Abstract
Introduction
Venous thromboembolism (VTE) with its two manifestations deep vein thrombosis (DVT) and pulmonary embolism (PE) is a major public health problem. The VTEval Project aims to investigate numerous research questions on diagnosis, clinical management, treatment and prognosis of VTE, which have remained uncertain to date.
Methods and analysis
The VTEval Project consists of three observational, prospective cohort studies on VTE comprising cohorts of individuals with a clinical suspicion of acute PE (with or without DVT), with a clinical suspicion of acute DVT (without symptomatic PE) and with an incidental diagnosis of VTE (PE or DVT). The VTEval Project expects to enrol a total of approximately 2000 individuals with subsequent active and passive follow-up investigations over a time period of 5 years per participant. Time points for active follow-up investigations are at months 3, 6, 12, 24 and 36 after diagnosis (depending on the disease cohort); passive follow-up investigations via registry offices and the cancer registry are performed 48 and 60 months after diagnosis for all participants. Primary short-term outcome is defined by overall mortality (PE-related death and all other causes of death), primary long-term outcome by symptomatic VTE (PE-related death, recurrence of non-fatal PE or DVT). The VTEval Project includes three ‘all-comer’ studies and involves the standardised acquisition of high-quality data, covering the systematic assessment of VTE including symptoms, risk profile, psychosocial, environmental and lifestyle factors as well as clinical and subclinical disease, and it builds up a large state-of-the-art biorepository containing various materials from serial blood samplings.
Ethics and dissemination
The VTEval Project has been approved by the local data safety commissioner and the responsible ethics committee (reference no. 837.320.12 (8421-F)). Trial results will be published in peer-reviewed journals and presented at national and international scientific meetings.
Trial registration number
Keywords: Venous thromboembolism, Deep vein thrombosis, Pulmonary embolism, Cohort study design, Biobanking, Diagnosis
Introduction
Venous thromboembolism (VTE) encompasses two clinical manifestations—deep vein thrombosis (DVT) and pulmonary embolism (PE)—and represents a major public health burden in developed countries.1 PE as a primary event or as a complication of a DVT is life-threatening and associated with substantial morbidity and mortality rates.1 With 245 events per 100 000 person-years (DVT: 148/100 000 person-years and PE: 95/100 000 person-years), the overall VTE incidence in Europe is considerably higher than in the USA (108/100 000);after ischaemic heart disease and stroke, VTE is considered the third most common cardiovascular disease in Western countries.1–3 While the incidence rates are higher in women during childbearing years, they are higher in men after age 45 and increase exponentially with age for both sexes.2–4 Once VTE occurs, the prognosis is characterised by a 5–10% risk of severe post-thrombotic syndrome (PTS) and other clinical complications, such as chronic thromboembolic pulmonary hypertension (CTEPH), which develops in approximately 4% of patients after PE.5 6 Healthcare costs related to recurrent VTE, PTS or exploratory end points are substantial as they represent nearly one-fifth of the all-cause costs.6
Despite increasing knowledge on VTE over the past decades, there are still several aspects of diagnosis, clinical management, treatment and prognosis with uncertainties that need to be addressed. VTE is still disregarded in geriatric medicine as well.7 The need for further research is fuelled by the availability of new developments in therapy, especially new medical drugs.8 With the therapeutic advancements, there is a shift of the research focus towards secondary prevention in VTE, as it will help to evaluate clinical diagnostics, clinical decision-making and treatment, as well as the set-up of specific preventive programmes for sequelae, advancing prognosis and quality of life.
The aim of the VTEval Project, which harbours three prospective cohorts of individuals with suspected and incidental VTE, is a systematic, high-quality assessment of VTE including symptoms, risk profiles, psychosocial, environmental and lifestyle factors as well as clinical and subclinical disease. In addition to the comprehensive phenotyping approach, VTEval is building up a large biobank containing various blood samples that are collected from individuals at serial time points. Thereby, VTEval offers the possibility for systems-oriented epidemiology integrating various data levels for ‘omic’-metrics including genome, transcriptome, proteome, metabolome and phenome. This will enable researchers to model multilevel causes of health and disease for diagnosis, management, treatment and prognosis of VTE.
Methods and results
Objectives
The major objectives of the VTEval Project are: (1) to evaluate diagnostics and assess clinical management strategies of VTE, (2) to evaluate outcome and improve risk stratification and (3) to set up a long-term resource for patient-oriented research in VTE.
Specific objectives are: (1) to evaluate risk factors, parameters of non-invasive imaging, humoral biomarkers for the diagnosis of acute VTE; identify determinants for clinical risk stratification; optimise pathways for ‘best practice’ management and generate scores for clinical decision-making; (2) to identify markers for risk stratification and prognosis; develop risk scores for primary and recurrent VTE; assess short-term and long-term outcome; evaluate the incidence of cardiovascular and non-cardiovascular outcome after VTE; analyse determinants for complications; investigate VTE risk profile, outcome and prognosis with regard to comorbidities, sex differences, ethnic groups and geriatric medicine; evaluate phenotypes of subclinical disease in VTE; (3) to develop a documentation system for high-quality and standardised data acquisition suitable for clinical and scientific purposes, including a quality management system; establish a large prospective cohort of individuals with suspected and incident, asymptomatic and symptomatic VTE; set up a large-scale biobank using highly standardised methods.
Additional objectives include the evaluation of VTE guideline adherence, the evaluation of distinct treatment regimens on different events of thromboembolic disease outcome, and the quantification of the net clinical benefit of medical therapy in VTE.
Study design and study population
The VTEval Project represents three investigator-initiated, prospective observational cohort studies to evaluate VTE diagnostics and management, treatment and outcome. It comprises a cohort of patients with suspected PE, a cohort with suspected DVT and a cohort with incidentally diagnosed VTE.
Eligible patients are individuals with a clinical suspicion of acute PE (with or without DVT) (1), individuals with a clinical suspicion of acute DVT (without symptomatic PE) (2), and individuals with incidental diagnosis of VTE (PE or DVT) (3). The inclusion and exclusion criteria for clinically suspected and incidental diagnosis of VTE are given in figure 1A–C.9 10 Eligible individuals are recruited in all units/sections via which they present at the hospital, such as emergency rooms, chest pain units and outpatient clinics; patients are ≥18 years of age and must have provided informed consent.
Figure 1.
