Abstract
We present a case of a patient with a diagnostic dilemma who was referred for possible faecal microbiota transplantation (FMT) for refractory diarrhoea secondary to Clostridium difficile infection (CDI). On detailed history, the patient was exposed to ipilimumab concomitantly while being treated for CDI, and was instead diagnosed with diarrhoea secondary to superimposed ipilimumab-associated colitis. Ipilimumab is an anti-CTLA4 monoclonal antibody approved for use in metastatic melanoma and under trial for other indications. Ipilimumab is associated with several immune-related adverse effects, of which diarrhoea and colitis are the most common. While FMT has shown tremendous efficacy in managing recurrent and refractory CDI, it was not offered in this case due to negative C. difficile testing showing a high degree of suspicion for ipilimumab-associated colitis due to recent drug use. Our patient was successfully managed with fluid resuscitation and steroids, and remains symptom free at last follow-up at 9 months.
Background
Clostridium difficile infection (CDI) is the common cause of hospital-acquired infection in the USA,1 with increasing incidence and severity, in the hospital as well as in community settings.2 Extraintestinal infections have also been recently reported.3 CDI can be associated with multiple recurrences and faecal microbiota transplantation (FMT) has emerged as an efficacious therapeutic option for recurrent and refractory CDI.4 Ipilimumab is an anti-CTLA4 monoclonal antibody that is being investigated in the treatment of several cancers. It upregulates the T-cell immune response and is associated with several immune-related adverse effects (irAEs).5 Autoimmune diarrhoea and colitis are among the most common irAEs reported with ipilimumab use.6 We present a patient suffering from refractory diarrhoea with recent CDI, who was referred for FMT, but diagnosed and managed as ipilimumab-associated colitis.
Case presentation
A 72-year-old man with a 6-month history of watery diarrhoea was referred for possible FMT for refractory CDI. His medical history was significant for high-grade non-Hodgkin's lymphoma, treated with rituximab and multiple chemotherapy agents including experimental drugs. Three months after onset of diarrhoea, his stool tested positive for CDI, and he was treated with two 14-day courses of metronidazole, two courses of 14-day oral vancomycin and one course of prolonged vancomycin taper, with minimal symptom improvement. During this time, two stool tests were positive and no tests were negative for CDI. A colonoscopy performed at an outside hospital demonstrated pancolitis (erythema, granularity and decreased vascularity); histology showed neutrophilic infiltration with no microscopic evidence of pseudomembranes. The patient denied abdominal pain or haematochezia. Further questioning revealed that he had been initiated on ipilimumab and nivolumab therapy (experimental use), 3 months earlier, after failing multiple approved lymphoma therapies. He received a total of four doses of ipilimumab (each dose 3 mg/kg intravenously) administered every 3 weeks, most recently 3 weeks before the present evaluation. His symptoms included 18–20 watery stools a day with faecal urgency and occasional incontinence.
General and systemic physical examination revealed evidence of dehydration and decreased rectal tone, but was otherwise normal. No skin rash was noted.
Investigations
Laboratory evaluation showed normocytic anaemia with haemoglobin 8.9 g/dL and C reactive protein 8.1 mg/L (normal <8 mg/dL). Liver function tests were in the normal range. Stool studies performed to rule out infectious causes of diarrhoea demonstrated presence of faecal leucocytes but were negative for C. difficile PCR, enteric pathogens culture, Cryptosporidium, Cyclospora and Microsporida. Given the unremitting severe and chronic nature of the symptoms, a decision to proceed with lower gastrointestinal endoscopy was taken. Flexible sigmoidoscopy demonstrated granularity and loss of vascular pattern, suggestive of colitis (figure 1). Biopsies demonstrated mild active chronic colitis with neutrophilic infiltration without crypt distortion. Cytomegalovirus immunostaining was negative.
Figure 1.
