Abstract
Prenatal pertussis has become a concern once again with the reappearance of the disease in the USA. A 30-year-old mother whose pregnancy was complicated with fetal arrhythmia was referred for further evaluation in the third trimester. After initial treatment with antiarrhythmic medications due to continued irregular rhythm, she was revisited for persistent hacking cough at 38 weeks gestational age. PCR examination confirmed pertussis diagnosis. Owing to increased risk of digoxin toxicity with concurrent antibiotic administration, antiarrhythmic medication was discontinued. Delivery was induced 2 days after the initiation of azithromycin therapy to prevent the transmission of the disease to the neonate. A well-planned delivery in a patient with prenatal diagnosis prevents neonatal infection while considering the obstetrical dilemma for concurrent management of the intrauterine arrhythmia and antibiotic administration.
Background
Pertussis has made a startling reappearance in recent years with the Centers for Disease Control and Prevention (CDC) reporting almost 50 000 cases in 2012 alone.1 Therefore, it is recommended that pregnant women receive tetanus, diphtheria and acellular pertussis (Tdap) booster vaccine during the third trimester of every pregnancy. Family and caregivers can also receive Tdap in order to ‘cocoon’ the newborn and provide protection through the vulnerable neonatal period until the infant receives the first diphtheria, tetanus and acellular pertussis vaccine at 2 months of age.2 Fetal or neonatal involvement from pertussis can result in severe complications and perinatal transmission of pertussis has been described. We report on a planned delivery in a patient with prenatal maternal pertussis complicated by ongoing treatment of a fetal arrhythmia in order to avoid collateral effects of combining medications; this is a challenging obstetrical dilemma.
Case presentation
A 30-year-old gravida 5 para 3 (3 prior vaginal deliveries) patient was referred by her primary obstetrician for irregular fetal heart rhythm noted on fetal Doppler studies. Fetal echocardiogram was performed at 29 weeks of gestation in our centre, revealing occasional premature atrial contractions (PAC), small pericardial effusion and mild constriction of the ductus arteriosus. The patient was advised to stop caffeine, avoid non-steroidal anti-inflammatory medications and periodically return for fetal heart rate monitoring. At 33 weeks gestation, the fetus was evaluated by the primary obstetrician who noted bradycardia in the 70s. Fetal echocardiography noted atrial bigeminy with a sinus beat followed by a non-conducted PAC with a ventricular rate of 72–78 bpm due to prolonged atrial bigeminy. Thus the noted bradycardia was benign due to blocked PAC occurring after the sinus beat. There was a rare conducted atrial couplet with a rate of 220 bpm. The fetus was intermittently in sinus rhythm rate ranging between 120 and 145 bpm. Two days later, the fetal echocardiography demonstrated new-onset right atrial enlargement, mild tricuspid and mitral regurgitation. There was persistent frequent PAC and bursts of non-sustained supraventricular tachycardia (SVT) at 250 bpm. Owing to the changes noted on the fetal echocardiography, in addition to non-sustained SVT, the patient was hospitalised for fetal heart rate monitoring and started on digoxin (250 mg every 8 h). During early hospitalisation, fetal echocardiography showed very irregular rhythm due to frequent PACs that were blocked and conducted. The digoxin trough level was in the therapeutic range of 1.2–1.7 ng/dL (0.9–2.0 ng/dL), when a follow-up echocardiogram documented bursts of atrial flutter (figure 1) with rates up to 496 bpm and 2:1 and 3:1 variable block, which resulted in a ventricular rate of 165.250 bpm. Owing to the non-sustained runs of atrial flutter, sotalol 120 mg every 12 h, was added and digoxin dose was continued at 250 µg every 8 h. Sotalol can prolong the QT interval, and maternal QT interval was monitored; it remained normal. The fetus continued with intermittent blocked and conducted PAC but no recurrence of the SVT or atrial flutter. The patient was discharged on the same doses of digoxin and sotalol.
Figure 1.
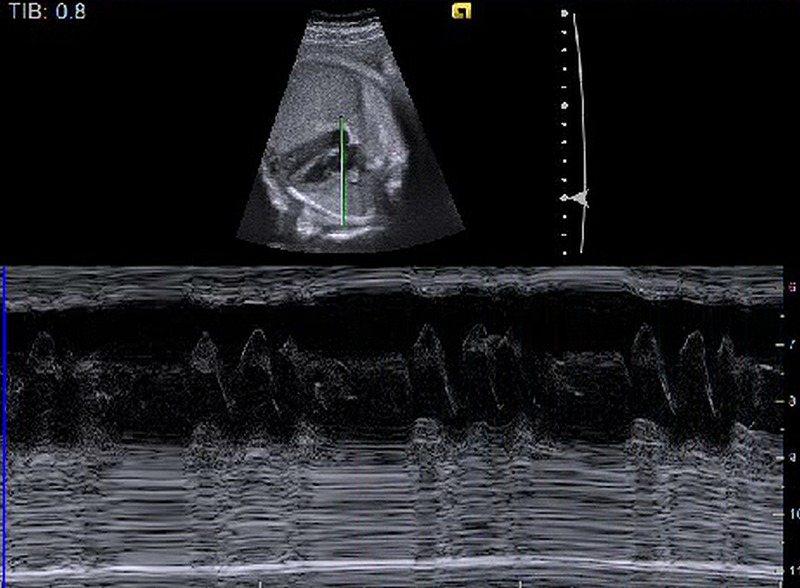
Fetal echocardiography showing arrhythmia.