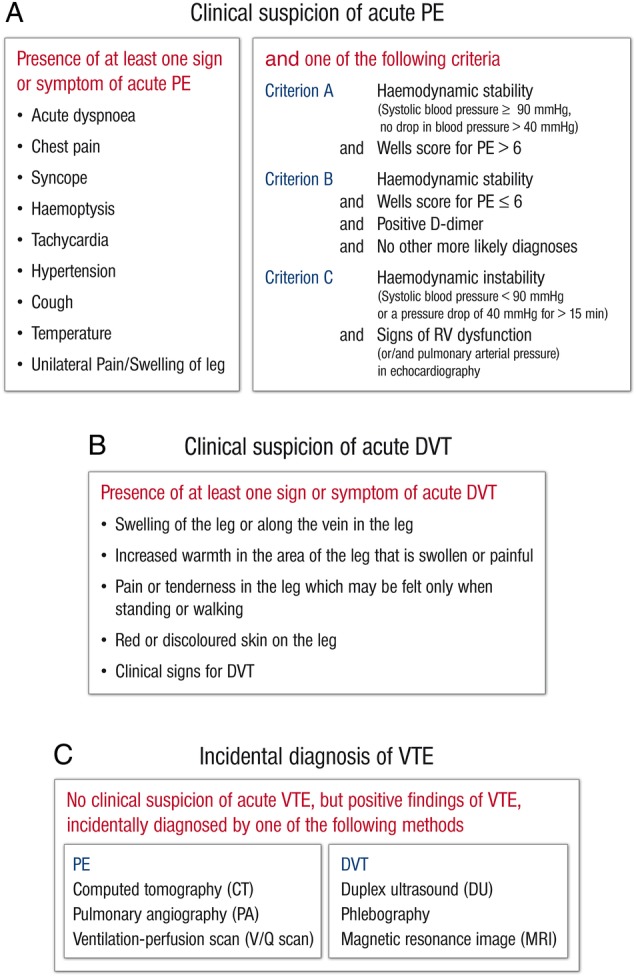
(A) Definition for clinical suspicion of acute PE. PE, pulmonary embolism; RV, right ventricular. (B) Definition for clinical suspicion of acute DVT. DVT, deep vein thrombosis. (C) Definition for clinical suspicion of incidental VTE. DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Investigational plan (active and passive follow-up examinations)
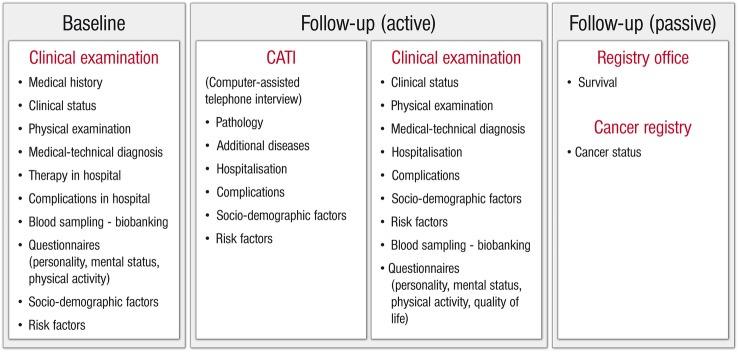
Individuals are followed over a period of 5 years. All study participants are followed actively with clinical examinations, including biosampling, structured interviews (standardised computer-assisted personal interview, CAPI) and self-administered questionnaires during the hospital stay and routine clinical follow-up visits, or computer-assisted telephone interviews (CATI). All interviews are conducted according to standard operating procedures (SOP). CAPIs are conducted by the certified personnel, including study physicians and study nurses; CATIs are also carried out by specifically trained study staff. The quality of measurements and computer-assisted interviews is regularly checked by internal quality controls. Interviews collect participant information on medical history, therapy and complications in hospital, sociodemographic data and risk factors. Data assessment is based on established questionnaire instruments and adapted according to guidelines (Centre for Survey Research and Methodology (GESIS-ZUMA, Mannheim, Germany), Robert Koch Institute (RKI, Berlin, Germany), Framingham Heart Study,11 KORA,12 SHIP,13 and the Gutenberg Health Study (GHS)14) and partly on proprietary development. Self-administered questionnaires request information on personality and mental status (DS14 and DS16;15 16 GAD-2;17 PHQ-9;18 Mini-SPIN;19 PHQ-15;20 CDS-221), physical activity (Short Questionnaire to Assess Health-enhancing Physical Activity, SQUASH,22), quality of life (Pulmonary Embolism Quality of Life, PEmb-QoL23) and treatment satisfaction (Anti-Clot Treatment Scale; ACTS24).
Questionnaires are recorded electronically in the database by the study staff by double data entry.
Patients are followed ‘passively’ via registry offices and cancer registries: Registry offices, that is, local governmental registries in which births and deaths are officially recorded provide information on patients’ vital status (on a regular basis as well as after 48 and 60 months for confirmed and incidental VTE and after 24, 36, 48 and 60 months for excluded VTE). Cancer registries give information on patients’ cancer status (at months 48 and 60 for confirmed and incidental VTE and at months 24, 36, 48 and 60 for excluded VTE). With a corresponding consent form, the patient authorises the study guidance to ask for an alignment of study data with information (if existing) from the Cancer Registry of Rhineland-Palatinate on her/his cancer status, giving permission for the use of personal data.
The general study flow is depicted in figure 2. Study documents have been approved by the local data safety commissioner and the responsible ethics committee (reference no. 837.320.12 (8421-F)). The studies were registered at ClinicalTrials.gov (unique identifier NCT02 156 401).
Figure 2.
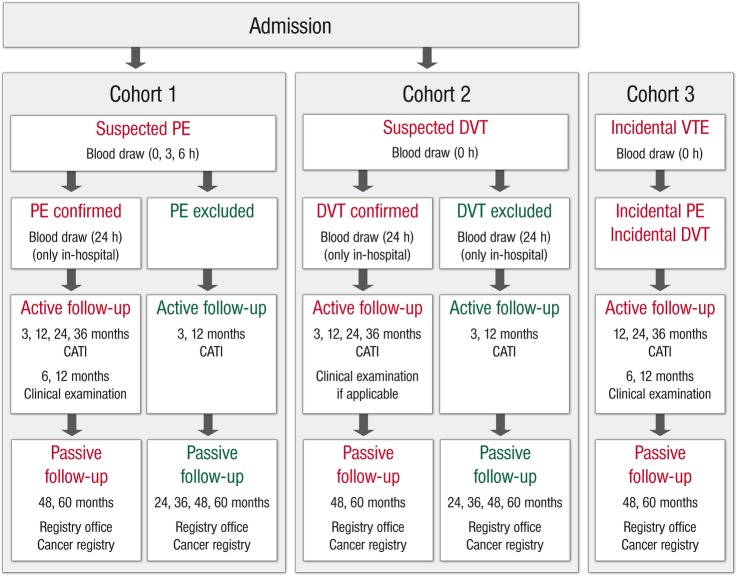
Flow chart of the VTEval Project.
