Abstract
The development of functional engineered tissue constructs depends on high cell densities and appropriate vascularization. In this study we implemented a four-compartment three-dimensional perfusion bioreactor culture model for studying the effects of medium perfusion on endothelial, hepatic, and hematopoietic cell populations of primary human fetal liver in an in vivo-like environment. Human fetal liver cells were cultured in bioreactors configured to provide either perfusion or diffusion conditions. Metabolic activities of the cultures were monitored daily by measuring glucose consumption and lactate production. Cell viability during culture was analyzed by lactate dehydrogenase activity. Hepatic functionality was determined by the release of albumin and alpha-fetoprotein (AFP) in culture medium samples. After 4 days of culture, cells were analyzed for the expression of a variety of endothelial, hepatic, and hematopoietic genes, as well as the surface marker expression of CD31 and CD34 in flow cytometry. We found that medium perfusion increased the gene expression of endothelial markers such as CD31, von Willebrand factor (vWF), CD140b, CD309, and CD144 while decreasing the gene expression of the erythrocyte-surface marker CD235a. Hepatic differentiation was promoted under perfusion conditions as demonstrated by lower AFP and higher albumin secretion compared with cultures not exposed to medium perfusion. Additionally, cultures exposed to medium perfusion gave higher rates of glucose consumption and lactate production, indicating increased metabolic activity. In conclusion, high-density bioreactors configured to provide constant medium perfusion significantly induced hepatic and endothelial cell differentiation and provided improved conditions for the culture of human fetal liver cells compared with cultures without perfusion.
Introduction
The shortage of donor organs for transplantation in chronic liver disease is a significant problem in medicine and limits the lifespan of patients in need of organ transplantation. This combined with the long waiting time for organ recipients suggests a clinical need for alternative therapies such as transplantable tissue constructs.1
Early liver cell cultures were introduced in the 1950s using monolayers of mouse cells2 and were followed by the development of suspension cultures.3 The introduction of sandwich cultures of liver cells in between two layers of collagen I4–6 more closely simulated the in vivo liver plate architecture. To move toward the higher cell densities that are required for the growth of liver tissue, more advanced three-dimensional (3D) culture systems that mimic the in vivo situation are required. Perfusion bioreactors are an example of such a system, combining three-dimensionality with medium perfusion to provide a tissue-like environment, and have been used to culture primary adult human liver cells,7 mouse8 and human fetal liver cells,9 human fetal hepatocytes,10 and were used to promote the hepatic differentiation of embryonic stem cells.11 While these systems are promising, one of the challenges facing further development is the requirement of proper vascularization to overcome mass exchange limitations and enable survival of large cell masses over extended periods of time in the bioreactors.
In the fetal liver, angiogenesis occurs in hematopoietic and hepatic tissues that develop simultaneously, making it a model system to study vascularization within an organ context. In particular, this is due to the presence of endothelial progenitor cell populations, which have been shown to form blood vessels in vivo.12,13 The liver is a highly perfused organ, with perfusion rates around 1 mL/min/g of tissue14 and blood velocities of 0.4–0.45 mm/s in the sinusoids,15 150 mm/s in the portal vein,16 and 600 mm/s in the hepatic artery.17 It has already been demonstrated that flow improves the culture and metabolism of rat hepatocytes in bioreactors,18–20 but no such studies exist for human fetal liver and its distinct cell populations. In addition, there are numerous studies detailing the beneficial effect of shear forces from flow on mature endothelial cells21–24 as well as endothelial progenitors derived from embryonic stem cells25 and the bone marrow.26 Since the developing fetal liver becomes vascularized and perfused, the forces that are generated by medium flow inside of a 3D perfusion bioreactor could be important for the differentiation of fetal liver cells into mature liver tissue.
The goal of this study is to examine the effects of perfusion conditions in an in vivo-like four-compartment 3D bioreactor culture model that provides integral oxygenation to human fetal liver cells and investigate the effects of such an environment on the specific cell types of the liver, that is, hepatic, hematopoietic, and endothelial populations.