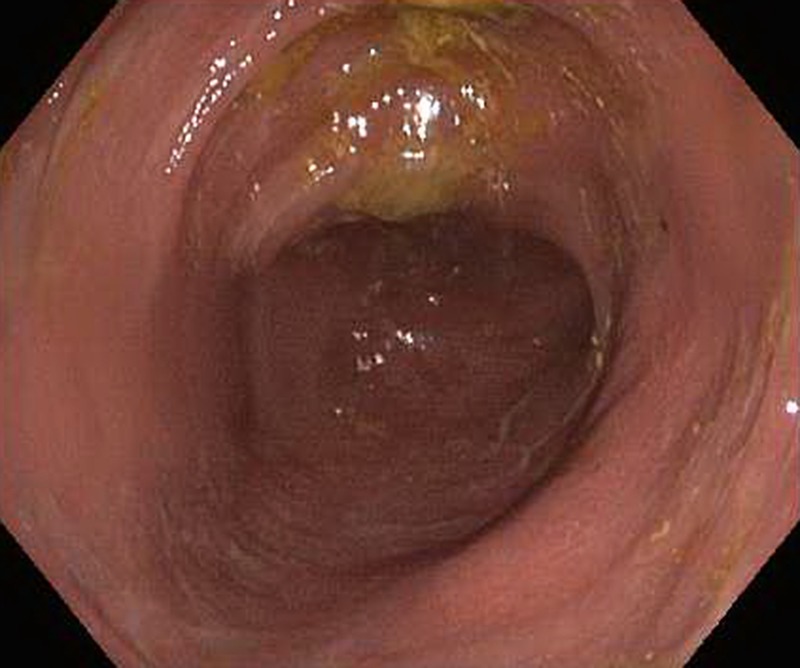
Initial flexible sigmoidoscopy demonstrating granularity and loss of vascular pattern in the sigmoid colon, suggestive of colitis.
Differential diagnosis
The patient had a known diagnosis history of CDI, which was diagnosed 3 months after onset of diarrhoea, and there was high suspicion of recurrent and refractory CDI. However, a stool C. difficile toxin assay was negative and multiple other stool tests did not reveal an infectious aetiology for the diarrhoea. The patient had experienced 3 months of diarrhoea symptoms (started 6 months before presentation) before being tested and diagnosed with CDI; it is likely that he had untreated CDI for this length of time, as untreated CDI can lead to chronic diarrhoea. However, since he lost response to CDI therapies and was exposed to ipilimumab (3 months before presentation), suspicion was raised for an alternate diagnosis. Furthermore, non-response to vancomycin is unusual in patients with CDI, which also supports alternative diagnoses.7 Given the ipilimumab exposure and timeline, negative infectious studies, and endoscopic and histopathological findings, a diagnosis of ipilimumab-associated colitis was entertained.
Treatment
As the patient was dehydrated, he required hospital admission; he was resuscitated with intravenous hydration and symptomatic therapy, and oral loperamide was initiated and continued as needed. Definitive therapy for ipilimumab-associated colitis included parenteral methylprednisone 60 mg daily for 4 days, followed by an oral prednisone taper (starting at 40 mg/day; tapered by 5 mg/week).8 9
Outcome and follow-up
The frequency and volume of diarrhoea improved considerably on the intravenous methylprednisone, and the oral prednisone was associated with further improvement of stool consistency and decreased frequency. A follow-up flexible sigmoidoscopy performed after completion of steroid therapy demonstrated a normal vascular pattern, but mildly erythematous mucosa in the sigmoid colon with mild colitis on biopsy (figure 2). The patient had two to three formed bowel movements a day at last follow-up. He was advised to watch for symptoms of recurrent diarrhoea and to report if the diarrhoea returned. Ipilimumab was not restarted in our patient.
Figure 2.