Study flow for cohort of patients with suspected PE (cohort 1)
Individuals with a clinical suspicion of PE are enrolled on admission to hospital. At baseline (0 h), clinical examination and venous blood sampling for biobanking with the routine blood withdrawal are carried out (figure 3). In all individuals, additional blood is drawn 3 and 6 h after baseline, and a further blood sample is taken 24 h after admission for the confirmed cases. With all individuals, an interview is conducted in the form of a standardised CAPI and self-administered questionnaires collecting additional information from the participant (medical history, therapy and complications in hospital, personality, mental status, physical activity, sociodemographic data, risk factors). After being discharged from hospital, patients with PE are followed at 3, 6 and 12 months after the first diagnosis of acute PE. At the 3-month follow-up examination, data are collected by a CATI which is carried out in a centralised manner. In study centres with expertise in clinical follow-up examination, data are collected after 6 and 12 months by means of medical examinations, CAPI and self-administered questionnaires as well.
Figure 3.
Description of the VTEval Project cohorts (from patient admission to follow-up examinations). CATI, computer-assisted telephone interview; DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
In the consecutive 2 years (months 24 and 36), participants will be interviewed via CATI. After this, they will be followed passively by checking for vital and cancer status via data from registry offices and the cancer registry (months 48 and 60).
In individuals with exclusion of acute PE, an active follow-up is carried out at 3 and 12 months after the presumed diagnosis of PE by a CATI. Then, follow-up examinations will be conducted every year by checking the participants’ vital and cancer status at registry offices and the cancer registry (same procedure as established for the confirmed PE cases).
Study flow for cohort of patients with suspected DVT (cohort 2)
Individuals with a clinical suspicion of acute DVT are enrolled on presentation to hospital. Clinical examination and blood sampling are conducted at baseline (0 h) (figure 3). A standardised interview is conducted by CAPI and self-administered questionnaires collecting additional data from the participant (medical history, therapy and complications in hospital, personality, mental status, physical activity, sociodemographic data, risk factors). After 24 h, an additional blood sampling is carried out for participants treated in hospital. Individuals with confirmed DVT are followed at 3, 12, 24 and 36 months after enrolment by CATI. Clinical examinations are performed 3 and 12 months after baseline. Individuals with an exclusion of DVT are followed by CATI 3 and 12 months after the baseline visit, and their vital and cancer status will be annually checked at registry offices and the cancer registry (months 48 and 60).
Study flow for cohort of patients with incidental VTE (cohort 3)
At baseline (0 h), individuals (outpatients or inpatients) with an incidental diagnosis of PE or DVT are subjected to medical-technical diagnostic examinations and a blood draw (figure 3). After 6 and 12 months, further medical-technical diagnostic examinations take place, and an interview (CAPI)/self-administered questionnaire is applied in each follow-up examination, respectively, assessing data on medical history, therapy and complications in hospital, personality, mental status, physical activity, sociodemographic and risk factors. After 24 and 36 months, the individuals are followed by CATI and, as with cohorts 1 and 2, vital and cancer status will be checked at registry offices and the cancer registry afterwards (months 48 and 60).
Outcome
Outcome of VTE is differentiated into short-term and long-term outcome. Short-term outcome events are related to acute VTE complications during the in-hospital phase (time between the clinical suspicion and hospital discharge). Long-term outcome events are related to VTE complications after 3 and ≥24 months after VTE suspicion or incidental diagnosis. The primary event of interest in short-term outcome is overall death. The primary long-term measure is symptomatic VTE defined as PE-related death, development or recurrence of nonfatal PE, and the development or recurrence of DVT.
Data management and statistical analysis
Data collection covers a multimodal approach: (1) patient-by data assessment (including available medical records) and (2) clinical examination in the clinical visit via an electronic case report form (eCRF), (3) standardised interview by CATI, data alignment of study data with the databases of (4) the local registry offices and (5) the local cancer registry. The eCRF includes a plausibility check by data entry. To ensure high quality data, a centralised data management performs quality control and plausibility checks according to predefined procedures. Project data are stored and analysed in a central database. Access to the study documentation system and the study database requires user authentication and is restricted to the responsible study staff.
For statistical analysis, individuals are stratified into the subgroups ‘confirmed VTE’ and ‘excluded VTE’ for cohorts 1 and 2; individuals in cohort 3 comprise cases of incidentally diagnosed VTE only. Overall, three settings are intended for statistical analysis of the three VTEval cohorts: cross-sectional (eg, prevalence and descriptive analysis), case–control (for cohorts 1 and 2 only; eg, matched by sex and age) and longitudinal (eg, survival analysis). Regarding PE, analyses will be carried out after the inclusion of n=150, n=300 and n=500 enrolled patients and subsequently performed after inclusion of another 250 individuals, respectively. Analyses on DVT will be performed after the inclusion of n=300, n=500, n=750 and n=1000 enrolled participants and subsequently after the inclusion of 250 individuals, respectively.
Study size estimation
The recruitment and follow-up period for the VTEval Project is planned for 5 years, envisaging the inclusion of approximately 2000 participants in total, consisting of an estimated 700 individuals with suspected PE (25% confirmed PE, 75% exclusions), 1200 individuals with suspected DVT (50% confirmed DVT, 50% exclusions) and 100 incident cases. The estimate and projected suspected-to-recruited ratios were calculated on the basis of (1) the total number of in-hospital patients with a primary or secondary diagnosis of VTE in the study base in 2011, (2) an estimate of individuals with an initial clinical suspicion of VTE, but no confirmation by objective examination methods, (3) an estimated number of individuals with an incidental diagnosis of VTE, and a loss of 40% for eligible participants who do not participate in the studies (20% due to refusal to participate and 20% due to other reasons). Using two sample binomial tests and assuming prevalence of exposure of 5% (in individuals with excluded VTE) and a power of 80% (α=0.05), minimum detectable differences of 6.4% and 4.2% between the subgroups ‘confirmed VTE’ and ‘exclusions’ for the expected sample sizes (175 confirmed PE/525 exclusions; 600 confirmed DVT/600 exclusions) are needed, respectively. With a prevalence of 40% for a selected trait, minimum detectable differences of 12.4% (PE) and 8.4% (DVT) are needed to achieve a power of at least 80%. When combining PE and DVT (775 confirmed VTE/1125 exclusions, α=0.05, 80% power), subgroup differences of 3.3% and 6.8% for a prevalence of 5% and 40% are required, respectively.