The patient contacted the clinic at 38 weeks gestation reporting a ‘hacking cough that just won't go away’, present for the last 4 days. Her three children were currently being treated with azithromycin for presumed pertussis after demonstrating similar symptoms over the past week. Pertussis was suspected and the patient was admitted to the hospital. Examination at that time showed oropharynx without erythema or exudate, no nasal exudate, clear eyes, lungs clear to auscultation bilaterally, and only mild cervical lymphadenopathy. Tdap vaccine was given. She had previously refused Tdap during her prenatal care but had planned to receive it post partum. Nasopharyngeal specimens collected for PCR returned positive for Bordetella pertussis.
Azithromycin can affect the cardiac conduction by prolonging the QTc. Owing to the concern of potential conduction side effects of the combination of azithromycin along with sotalol and digoxin increasing the risk of QTc prolongation, Torsade de pointe, ventricular fibrillation and bradycardia, we discontinued the sotalol and digoxin. Owing to the late gestational age, we proposed to induce labour and begin azithromycin. Since the patient and any affected family members would only be able to have contact with the newborn once the 5-day treatment course was completed, we planned to start cervical ripening and labour induction at 38 weeks and 2 days. Sotalol and digoxin were discontinued and azithromycin was initiated. Cervical ripening was achieved by using misoprostol 25 µg Q6. The patient delivered a healthy 2804 g female infant in an isolated room; the baby's Apgar score was 9 and 9 at first and fifth minutes of life, and under droplet precautions after 2 days of azithromycin treatment initiation. The infant was transferred to the neonatal intensive care unit for monitoring of heart rhythm but did not demonstrate any signs of pertussis. The infant continued to have isolated PAC but no recurrence of the atrial flutter or SVT, and remained off antiarrhythmic medication. At 3 weeks of age, the infant had follow-up Holter and was having non-sustained runs of SVT at the rate 300 bpm. The patient, her husband and her three children all completed an antibiotic course, and were able to be in contact with the infant after treatment was completed. The infant and mother were both discharged without any complication.
Discussion
We presented a case of prenatal maternal pertussis that reflects the trend of increasing incidence of the disease in the USA. It is always of great concern how to manage different diseases, such as several types of cancers, infections or other conditions, during pregnancy, since some treatments may affect both the mother and the fetus. Treatment in this particular case was complicated by ongoing treatment of fetal SVT and atrial flutter preventing concurrent treatment with usual macrolide antibiotic, which is a challenging decision in obstetrics. This plan could impose a significant risk of maternal arrhythmia, especially QTc prolongation, causing torsade de pointe, ventricular arrhythmia or maternal bradycardia.3 Gomes et al3 demonstrated, in their population-based study, that combination of macrolides with digoxin induced digoxin toxicity with OR up to 14.8. Slightly lower risk was attributed to azithromycin with OR of toxicity of 3.7. Initiation of azithromycin therapy after discontinuing sotalol and digoxin turned out to be successful in the treatment for mother and baby in our case. Stopping sotalol and digoxin increased the risk of recurrence of fetal arrhythmia, especially after a few days, because of the half-life of medications. Therefore, the plan was to deliver the fetus within 2–3 days. Meanwhile, we started the azithromycin to treat pertussis, and our goal was to continue treatment during labour induction for 2–3 days, while also avoiding a prolonged isolation between mother and infant. Since our patient had three prior vaginal deliveries, our goals were successfully achieved.
The recent resurgence of pertussis in the USA has focused attention on infants too young to complete their primary immunisation series, particularly those aged 3 months and younger, because these infants are at highest risk of pertussis infection and pertussis-related morbidity and mortality.2 Contributing to this pertussis resurgence is the fact that while natural and vaccine-induced pertussis antibodies wane, they wane more rapidly after receipt of the less reactogenic acellular pertussis vaccines used in many resource-rich countries, than with whole cell pertussis vaccines.1 4 Further, it is estimated that approximately 75% of pertussis-infected infants acquire their infection from a household contact, most commonly (33% of cases) from their mother.5 The CDC first recommended targeted immunisation of postpartum women and infant contacts (cocooning) to prevent infant pertussis in 2006. Logistical and financial barriers precluded implementation of cocooning at a national level, however, and postpartum vaccination alone did not reduce infant infection.6 This resulted in the consideration of other strategies, specifically immunisation of the pregnant woman.