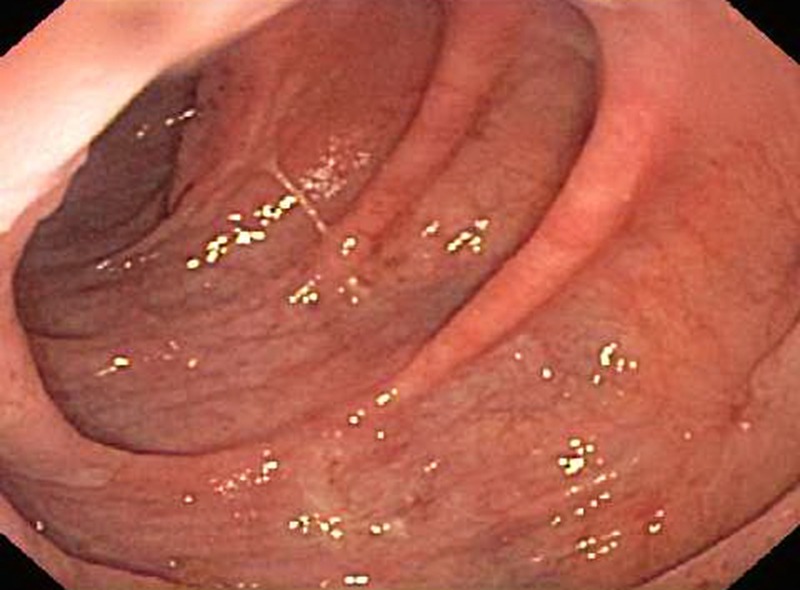
Follow-up flexible sigmoidoscopy demonstrating a normal vascular pattern with mildly erythematous mucosa in the sigmoid colon.
Discussion
Studies have highlighted increasing incidence and severity of CDI,10 particularly in patients with cancer, who possess multiple risk factors for severe and recurrent disease.11 Clinicians must maintain a low threshold for C. difficile testing in high-risk patients. Our patient initially tested positive for C. difficile and was treated with multiple courses of medications for CDI, with minimal symptom improvement. However, the diarrhoea did not resolve and subsequent C. difficile testing was negative. In the interim, he was also exposed to ipilimumab.
Ipilimumab is an anti-CTLA4 agent with a novel immunostimulatory mechanism of action, which predisposes patients to developing irAEs.5 In a patient with a history of anti-CTLA4 medication administration and diarrhoea, a high degree of suspicion for a gastrointestinal irAE should be maintained. The time from ipilimumab administration to developing diarrhoea or colitis is variable, with reports of symptom onset after anywhere from 1 to 10 doses of ipilimumab, with no predictable pattern.5 The most common symptom is watery diarrhoea and, less commonly, abdominal pain, bloody diarrhoea, nausea, vomiting or fever.5
Nivolumab is a monoclonal antibody directed against the programmed death (PD) receptor, approved for use in melanoma and non-small cell lung cancer. The frequencies of irAEs have generally been reported to be lower compared with anti-CTLA4 antibodies.12 Toxicities reported for anti-PD-1 agents include rash, pruritus, arthralgia and diarrhoea and colitis, in less than 1% patients.13 14
In patients who are exposed to ipilimumab who present with diarrhoea, infectious causes must be ruled out; and stool tests for enteric pathogens and C. difficile must be performed. Ipilimumab-associated colitis and infection may coexist, as demonstrated in a case of a biopsy confirming neutrophilic colitis with stool cultures growing Salmonella spp.15
Given our patients’ history of positive C. difficile PCR, current negative C. difficile PCR assay and unresolved symptoms with antimicrobial therapy, which have over 95% resolution rates, he may have initially had a true CDI that was treated but did not lead to complete symptom relief due to concomitant ipilimumab-associated colitis. CDI alone did not explain his ongoing symptoms, and the temporal relationship with ipilimumab administration, endoscopic findings and improvement with steroids, supported the diagnosis of ipilimumab-associated colitis. It is also important to look for non-colitis related irAEs in these patients, as these may frequently coexist with colitis.6 8 Skin rash and liver dysfunction can occur, and these were ruled out by a careful examination and laboratory testing, respectively.
Treatment of grades 1 and 2 diarrhoea in ipilimumab-associated colitis is largely supportive, with fluid and electrolyte replenishment, and antidiarrhoeals.8 9 Patients with persistent grade 2 symptoms or grades 3 and 4 symptoms deserve endoscopic evaluation. Our patient presented with grade 3 diarrhoea (an increase of >7 stools/day over baseline; Common Terminology Criteria for Adverse Events, V.4).16 Extensive diffuse inflammatory changes such as exudates, granularity, loss of vascular pattern and ulcerations, may be seen, although a normal endoscopic examination does not rule out a diagnosis of ipilimumab-associated colitis.6 If an endoscopic examination is performed, mucosal biopsies must be obtained. Histopathology often reveals features of diffuse active colitis with infiltrates of neutrophils, lymphocytes and plasma cells in the lamina propria, together with crypt abscesses and mucosal ulcerations, although these are not pathognomic.17
Patients with grade 3 or 4 diarrhoea require systemic corticosteroid therapy. Intravenous methylprednisolone is usually the first line of therapy, followed by an oral prednisone course, tapered over 6–8 weeks. Patients not responding to steroid therapy may benefit from a single dose of infliximab.18
Although most patients have a mild presentation and respond to appropriate therapy, severe colitis requiring colectomy and sometimes causing death have been reported.19 Our patient's symptoms responded well to steroid therapy with follow-up sigmoidoscopy demonstrating improvement. Clinicians must be aware of irAEs and their management in patients exposed to ipilimumab.