In the literature, the incidence rate of VTE recurrence is 4.9 (95% CI 4.7 to 5.0) per 100 person-years, with 4.7/100 person-years following first DVT and 5.1/100 person-years following first PE.25 At the same time, incidence rates for provoked and unprovoked VTE were 3.8 (95% CI 3.6 to 4.1) and 5.6 (95% CI 5.4 to 5.8) per 100 person-years, respectively.25 For each VTEval cohort, we calculated approximate confidence precision intervals to predict disease incidence for the estimated number of cases by assuming Poisson distribution. Given the estimated numbers of cases in the VTEval Project (175 confirmed PE, 600 confirmed DVT and 100 incident cases, and 800 provoked, 1200 unprovoked VTE, respectively), 95% precision intervals are (2.0–10) for a PE incidence of 5.1, (2.8–6.9) for a DVT incidence of 4.7 and (3.2–6.8) for the assessed VTE incidence of 4.9. 95% precision intervals are (2.4–5.5) for the incidence of 3.8 for provoked VTE and (4.1–7.2) for the incidence of 5.6 for unprovoked VTE.
Biobanking
A central goal of the VTEval Project is to establish a centralised biodatabank including blood from each individual at serial time points. Blood samples are collected, transported, processed and stored according to SOPs. Basically, EDTA plasma, Na-citrated plasma and serum samples as well as DNA and RNA are collected. Two-dimensional-barcoded polypropylene tubes are utilised for storage. The biorepository is controlled by sample management software containing the sample-specific information and has a semiautomatic storage system with electronic temperature monitoring. Biomaterial of each individual is splitted in two identical racks for storage in separated locations.
At first, the VTEval Project envisages the measurement and evaluation of routine laboratory markers (ie, D-dimer and C reactive protein, CRP), numerous parameters involved in coagulation and inflammation (eg, factor VIII (FVIII), interleukin 6 or resistin), platelet and leucocyte activation (eg, soluble P-selectin (sP-Sel)) as well as regulatory microRNAs (ie, miR-126, miR-155 and miR-200c) and their associations with DVT.26–36
Study implementation
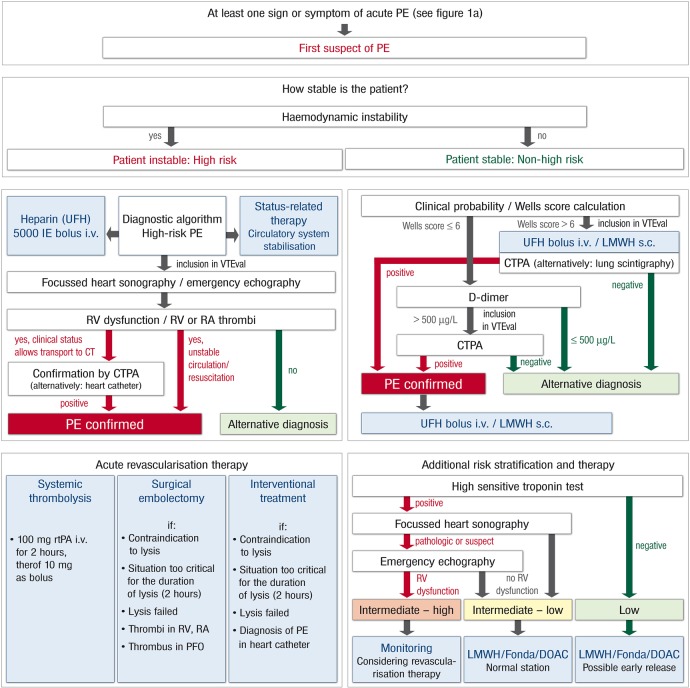
The VTEval Project has been initiated by the Department of Clinical Epidemiology of the Center for Thrombosis and Hemostasis (CTH)—a model centre to support research, treatment and medical education in the field of thrombosis and haemostasis at the University Medical Center Mainz in Western Mid-Germany. After establishing infrastructure and logistics for the CTH cohort studies in the single centre setting at the University Medical Center, the extension of VTEval to multicentre studies is planned. The recruitment of the first patients for the VTEval Project started in April 2013. The study set up profits from the existing network of study centres within the CTH (eg, the thrombEVAL study programme37). To enlarge the CTH cohort, a modular model for data acquisition will be designed comprising a basic module and complementary modules taking into account a varying depth of phenotyping (ie, data acquisition on individuals) in the participating study centres. To ensure the comparability and high quality of data, SOPs for the screening of acute VTE were predefined for all medical and study-related procedures (figures 4 and 5).
Figure 4.
Standard operating procedure (SOP) and risk-adjusted therapeutic strategies in acute pulmonary embolism. CTPA, computed tomographic pulmonary angiography; DOAC, direct oral anticoagulant; Fonda, fondaparinux; i.v., intravenous; LMWH, low-molecular-weight heparin; PE, pulmonary embolism; PFO, patent foramen ovale; RA, right atrial; rtPA, recombinant tissue plasminogen activator; RV, right ventricular; s.c., subcutanous; UFH, unfractionated heparin.
Figure 5.