There is indirect evidence from the prevaccine era and from maternal immunisation studies in the 1940s, that if the concentration of pertussis-specific IgG in newborn infants is high, infants are protected from acquiring pertussis during the first few months of life. In addition, numerous studies demonstrate that both natural and Tdap-induced pertussis-specific IgG is both passively and actively transported across the placenta.5 7 In 2011, the CDC recommended Tdap for pregnant women who had not previously received it. Reports that pertussis antibodies in infants of mothers who received Tdap within the prior 2 years were likely too low for infant protection,8 combined with continued pertussis-related infant morbidity and mortality, resulted in the updated CDC recommendation that Tdap vaccine be administered during every pregnancy, preferably between weeks 27 and 36 of gestation. This timing of vaccination promotes optimal placental transfer of maternal antibodies to the fetus via the placenta, and studies are ongoing to demonstrate the efficacy of this approach in preventing neonatal infection. Our patient had refused Tdap vaccination during prenatal care so she was vulnerable to acquiring infection, leading to increased risk for her and her fetus and the management issues outlined here. This case highlights the importance of administering Tdap vaccine during pregnancy to prevent maternal morbidity and life-threatening infant infection.
Transmission of pertussis to a newborn may be unrecognised, because the infected adult may have mild illness and the incubation period ranges from 5 to 21 days. Newborns are most often infected by their acutely ill mothers, who tend to have lingering and sometimes debilitating symptoms for weeks after bacteria are cleared. If delivery is to occur while the mother is in the infectious stage, the following treatment is recommended: isolate infant from mother until mother is no longer infectious; antibiotic prophylaxis to mother to decrease length of infectivity and to the infant; rapid and efficient identification and prophylaxis for exposed family members, contacts and healthcare workers.9
Our case is of particular clinical interest since the planned vaginal delivery was important to minimise the risks of the combination of medications (azithromycin and sotalol/digoxin), to reduce the duration of absent fetal antiarrhythmic treatment and to decrease the isolation time between mother and newborn.
Learning points.
A well-planned delivery in a patient with prenatal diagnosis of maternal infection prevents neonatal infection.
Prenatal care should include vaccination history and administration if not performed earlier.
Concurrent management of the intrauterine arrhythmia and antibiotic administration may be required for a short period of time during pregnancy.
Acknowledgments
We acknowledge Nancy A. Ayres M.D. and Catherine M. Healy M.D. for their mentoring in preparing this manuscript.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Allen A. Public health. The pertussis paradox. Science 2013;341:454–5. 10.1126/science.341.6145.454 [DOI] [PubMed] [Google Scholar]
- 2.[No authors listed] ACOG Committee Opinion No. 566: update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol 2013;121:1411–14. 10.1097/01.AOG.0000431054.33593.e3 [DOI] [PubMed] [Google Scholar]
- 3.Gomes T, Mamdani M, Juurlink D. Macrolide-induced digoxin toxicity: a population-based study. Clin Pharmacol Ther 2009;86:383–6. 10.1038/clpt.2009.127 [DOI] [PubMed] [Google Scholar]
- 4.Misegades LK, Winter K, Harriman K et al. Association of childhood pertussis with receipt of 5 doses of pertussis vaccine by time since last vaccine dose, California, 2010. JAMA 2012;308:2126–32. 10.1001/jama.2012.14939 [DOI] [PubMed] [Google Scholar]
- 5.Healy CM, Baker CJ. Infant pertussis: what to do next? Clin Infect Dis 2012;54:328–30. 10.1093/cid/cir846 [DOI] [PubMed] [Google Scholar]
- 6.Castagnini LA, Healy CM, Rench MA et al. Impact of maternal postpartum tetanus and diphtheria toxoids and acellular pertussis immunization on infant pertussis infection. Clin Infect Dis 2012;54:78–84. 10.1093/cid/cir765 [DOI] [PubMed] [Google Scholar]
- 7.Van Rie A, Wendelboe AM, Englund JA. Role of maternal pertussis antibodies in infants. Pediatr Infect Dis J 2005;24:S62–5. 10.1097/01.inf.0000160915.93979.8f [DOI] [PubMed] [Google Scholar]
- 8.Healy CM, Rench MA, Baker CJ. Importance of timing of maternal Tdap immunization and protection of young infants. Clin Infect Dis 2013;56:539–44. 10.1093/cid/cis923 [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Pediatrics. Pertussis. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. Red book:2012 report of the committee on infectious diseases. 29th edn Elk Grove Village, IL: American Academy of Pediatrics, 2012:553–65. [Google Scholar]


