Abstract
Hypercalcaemia is frequently observed in patients with sarcoidosis. This is classically attributed to ectopic production of 1,25 dihydroxy vitamin D by sarcoid granulomas. We present a case of sarcoidosis-related hypercalcaemia with normal vitamin D levels. In this patient, production of parathyroid hormone-related peptide (PTHrp) was the cause for sarcoidosis-induced hypercalcaemia. As such, plasma PTHrp levels were increased and bone marrow granulomas stained positively for PTHrp expression. Medium-dose prednisolone treatment improved symptoms of sarcoidosis and normalised serum calcium, and PTHrp concentrations. Thus, production of PTHrp may be the cause for hypercalcaemia in some patients with sarcoidosis.
Background
Sarcoidosis is an inflammatory disease of unknown aetiology, characterised by the presence of non-caseating granulomas, that primarily affects the lungs, although it may also manifest at extrapulmonary sites.1 Hypercalcaemia, present in 10–20% of patients, is due to parathyroid hormone (PTH)-independent production of 1,25-dihydroxy vitamin D by granuloma histiocytes.2 We describe an alternative cause for hypercalcaemia in a patient with sarcoidosis.
Case presentation
A 67-year-old man was referred to our department with fatigue and weight loss. His history included myocardial infarction and peripheral artery occlusive disease more than a decade ago, for which he used antihypertensive agents, salicylic acid and a statin. Nearly 6 months prior, he developed fatigue and loss of appetite resulting in a 20 kg weight loss. No other symptoms were noted, particularly no fever or night sweats. Physical examination revealed a moderately ill man with liver and spleen palpable 3 cm below the diaphragm. No lymphadenopathy was observed and the joints and skin examination was unremarkable.
Investigations
Laboratory analysis revealed moderate inflammation (erythrocyte sedimentation rate, ESR: 20 mm/h; reference range: <15 mm/h), pancytopenia with haemoglobin 7.6 mmol/L (reference range: 8.5–11.0 mmol/L), leucocytes 2.0×109/L (reference range: 4.0–10.0×109/L) and thrombocytes 107×109/L (reference range: 150–400×109/L), impaired kidney function (estimated glomerular filtration rate, eGFR 48 mL/min/L×1.73 m2; normal value: >60 mL/min/L×1.73 m2) and hypercalcaemia (albumin-corrected calcium 3.13 mmol/L; normal range: 2.1–2.6 mmol/L) with other electrolytes including phosphate in the normal range. The chest X-ray was normal. The patient was treated with isotonic saline, loop diuretics and bisphosphonates intravenously, following which calcium levels normalised.
Differential diagnosis
Our differential diagnoses included hypercalcaemia related to a solid malignancy (through destructive bone metastases or production of parathyroid hormone-related peptide (PTHrp)), a lymphoproliferative disorder (through destructive bone lesions or production of PTHrp/1,25-dihydroxy vitamin D) and granulomatous disorders, such as sarcoidosis or tuberculosis, which may cause hypercalcaemia due to production of 1,25-dihydroxy vitamin D. Finally, we considered multiple myeloma. A total body CT showed no signs of malignancy, while monoclonal gammopathy could not be detected. A bone marrow biopsy demonstrated multiple non-caseating granulomas (figure 1A). With angiotensin converting enzyme (ACE) at three times the upper limit and diagnostic tests for tuberculosis negative, we concluded sarcoidosis with reticuloendothelial localisation. Regarding the hypercalcaemia, PTH was low (0.7 mmol/L; reference range: 1.9–10.2 pmol/L), while, remarkably, both 25-hydroxy vitamin D (69 nmol/L; reference range: 30–130 nmol/L) and 1,25-dihydroxy vitamin D concentrations (130 pmol/L; reference range: 48–161 pmol/L) were normal. However, serum PTHrp concentration was strongly increased (13.0 pmol/L; reference range <0.6 pmol/L). Immunohistochemical staining of the bone marrow biopsy confirmed PTHrp expression by the granulomas (figure 1B).
Figure 1.
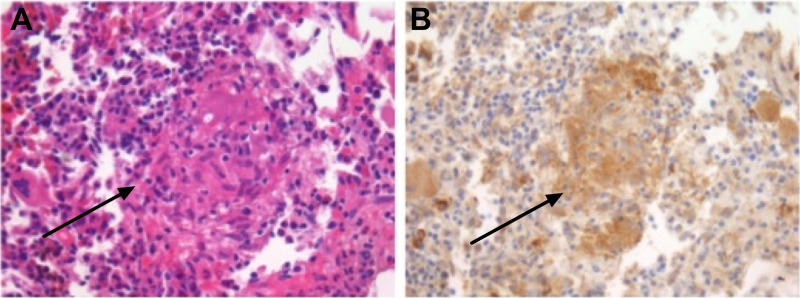
(A) Bone marrow biopsy shows the presence of a non-caseating granuloma (arrow) (H&E stain). (B) Parathyroid hormone-related peptide (PTHrp) immunohistochemistry demonstrating PTHrp expression by the granuloma.
Treatment
The patient was subsequently treated with medium-dose glucocorticoid therapy.
Outcome and follow-up
A number of months later, the patient's symptoms had subsided and all laboratory abnormalities, including calcium and PTHrp concentrations, had normalised. Levels of 1,25 dihydroxy vitamin D remained unchanged during treatment. Now, over 1.5 years after the diagnosis and with tapering glucocorticoid dosages, the patient is doing well.