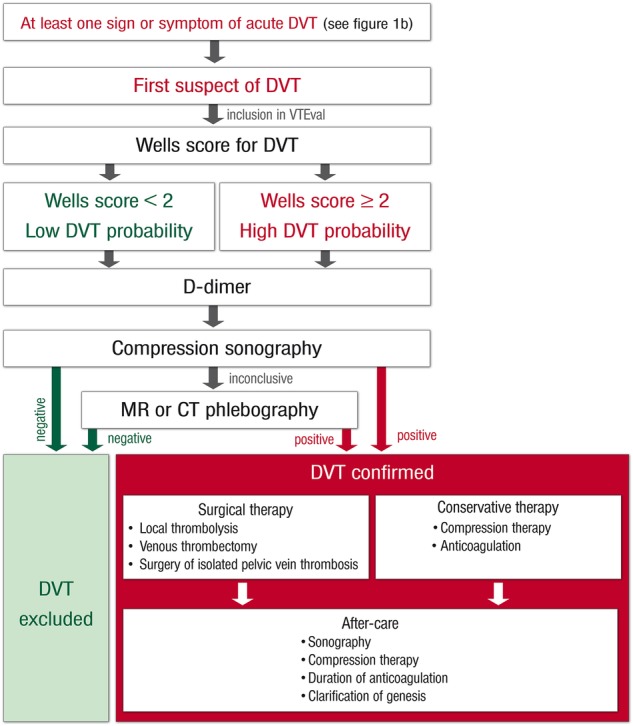
Standard operating procedure (SOP) and risk-adjusted therapeutic strategies in acute deep vein thrombosis. DVT, deep vein thrombosis.
Discussion
VTE represents a relevant public health burden. Despite increasing knowledge on VTE, various clinical aspects of the disease are still unclear and advancements in diagnostic tools and medical therapy generate new unknown issues and uncertainties. The VTEval Project encompasses three large, prospective, observational cohort studies including biobanking for the evaluation and improvement of VTE diagnostics, management, treatment and prognosis. The enrolment of a primary sample of 2000 suspected and incident cases will expedite the analysis of clinical and laboratory measures of VTE, thus helping to elucidate unsettled issues.
Considerable uncertainties in diagnosing VTE do still exist, rendering the efficient, fast and reliable detection of VTE a major clinical challenge. D-dimer testing has been extensively studied as a very efficient step in the diagnostic algorithm for patients with a suspected first episode of PE or DVT.38 As the currently best-recognised biomarker for the initial assessment of VTE, a negative value of D-dimer may safely ‘rule out’ both PE and DVT due to its high sensitivity of about 95%.27 D-dimer indicates fibrin degradation with high specificity. However, fibrin degradation is not very specific for VTE as it frequently occurs in the setting of an underlying malignant disease or non-specific inflammatory responses, for example, pregnancy, surgery and trauma, giving false-positive results.27 39 Consequently, D-dimer testing does not necessarily ‘rule in’ VTE, making imaging procedures such as compression duplex ultrasound and computed tomographic pulmonary angiography the gold standard for the diagnosis of DVT and PE, respectively.27 35 40 Despite this, recent evidence suggests using age-adjusted cut-offs to improve the performance of D-dimer testing in the elderly.40 41 Notably, D-dimer was shown to have a lower sensitivity and a lower negative predictive value for isolated distal deep vein thromboses (IDDVT), which are confined to the infrapopliteal veins of the lower limbs.42 43 To date, the diagnostic value of D-dimer in the exclusion of proximal DVT is well established but neither well studied nor officially approved for the exclusion of IDDVT.44
To enable a more accurate diagnosis of VTE, genetic background and predisposition, states of inflammation or immunity, haemodynamic factors as well as profiles of epigenetics or circulating microRNA levels may support diagnostics as they, individually or combined, may modulate or falsify test results.45–48 So far, a number of VTE biomarkers have been proposed in addition to or together with D-dimer, including CRP, sP-Sel, FVIII, tissue factor-positive microparticles and leucocytes as promising candidates.27 35 39 49–51 The validity of these markers remains to be determined.
In diagnostics by CT angiography, the value and clinical significance of subsegmental PE is currently under discussion.40 41 A standardised definition of subsegmental PE is not yet in use.41 An important issue is also the incidental discovery of clinically unsuspected PE by CT angiography, primarily occurring in patients with cancer. There is no evidence available as yet for the management of incident cases, especially if PE is limited to segmental or subsegmental branches.40 41 Indeed, various combinations of clinical findings, echocardiography and laboratory biomarkers have been proposed and investigated, but the ultimate validation of these modalities and scores with respect to risk stratification is lacking and considered top priority.
The commonly accepted standard of care for most patients with VTE is the initial administration of low-molecular-weight heparin, overlapped and followed by a vitamin K antagonist (VKA) or new oral anticoagulants (NOACs).41 52 The effectiveness of VKAs has been well described in the short-term treatment of VTE with the risks of recurrence reduced by around 82% after primary treatment is stopped.53 54 This regimen, however, is complex to implement in clinical practice, and due to the numerous limitations, patients’ quality of life is negatively affected.55 56 NOACs including direct thrombin (factor IIa) inhibitors (dabigatran) and selective factor Xa inhibitors (rivaroxaban, apixaban and edoxaban) have emerged as promising alternatives with the potential to overcome the limitations of traditional treatments.57–60 However, long-term experience on optimal treatment and duration of therapy is not available. Notably, outpatient or home treatment of acute PE and/or DVT represents a first step towards lowering patients’ burden, and it may be effective and safe in appropriately selected patients with predefined and easy-to-use criteria as observed VTE recurrence, mortality and bleeding rates are low.61 62
Anticoagulant therapy is effective, but it entails expense, inconvenience, effect on quality of life and the risk of major haemorrhage, that is, harms and benefits need to be carefully elucidated.8 63 Lifelong treatment with anticoagulation is neither uniformly applied nor recommended as it exposes patients to a potentially unnecessary long-term therapy and a substantial risk of bleeding.64 Prolonged anticoagulation carries an annual risk of major bleeding of up to 3%, and the risk of fatal bleeding is 0.25%.64 Among the many different bleeding events, intracranial haemorrhage is by far the most severe due to the increased morbidity and lethality.65 The duration of anticoagulation is primarily influenced by the underlying cause of VTE.64 66 Patients who had a VTE after surgery (provoked) have a very low annual risk of recurrence (<1%) and can safely discontinue anticoagulant therapy.64 In contrast, patients with an unprovoked VTE who discontinue anticoagulation after 3–6 months have a risk of recurrence in the first year of 5–27% and 2–3.8% for each subsequent year.64 Several algorithms, such as the HERDOO2, the Vienna prediction model and the DASH (D-dimer level measured 1 month after anticoagulation withdrawal, young Age, male Sex, and Hormonal therapy associated with the index VTE event) score, have been proposed67 but remain to be externally validated in large cohorts of patients after the first episode of PE or DVT.