Learning points.
Ipilimumab is an anti-CTLA4 monoclonal antibody, used in melanoma, that upregulates T-cell immune response and may be associated with several immune-related adverse effects (irAEs), of which diarrhoea and colitis are the most common.
In patients with a history of anti-CTLA4 exposure and diarrhoea, gastrointestinal irAEs should always be suspected.
Infectious causes of diarrhoea must be ruled out before a diagnosis of ipilimumab-induced diarrhoea/colitis can be made.
Treatment of grades 1 and 2 diarrhoea in ipilimumab-associated colitis is largely supportive with fluid and electrolyte repletion, and loperamide.
Patients with grade 3 or 4 diarrhoea require systemic corticosteroid therapy, and steroid-resistant cases may benefit from a single dose of infliximab.
Footnotes
Contributors: AG and SK were involved in the conception and design, acquisition of data or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and gave final approval of the version published.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Magill SS, Edwards JR, Bamberg W et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014;370:1198–208. 10.1056/NEJMoa1306801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Khanna S. Community-acquired Clostridium difficile infection: an increasing public health threat. Infect Drug Resist 2014;7:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Patel R, Baddour LM et al. Extraintestinal Clostridium difficile infections: a single-center experience. Mayo Clin Proc 2014;89:1525–36. 10.1016/j.mayocp.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 4.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011;53:994–1002. 10.1093/cid/cir632 [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck KE, Blansfield JA, Tran KQ et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006;24:2283–9. 10.1200/JCO.2005.04.5716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna S, Pardi DS. Clostridium difficile infection: management strategies for a difficult disease. Therap Adv Gastroenterol 2014;7:72–86. 10.1177/1756283X13508519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Felice KM, Gupta A, Rakshit S et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res 2015. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, De Felice KM, Loftus EV Jr et al. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther 2015; in press. [DOI] [PubMed] [Google Scholar]
- 10.Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol 2010;4:409–16. 10.1586/egh.10.48 [DOI] [PubMed] [Google Scholar]
- 11.Chopra T, Alangaden GJ, Chandrasekar P. Clostridium difficile infection in cancer patients and hematopoietic stem cell transplant recipients. Expert Rev Anti Infect Ther 2010;8:1113–19. 10.1586/eri.10.95 [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol 2014;11:91–9. 10.1038/nrclinonc.2013.245 [DOI] [PubMed] [Google Scholar]
- 14.Gibney GT, Kudchadkar RR, DeConti RC et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res 2015;21:712–20. 10.1158/1078-0432.CCR-14-2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCutcheon JL, McClainb CM, Puzanovc I et al. Infectious colitis associated with ipilimumab therapy. Gastroenterol Res 2014;7:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. US DEPARTMENT OF HEALTH AND HUMAN SERVICES. National Institutes of Health. National Cancer Institute [cited 28 May 2009 (v4.03: 14 June, 2010)]. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5×11.pdf.
- 17.Berman D, Parker SM, Siegel J et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun 2010;10:11. [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston RL, Lutzky J, Chodhry A et al. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig Dis Sci 2009;54:2538–40. 10.1007/s10620-008-0641-z [DOI] [PubMed] [Google Scholar]
- 19.Babi C, Jaclyn S, Mark N, John H et al. Ipilimumab induced enterocolitis: a fatal immune-related adverse event of melanoma treatment. J Gastroenterol Hepatol Res 2013;2:934–6. [Google Scholar]