Materials and Methods
Liver cell isolation
Human fetal liver cells of 16–20 weeks of gestational age were obtained as an anatomical gift from the Allegheny Reproductive Health Center (Pittsburgh, PA). Organs were retrieved from abortions after informed consent of the donor family and approval of the local Institutional Review Board. Liver cells were isolated from the primary tissue as described previously with slight modifications.27 The livers were digested with collagenase type IV (Sigma-Aldrich, St. Louis, MO) at 37°C and resuspended in a supplemented RMPI 1640 medium27; supplements included 0.1% bovine serum albumin (BSA; fatty acid-free), 30 nM selenium, 540 μg/mL niacinamide, 5 ng/mL insulin, 10 ng/mL transferrin, free fatty acid mixture (2.36 μM palmitic acid, 0.21 μM palmitoleic acid, 0.88 μM stearic acid, 1.02 μM oleic acid, 2.71 μM linoleic acid, 0.43 μM linolenic acid), 0.1 μM hydrocortizone, 50 μM β-mercaptoethanol (Sigma-Aldrich), 2 mM glutamax, and antibiotic–antimycotic mixture (Life Technologies, Carlsbad, CA). The total cell number and viability were determined using a Neubauer chamber and Trypan Blue (Life Technologies) exclusion.
Bioreactor and perfusion system
The hollow fiber-based multicompartment bioreactor used in this study is a scaled-down two-capillary layer prototype of the clinical and laboratory scale reactors that have been described previously11 and consists of three independent yet interwoven hollow fiber capillary systems that provide integral oxygenation and a 3D network for cell immobilization and perfusion in the extracapillary space. Two sets of hydrophilic capillaries made of polyethersulfone (MicroPES TF 10; Membrana, Wuppertal, Germany) with a maximum pore size of 0.5±0.1 μm, an outer diameter of 500±40 μm, and an inner diameter of 300±40 μm were used for medium supply whereas a hydrophobic composite hollow fiber membrane system (Mitsubishi, Tokyo, Japan) with an outer diameter of 280±10 μm and an inner diameter of 200±10 μm was used to enable gas exchange. The capillary systems were integrated into a bioreactor housing fabricated from a two-component medical grade polyurethane (PUR-System 725A/725B; Rohm and Haas, Bremen, Germany). Such a configuration exposes cells that are located in the extracapillary space to decentralized medium supply with high mass exchange rates and direct membrane oxygenation. Injection of the cells into the bioreactor was accomplished using a flow head connected to silicone rubber capillaries leading directly into a 1 mL cell compartment. The small cell compartment allows lower cell numbers to be inoculated for culture. Sterilization of the bioreactors and associated tubing was performed with ethylene oxide according to clinical standards. Details of the perfusion system used to control the medium and gas flow in the system have been described in detail previously.11
Three-dimensional perfusion bioreactor cultures
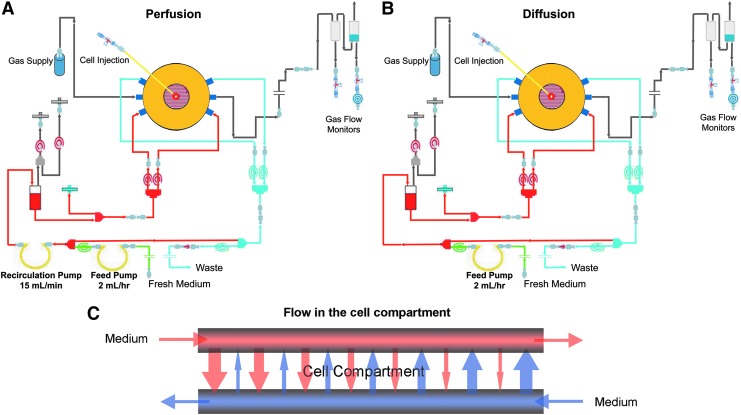
A total of 400 million human fetal liver cells in 1 mL of supplemented RPMI 1640 medium with 20 mM HEPES buffer were inoculated into each bioreactor (StemCell Systems, Berlin, Germany) using a needle and syringe. Two bioreactor flow configurations were used per experimental repeat: one with and one without medium recirculation at a rate of 15 mL/min. Both bioreactors were supplied with fresh medium at a feed rate of 2 mL/h. Figure 1 illustrates the medium flow path for bioreactor configurations with and without medium recirculation, hereafter referred to as perfusion and diffusion conditions, respectively.
FIG. 1.
Schematics of three-dimensional (3D) bioreactor configurations designed to provide cells with (A) perfusion and (B) diffusion conditions under a constant medium feed rate. Cells in configuration (A) were subjected to a constant overall medium recirculation rate of 15 mL/min whereas those in configuration (B) were not exposed to such conditions due to the lack of a recirculation pump. Both reactors received fresh medium at a rate of 2 mL/h (green lines). The constant medium recirculation in (A) exposes cells to counter-directional cross-flow perfusion in the cell compartment whereas cells in (B) are only exposed to a slow, diffusive flow from the medium feed pump. (C) Detail of the bioreactor cell compartment illustrating medium and oxygenation fiber arrangement as well as the principle of medium perfusion in the cell compartment.