Patients with PE are at an increased risk of serious long-term complications such as pulmonary hypertension (PH), a severe and progressive sequela that is associated with significant morbidity and mortality.68–70 CTEPH is a potentially curable cause of PE, with a reported cumulative incidence of 0.1–9.1% within the first 2 years after a symptomatic PE event.40 41 The imprecision of the estimate may be due to referral bias, lack of early symptoms and difficulty in differentiating acute PE from an acute episode superimposed on pre-existing CTEPH. Pulmonary endarterectomy is the given treatment of choice, involving substantial relief from symptoms and near-normalisation of haemodynamics.41 Yet optimisation of CTEPH diagnosis could refer patients earlier to life-saving treatment, pointing to the need for improved diagnostics and identification.41 71
Studies as implemented in the VTEval Project are essential to address contemplated uncertainties in VTE diagnostics, management, treatment and prognosis. The strengths of the project include the use of predefined SOP to ensure a standardised and comparable observation, and an electronic documentation system for high-quality data acquisition, as well as the inclusion of a large biobank. To date, there are very few observational cohort studies which are equipped with major concomitant biobank data on VTE. The VTEval Project is offering an innovative design with state-of-the-art technology, allocating and combining large-scale biobanking with comprehensive phenotypic data in VTE. In contrast to numerous clinical studies, study participants are not selected, that is, all-comers are included, and the observational character of the project provides a representative picture of the disease. To avoid bias and to ensure the representative character of the project, relatives of potential participants will be asked for the alleged will of the patient initially to give written consent in cases where the patient is not able to do so himself/herself at the moment of enrolment. Written consent must be provided by the patient or his/her legal representative within 7 days of admission.
Data recording between the three VTE cohorts is harmonised, and all individuals with an exclusion of VTE are followed up as an optimal control cohort for longitudinal analyses. Investigations on data from VTEval might contribute to answering numerous open research questions. Efforts can address diagnostic uncertainties, that is, the wealth of exploitable phenotypic data along with corresponding biomaterial may facilitate the investigation of established and novel biomarkers and elucidate their specification and applicability in clinical practice. By generating and improving scores for clinical decision-making and by establishing risk-benefit analyses, the individual risk of recurrence may be evaluated and the feasibility of ambulatory or even home treatment of patients to increase patients’ quality of life can be analysed. Treatment algorithms and mature pharmaceuticals for VTE and VTE sequelae may be improved and directly implemented into clinical practice, tapping their full potential.
Footnotes
Collaborators: VTEval study group. Steering committee: Philipp S. Wild (coordinator and speaker), Stavros V. Konstantinides, Thomas Münzel, Karl J. Lackner, Christine Espinola-Klein (all Mainz, Germany). Principal investigators: Philipp S. Wild, Stavros V. Konstantinides (Mainz, Germany). Scientific study management: Bernd Frank (Mainz, Germany).
Contributors: PSW received the funding for the project. PSW, SVK, TM, KJL and UW contributed to conception and design; PSW, SVK, TM, CE-K and GW defined SOPs. PSW, JHP, VG, HL and LA managed the implementation of the study. HL and FK were responsible for data management analysis. PSW, KJL and VG established the centralised biodatabank. AU carried out statistical planning; AU and JHP performed database structuring. BF and PSW drafted the manuscript which was critically revised for important intellectual content by all authors. All authors approved the final version of the manuscript to be published.
Funding: The VTEval Project is funded by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF). BF, LA, HL, VG, JP, AU, UW, SK and PSW are fully or partly funded by the German Federal Ministry of Education and Research (Grant 01EO1003).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The VTEval Project has been approved by the local data safety commissioner and the responsible ethics committee (reference no. 837.320.12 (8421-F)).
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Contributor Information
Collaborators: for the VTEval study group, Philipp S. Wild, Stavros V. Konstantinides, Thomas Münzel, Karl J. Lackner, and Christine Espinola-Klein
References
- 1.Cohen AT, Agnelli G, Anderson FA et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756–64. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28–92. 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverstein MD, Heit JA, Mohr DN et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998;158:585–93. 10.1001/archinte.158.6.585 [DOI] [PubMed] [Google Scholar]
- 4.White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(23 Suppl 1):I4–8. 10.1161/01.CIR.0000078468.11849.66 [DOI] [PubMed] [Google Scholar]
- 5.Pengo V, Lensing AW, Prins MH et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257–64. 10.1056/NEJMoa032274 [DOI] [PubMed] [Google Scholar]
- 6.Lefebvre P, Laliberte F, Nutescu EA et al. All-cause and potentially disease-related health care costs associated with venous thromboembolism in commercial, Medicare, and Medicaid beneficiaries. J Manag Care Pharm 2012;18:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siccama RN, Janssen KJ, Verheijden NA et al. Systematic review: diagnostic accuracy of clinical decision rules for venous thromboembolism in elderly. Ageing Res Rev 2011;10:304–13. 10.1016/j.arr.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 8.Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA 2014;311:717–28. 10.1001/jama.2014.65 [DOI] [PubMed] [Google Scholar]
- 9.Wells PS, Anderson DR, Bormanis J et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795–8. 10.1016/S0140-6736(97)08140-3 [DOI] [PubMed] [Google Scholar]