Discussion
We describe a case of sarcoidosis-related hypercalcaemia caused by granuloma-induced production of PTHrp. Only a single similar case report exists in the literature.3 The autonomous production of 1,25-dihydroxy vitamin D by granuloma macrophages is generally accepted to be the cause for hypercalcaemia due to increased activity of 1α-hydroxylase, the enzyme that catalyses the conversion from inactive to active vitamin D.
Inappropriate PTHrp production is typically associated with malignancies, where it forms the primary cause for hypercalcaemia in non-metastatic solid tumours and indicates a poor prognosis.4 PTHrp shares many actions with PTH due to its homology, including enhancing bone resorption and tubular calcium reabsorption, leading to hypercalcaemia. PTHrp also stimulates 1α-hydroxylase resulting in increased 1,25-dihydroxy vitamin D concentrations, however, not to the same extent as PTH, as was elegantly demonstrated in both studies in healthy humans5 6 and a mechanistic study in rodents.7 This likely explains why 1,25-dihydroxy vitamin D concentrations, despite being in the normal range, were relative high for the given calcium concentrations. On the other hand, we feel it is unlikely that a normal 1,25-dihydroxy vitamin D level was a strong contributor to the observed hypercalcaemia. Strikingly, following treatment, calcium levels normalised and PTHrp became undetectable, whereas 1,25-dihydroxy vitamin D concentrations remained unchanged.
The source for PTHrp in sarcoid granulomas remains unclear. Notably, it was shown that the inflammatory mediators tumour necrosis factor-α and interleukin-6, which are increased in sarcoidosis,8 may stimulate PTHrp expression.9 Glucocorticoids, on the other hand, may restore normocalcaemia by reducing the inflammatory response and by directly suppressing PTHrp expression.10
We conclude that sarcoidosis-related hypercalcaemia is not always caused by increased vitamin D concentrations, but may also be consequential to increased PTHrp production; the mechanisms that underlie this phenomenon are not understood.
Learning points.
Hypercalcaemia is a common finding in sarcoidosis due to increased production of 1,25-dihydroxy vitamin D.
Parathyroid hormone-related peptide (PTHrp) production by sarcoid granulomas can also be a cause of hypercalcaemia.
PTHrp production is particularly known in malignant diseases and indicates a poor prognosis. Autoimmune diseases such as sarcoidosis may also cause PTHrp production.
Acknowledgments
The authors thank professor J Bovée, department of pathology of the Leiden University Medical Centre, for kindly providing the PTHrp antibodies, and Mr F Morsink, department of pathology of the University Medical Centre, Utrecht, for his technical assistance.
Footnotes
Contributors: DR treated the patient and wrote the manuscript. SG helped to analyse the laboratory values, together with EK; they also performed the PTHrp values and helped with the literature search relating PHTrp to sarcoidosis. In addition, they contributed to writing the manuscript. LAAB performed the analysis of the bone marrow biopsies with additional IHC staining. Reinier ten Kate was clinical supervisor at the outpatient clinic, was involved in the treatment of the patient, and supervised DR. He was also involved in writing the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Baughman RP, Teirstein AS, Judson MA et al. . Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001;164:1885–9. 10.1164/ajrccm.164.10.2104046 [DOI] [PubMed] [Google Scholar]
- 2.Adams JS, Singer FR, Gacad MA et al. . Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J Clin Endocrinol Metab 1985;60:960–6. 10.1210/jcem-60-5-960 [DOI] [PubMed] [Google Scholar]
- 3.Krikorian A, Shah S, Wasman J. Parathyroid hormone-related protein: an unusual mechanism for hypercalcemia in sarcoidosis. Endocr Pract 2011;17:e84–6. 10.4158/EP11060.CR [DOI] [PubMed] [Google Scholar]
- 4.Wysolmerski JJ. Parathyroid hormone-related protein: an update. J Clin Endocrinol Metab 2012;97:2947–56. 10.1210/jc.2012-2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwitz MJ, Tedesco MB, Sereika SM et al. . Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) [hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J Clin Endocrinol Metab 2003;88:1603–9. 10.1210/jc.2002-020773 [DOI] [PubMed] [Google Scholar]
- 6.Horwitz MJ, Tedesco MB, Sereika SM et al. . Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J Bone Miner Res 2005;20:1792–803. 10.1359/JBMR.050602 [DOI] [PubMed] [Google Scholar]
- 7.Michigami T, Yamato H, Suzuki H et al. . Conflicting actions of parathyroid hormone-related protein and serum calcium as regulators of 25-hydroxyvitamin D3-1a-hydroxylase expression in a nude rat model of humoral hypercalcemia of malignancy. J Endocrinol 2001;171:249–57. 10.1677/joe.0.1710249 [DOI] [PubMed] [Google Scholar]
- 8.Bost TW, Riches DW, Schumacher B et al. . Alveolar macrophages from patients with beryllium disease and sarcoidosis express increased levels of mRNA for tumor necrosis factor-alpha and interleukin-6 but not interleukin-1 beta. Am J Respir Cell Mol Biol 1994;10:506–13. 10.1165/ajrcmb.10.5.8179912 [DOI] [PubMed] [Google Scholar]
- 9.Rizzoli R, Feyen JH, Grau G et al. . Regulation of parathyroid hormone-related protein production in a human lung squamous cell carcinoma line. J Endocrinol 1994;14:333–41. 10.1677/joe.0.1430333 [DOI] [PubMed] [Google Scholar]
- 10.Ahlström M, Pekkinen M, Lamberg-Allardt C. Dexamethasone downregulates the expression of parathyroid hormone-related protein (PTHrP) in mesenchymal stem cells. Steroids 2009;74:277–82. 10.1016/j.steroids.2008.12.002 [DOI] [PubMed] [Google Scholar]


