith A. E., Donoghue D. J. Cellular transformation by a transmembrane peptide: structural requirements for the bovine papillomavirus E5 oncoprotein. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4634–4638. doi: 10.1073/pnas.91.11.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloso M., Mazzotti M., Vass W. C., Beguinot L. SHC and GRB-2 are constitutively by an epidermal growth factor receptor with a point mutation in the transmembrane domain. J Biol Chem. 1995 Aug 18;270(33):19557–19562. doi: 10.1074/jbc.270.33.19557. [DOI] [PubMed] [Google Scholar]
- Oberklaid F., Danks D. M., Jensen F., Stace L., Rosshandler S. Achondroplasia and hypochondroplasia. Comments on frequency, mutation rate, and radiological features in skull and spine. J Med Genet. 1979 Apr;16(2):140–146. doi: 10.1136/jmg.16.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K., Ornitz D., Werner S., Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol. 1993 Feb;155(2):423–430. doi: 10.1006/dbio.1993.1040. [DOI] [PubMed] [Google Scholar]
- Ponseti I. V. Skeletal growth in achondroplasia. J Bone Joint Surg Am. 1970 Jun;52(4):701–716. [PubMed] [Google Scholar]
- Reardon W., Winter R. M., Rutland P., Pulleyn L. J., Jones B. M., Malcolm S. Mutations in the fibroblast growth factor receptor 2 gene cause Crouzon syndrome. Nat Genet. 1994 Sep;8(1):98–103. doi: 10.1038/ng0994-98. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Bonaventure J., Legeai-Mallet L., Pelet A., Rozet J. M., Maroteaux P., Le Merrer M., Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994 Sep 15;371(6494):252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- Rutland P., Pulleyn L. J., Reardon W., Baraitser M., Hayward R., Jones B., Malcolm S., Winter R. M., Oldridge M., Slaney S. F. Identical mutations in the FGFR2 gene cause both Pfeiffer and Crouzon syndrome phenotypes. Nat Genet. 1995 Feb;9(2):173–176. doi: 10.1038/ng0295-173. [DOI] [PubMed] [Google Scholar]
- Scott G. K., Dodson J. M., Montgomery P. A., Johnson R. M., Sarup J. C., Wong W. L., Ullrich A., Shepard H. M., Benz C. C. p185HER2 signal transduction in breast cancer cells. J Biol Chem. 1991 Aug 5;266(22):14300–14305. [PubMed] [Google Scholar]
- Segatto O., King C. R., Pierce J. H., Di Fiore P. P., Aaronson S. A. Different structural alterations upregulate in vitro tyrosine kinase activity and transforming potency of the erbB-2 gene. Mol Cell Biol. 1988 Dec;8(12):5570–5574. doi: 10.1128/mcb.8.12.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiang R., Thompson L. M., Zhu Y. Z., Church D. M., Fielder T. J., Bocian M., Winokur S. T., Wasmuth J. J. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994 Jul 29;78(2):335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Shih C., Padhy L. C., Murray M., Weinberg R. A. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981 Mar 19;290(5803):261–264. doi: 10.1038/290261a0. [DOI] [PubMed] [Google Scholar]
- Spivak-Kroizman T., Lemmon M. A., Dikic I., Ladbury J. E., Pinchasi D., Huang J., Jaye M., Crumley G., Schlessinger J., Lax I. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell. 1994 Dec 16;79(6):1015–1024. doi: 10.1016/0092-8674(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Stern D. F., Heffernan P. A., Weinberg R. A. p185, a product of the neu proto-oncogene, is a receptorlike protein associated with tyrosine kinase activity. Mol Cell Biol. 1986 May;6(5):1729–1740. doi: 10.1128/mcb.6.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kamps M. P., Cao H. Oncogenic activation of p185neu stimulates tyrosine phosphorylation in vivo. Mol Cell Biol. 1988 Sep;8(9):3969–3973. doi: 10.1128/mcb.8.9.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg M. J., Gullick W. J. A sequence motif in the transmembrane region of growth factor receptors with tyrosine kinase activity mediates dimerization. Protein Eng. 1990 Mar;3(4):245–248. doi: 10.1093/protein/3.4.245. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Gullick W. J. Neu receptor dimerization. Nature. 1989 Jun 22;339(6226):587–587. doi: 10.1038/339587a0. [DOI] [PubMed] [Google Scholar]
- Stoll C., Dott B., Roth M. P., Alembik Y. Birth prevalence rates of skeletal dysplasias. Clin Genet. 1989 Feb;35(2):88–92. doi: 10.1111/j.1399-0004.1989.tb02912.x. [DOI] [PubMed] [Google Scholar]
- Tavormina P. L., Shiang R., Thompson L. M., Zhu Y. Z., Wilkin D. J., Lachman R. S., Wilcox W. R., Rimoin D. L., Cohn D. H., Wasmuth J. J. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat Genet. 1995 Mar;9(3):321–328. doi: 10.1038/ng0395-321. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Weiner D. B., Liu J., Cohen J. A., Williams W. V., Greene M. I. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989 May 18;339(6221):230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- Wides R. J., Zak N. B., Shilo B. Z. Enhancement of tyrosine kinase activity of the Drosophila epidermal growth factor receptor homolog by alterations of the transmembrane domain. Eur J Biochem. 1990 May 20;189(3):637–645. doi: 10.1111/j.1432-1033.1990.tb15532.x. [DOI] [PubMed] [Google Scholar]
- Wilkie A. O., Slaney S. F., Oldridge M., Poole M. D., Ashworth G. J., Hockley A. D., Hayward R. D., David D. J., Pulleyn L. J., Rutland P. Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nat Genet. 1995 Feb;9(2):165–172. doi: 10.1038/ng0295-165. [DOI] [PubMed] [Google Scholar]
- Xu Y. F., Meyer A. N., Webster M. K., Lee B. A., Donoghue D. J. The v-sis protein retains biological activity as a type II membrane protein when anchored by various signal-anchor domains, including the hydrophobic domain of the bovine papilloma virus E5 oncoprotein. J Cell Biol. 1993 Nov;123(3):549–560. doi: 10.1083/jcb.123.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Goncalves E., Kahn C. R., Shoelson S. E. Substitution of the insulin receptor transmembrane domain with the c-neu/erbB2 transmembrane domain constitutively activates the insulin receptor kinase in vitro. J Biol Chem. 1992 Jun 25;267(18):12452–12461. [PubMed] [Google Scholar]