- 10.Wells PS, Anderson DR, Rodger M et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 2000;83:416–20. [PubMed] [Google Scholar]
- 11.Velagaleti RS, Gona P, Chuang ML et al. Relations of insulin resistance and glycemic abnormalities to cardiovascular magnetic resonance measures of cardiac structure and function: the Framingham Heart Study. Circ Cardiovasc Imaging 2010;3:257–63. 10.1161/CIRCIMAGING.109.911438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koenig W, Karakas M, Zierer A et al. Oxidized LDL and the risk of coronary heart disease: results from the MONICA/KORA Augsburg Study. Clin Chem 2011;57:1196–200. 10.1373/clinchem.2011.165134 [DOI] [PubMed] [Google Scholar]
- 13.Alte D, Ludemann J, Piek M et al. Distribution and dose response of laboratory markers to alcohol consumption in a general population: results of the study of health in Pomerania (SHIP). J Stud Alcohol 2003;64:75–82. 10.15288/jsa.2003.64.75 [DOI] [PubMed] [Google Scholar]
- 14.Wild PS, Zeller T, Beutel M et al. The Gutenberg health study. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2012;55:824–9. 10.1007/s00103-012-1502-7 [DOI] [PubMed] [Google Scholar]
- 15.Grande G, Jordan J, Kummel M et al. Evaluation of the German Type D Scale (DS14) and prevalence of the Type D personality pattern in cardiological and psychosomatic patients and healthy subjects. Psychother Psychosom Med Psychol 2004;54:413–22. 10.1055/s-2004-828376 [DOI] [PubMed] [Google Scholar]
- 16.Denollet J. Personality and coronary heart disease: the type-D scale-16 (DS16). Ann Behav Med 1998;20:209–15. 10.1007/BF02884962 [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146:317–25. 10.7326/0003-4819-146-5-200703060-00004 [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor KM, Kobak KA, Churchill LE et al. Mini-SPIN: a brief screening assessment for generalized social anxiety disorder. Depress Anxiety 2001;14:137–40. 10.1002/da.1055 [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258–66. 10.1097/00006842-200203000-00008 [DOI] [PubMed] [Google Scholar]
- 21.Michal M, Zwerenz R, Tschan R et al. Screening for depersonalization-derealization with two items of the cambridge depersonalization scale. Psychother Psychosom Med Psychol 2010;60:175–9. 10.1055/s-0029-1224098 [DOI] [PubMed] [Google Scholar]
- 22.Wendel-Vos GC, Schuit AJ, Saris WH et al. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 2003;56:1163–9. 10.1016/S0895-4356(03)00220-8 [DOI] [PubMed] [Google Scholar]
- 23.Klok FA, Cohn DM, Middeldorp S et al. Quality of life after pulmonary embolism: validation of the PEmb-QoL Questionnaire. J Thromb Haemost 2010;8:523–32. 10.1111/j.1538-7836.2009.03726.x [DOI] [PubMed] [Google Scholar]
- 24.Cano SJ, Lamping DL, Bamber L et al. The Anti-Clot Treatment Scale (ACTS) in clinical trials: cross-cultural validation in venous thromboembolism patients. Health Qual Life Outcomes 2012;10:120 10.1186/1477-7525-10-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez C, Cohen AT, Bamber L et al. Epidemiology of first and recurrent venous thromboembolism: a population-based cohort study in patients without active cancer. Thromb Haemost 2014;112:255–63. 10.1160/TH13-09-0793 [DOI] [PubMed] [Google Scholar]
- 26.Bittar LF, Mazetto BM, Orsi FL et al. Long-term increased factor VIII levels are associated to interleukin-6 levels but not to post-thrombotic syndrome in patients with deep venous thrombosis. Thromb Res 2015;135:497–501. 10.1016/j.thromres.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 27.Ghozlan MF, Osman AA, Mahmoud HM et al. Comprehensive study on laboratory biomarkers for prediction and diagnosis of deep venous thrombosis. Blood Coagul Fibrinolysis 2015;26:255–60. 10.1097/MBC.0000000000000164 [DOI] [PubMed] [Google Scholar]
- 28.Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein and risk of venous thromboembolism in the general population. Arterioscler Thromb Vasc Biol 2010;30:1672–8. 10.1161/ATVBAHA.109.198473 [DOI] [PubMed] [Google Scholar]
- 29.Folsom AR, Lutsey PL, Astor BC et al. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thromb Haemostasis 2009;102:615–19. 10.1160/TH09-04-0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magenta A, Greco S, Gaetano C et al. Oxidative stress and microRNAs in vascular diseases. Int J Mol Sci 2013;14:17319–46. 10.3390/ijms140917319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magenta A, Cencioni C, Fasanaro P et al. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ 2011;18:1628–39. 10.1038/cdd.2011.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao L, Wang XG, Cheng JD et al. The up-regulation of endothelin-1 and down-regulation of miRNA-125a-5p, -155, and -199a/b-3p in human atherosclerotic coronary artery. Cardiovasc Pathol 2014;23:217–23. 10.1016/j.carpath.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 33.Reddy MA, Jin W, Villeneuve L et al. Pro-inflammatory role of microrna-200 in vascular smooth muscle cells from diabetic mice. Arterioscler Thromb Vasc Biol 2012;32:721–9. 10.1161/ATVBAHA.111.241109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramacciotti E, Blackburn S, Hawley AE et al. Evaluation of soluble P-selectin as a marker for the diagnosis of deep venous thrombosis. Clin Appl Thromb Hemost 2011;17:425–31. 10.1177/1076029611405032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandy FC, Stabler C, Eliassen AM et al. Soluble P-selectin for the diagnosis of lower extremity deep venous thrombosis. J Vasc Surg Venous Lymphat Disord 2013;1:117–25. 10.1016/j.jvsv.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabinovich A, Cohen JM, Cushman M et al. Inflammation markers and their trajectories after deep vein thrombosis in relation to risk of postthrombotic syndrome. J Thromb Haemost 2015;13:398–408. 10.1111/jth.12814 [DOI] [PubMed] [Google Scholar]
- 37.Prochaska JH, Coldewey M, Gobel S et al. Evaluation of oral anticoagulation therapy: rationale and design of the thrombEVAL study programme. Eur J Prev Cardiol 2015;22:622–8. 10.1177/2047487314527852 [DOI] [PubMed] [Google Scholar]
- 38.Schellong SM. Diagnosis of recurrent deep vein thrombosis. Hamostaseologie 2013;33:195–200. 10.5482/HAMO-13-06-0029 [DOI] [PubMed] [Google Scholar]
- 39.Hou H, Ge Z, Ying P et al. Biomarkers of deep venous thrombosis. J Thromb Thrombolysis 2012;34:335–46. 10.1007/s11239-012-0721-y [DOI] [PubMed] [Google Scholar]
- 40.Konstantinides S, Torbicki A. Management of venous thrombo-embolism: an update. Eur Heart J 2014;35:2855–63. 10.1093/eurheartj/ehu243 [DOI] [PubMed] [Google Scholar]
- 41.Konstantinides SV, Torbicki A, Agnelli G et al. , Authors/Task Force M. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)Endorsed by the European Respiratory Society (ERS). Eur Heart J 2014;35:3033–69. 10.1093/eurheartj/ehu283 [DOI] [PubMed] [Google Scholar]
- 42.Goodacre S, Sampson FC, Sutton AJ et al. Variation in the diagnostic performance of D-dimer for suspected deep vein thrombosis. QJM 2005;98:513–27. 10.1093/qjmed/hci085 [DOI] [PubMed] [Google Scholar]
- 43.Sartori M, Cosmi B, Legnani C et al. The Wells rule and D-dimer for the diagnosis of isolated distal deep vein thrombosis. J Thromb Haemost 2012;10:2264–9. 10.1111/j.1538-7836.2012.04895.x [DOI] [PubMed] [Google Scholar]
- 44.Luxembourg B, Schwonberg J, Hecking C et al. Performance of five D-dimer assays for the exclusion of symptomatic distal leg vein thrombosis. Thromb Haemost 2012;107:369–78. 10.1160/TH11-07-0511 [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Zhao J, Evan G et al. Circulating microRNAs: novel biomarkers for cardiovascular diseases. J Mol Med 2012;90:865–75. 10.1007/s00109-011-0840-5 [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie 2010;30:5–6, 8–9. [PubMed] [Google Scholar]
- 47.Morange PE, Tregouet DA. Lessons from genome-wide association studies in venous thrombosis. J Thromb Haemost 2011;9(Suppl 1):258–64. 10.1111/j.1538-7836.2011.04311.x [DOI] [PubMed] [Google Scholar]
- 48.Zoller B, Li X, Sundquist J et al. Familial transmission of venous thromboembolism: a cohort study of 80 214 Swedish adoptees linked to their biological and adoptive parents. Circ Cardiovasc Genet 2014;7:296–303. 10.1161/CIRCGENETICS.113.000341 [DOI] [PubMed] [Google Scholar]
- 49.Mannhalter C. Biomarkers for arterial and venous thrombotic disorders. Hamostaseologie 2014;34:115–20, 22–6, 28–30, passim 10.5482/HAMO-13-08-0041 [DOI] [PubMed] [Google Scholar]
- 50.Ramacciotti E, Hawley AE, Wrobleski SK et al. Proteomics of microparticles after deep venous thrombosis. Thromb Res 2010;125:e269–74. 10.1016/j.thromres.2010.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bucek RA, Reiter M, Quehenberger P et al. C-reactive protein in the diagnosis of deep vein thrombosis. Br J Haematol 2002;119:385–9. 10.1046/j.1365-2141.2002.03886.x [DOI] [PubMed] [Google Scholar]
- 52.Cohen AT, Imfeld S, Rider T. Phase III trials of new oral anticoagulants in the acute treatment and secondary prevention of VTE: comparison and critique of study methodology and results. Adv Ther 2014;31:473–93. 10.1007/s12325-014-0119-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:1794–801. 10.1182/blood-2013-12-512681 [DOI] [PubMed] [Google Scholar]
- 54.Ghanny S, Crowther M. Treatment with novel oral anticoagulants: indications, efficacy and risks. Curr Opin Hematol 2013;20:430–6. 10.1097/MOH.0b013e328363c170 [DOI] [PubMed] [Google Scholar]
- 55.Kearon C, Akl EA, Comerota AJ et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e419S–94S. 10.1378/chest.11-2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thavarajah D, Wetherill M. Implementing NICE guidelines on risk assessment for venous thromboembolism: failure, success and controversy. Int J Health Care Qual Assur 2012;25:618–24. 10.1108/09526861211261217 [DOI] [PubMed] [Google Scholar]
- 57.Investigators E, Bauersachs R, Berkowitz SD et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510. 10.1056/NEJMoa1007903 [DOI] [PubMed] [Google Scholar]
- 58.Agnelli G, Buller HR, Cohen A et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808. 10.1056/NEJMoa1302507 [DOI] [PubMed] [Google Scholar]
- 59.Raskob G, Buller H, Prins M et al. Edoxaban for the long-term treatment of venous thromboembolism: rationale and design of the Hokusai-venous thromboembolism study–methodological implications for clinical trials. J Thromb Haemost 2013;11:1287–94. 10.1111/jth.12230 [DOI] [PubMed] [Google Scholar]
- 60.Schulman S, Kakkar AK, Goldhaber SZ et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764–72. 10.1161/CIRCULATIONAHA.113.004450 [DOI] [PubMed] [Google Scholar]
- 61.Lozano F, Trujillo-Santos J, Barron M et al. Home versus in-hospital treatment of outpatients with acute deep venous thrombosis of the lower limbs. J Vasc Surg 2014;59:1362–7 e1. 10.1016/j.jvs.2013.11.091 [DOI] [PubMed] [Google Scholar]
- 62.Zondag W, Mos IC, Creemers-Schild D et al. Outpatient treatment in patients with acute pulmonary embolism: the Hestia Study. J Thromb Haemost 2011;9:1500–7. 10.1111/j.1538-7836.2011.04388.x [DOI] [PubMed] [Google Scholar]
- 63.Bates SM, Jaeschke R, Stevens SM et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e351S–418S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodger MA, Kahn SR, Wells PS et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008;179:417–26. 10.1503/cmaj.080493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caldeira D, Barra M, Pinto FJ et al. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 2015;262:516–22. 10.1007/s00415-014-7462-0 [DOI] [PubMed] [Google Scholar]
- 66.Wells P, Anderson D. The diagnosis and treatment of venous thromboembolism. Hematology Am Soc Hematol Educ Program 2013;2013:457–63. 10.1182/asheducation-2013.1.457 [DOI] [PubMed] [Google Scholar]
- 67.Poli D, Palareti G. Assessing recurrence risk following acute venous thromboembolism: use of algorithms. Curr Opin Pulm Med 2013;19:407–12. 10.1097/MCP.0b013e328363ed7c [DOI] [PubMed] [Google Scholar]
- 68.Prandoni P. Anticoagulant treatment of pulmonary embolism: impact and implications of the EINSTEIN PE study. Eur J Haematol 2012;89:281–7. 10.1111/ejh.12002 [DOI] [PubMed] [Google Scholar]
- 69.Ozsu S, Cinarka H. Chronic thromboembolic pulmonary hypertension: Medical treatment. Pulm Circ 2013;3:341–4. 10.4103/2045-8932.114761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guerin L, Couturaud F, Parent F et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost 2014;112:598–605. 10.1160/TH13-07-0538 [DOI] [PubMed] [Google Scholar]
- 71.Kim NH, Delcroix M, Jenkins DP et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013;62(25 Suppl):D92–9. 10.1016/j.jacc.2013.10.024 [DOI] [PubMed] [Google Scholar]