Culture pH was monitored daily by analyzing a sample of the culture medium from the reactor circuit on a clinical bloodgas analyzer (Cobas b 221; Roche, Indianapolis, IN) and was maintained in the physiological range of 7.35–7.45 by the action of HEPES buffer present in the culture medium.
Indirect cell viability assay
Viability of the cells during culture was indirectly measured by monitoring lactate dehydrogenase (LDH) activity in daily culture medium samples using the QuantiChrom LDH kit (BioAssay Systems, Hayward, CA) according to the manufacturer's protocol. LDH activities were normalized against the medium feed rate and system volume.
Measurement of metabolic parameters
The metabolic activity of cells in the bioreactor culture was measured daily using samples drawn from the bioreactor medium circuit. Glucose and lactate were simultaneously quantified using a clinical bloodgas analyzer (Cobas b221; Roche). Concentrations of these parameters were normalized against the medium feed rate and total system volume.
Bioreactor harvest
At the end of the culture period the bioreactors were removed from the perfusion devices and all tubing was disconnected. The reactors were transferred into a biosafety cabinet and the upper lid of the cell compartment was opened. A portion of the cells and fibers were removed for immunohistochemical analysis and the remainder of the cells and capillary layers were removed and placed into 0.05% trypsin-EDTA (Life Technologies) to dissociate cells into a single cell suspension for further analysis.
Alpha-fetoprotein and albumin enzyme-linked inmmunosorbent assays
Cell culture supernatants were analyzed for secreted albumin and alpha-fetoprotein (AFP) by sandwich enzyme-linked immunosorbent assays (ELISAs). The MaxiSorp Immunoplates (Nunc, Rochester, NY) were coated with anti-human albumin antibody (Bethyl Laboratories, Montgomery, TX) or anti-human AFP antibody (Abcam, Cambridge, United Kingdom) and incubated with samples or standards (albumin [Bethyl Laboratories]; AFP [MP Biomedicals, Solon, OH]) and conjugated with a goat anti-human albumin horseradish peroxidase-conjugated antibody (Bethyl Laboratories) or mouse anti-human AFP horseradish peroxidase-conjugated antibody (Abcam). Tetramethylbenzidine substrate solution was incubated for up to 10 min, the enzymatic reaction was stopped with 2 M sulfuric acid (Fisher Scientific, Pittsburgh, PA), and the absorbance was read at 450 nm with a Synergy H1 hybrid reader equipped with the Gen5 software version 2.00 (Bio-Tek, Winooski, VT).
Normalization of metabolic data
To account for metabolite dilution in the recirculation caused by fresh medium feed and to ensure that the metabolic data from the individual bioreactor runs were comparable, the raw data for glucose consumption and lactate production, as well as AFP and albumin secretion and LDH liberation were normalized by the system volume and feed rate for each bioreactor using the following calculation:
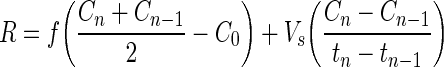 |
R, metabolite production rate (mg/h); f, medium feed rate (mL/h); Cn, metabolite concentration at time n (mg/mL); C0, concentration of metabolite at time 0 (mg/mL); Vs, volume of bioreactor plus tubing (mL).
This normalization accounts for any variations in system volumes and daily bioreactor medium feed rates.
Gene expression analysis
Gene expression analysis was performed on freshly isolated human fetal liver cells (day 0) as well as fractions harvested from the cell compartment of the bioreactors on day 4 of culture. Cells were lysed with RLT buffer and total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA). RNA was reverse transcribed to cDNA with a high capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). Gene expression was analyzed by real-time PCR using the StepOnePlus Real-Time PCR system and software version 2.0 (Applied Biosystems). Predesigned TaqMan probe and primer sets (all from Applied Biosystems) were used to quantify gene expression for CD34 (Hs00990732_ml), PTPRC (protein tyrosine phosphatase, receptor type, c, or CD45 [Hs04189704_ml]), GYPA (glycophorin A, or CD235a [Hs00266777_ml]), AFP (Hs_00173490_ml), ALB (albumin [Hs00609411_ml]), KRT19 (keratin 19, or CK19 [Hs00761767_sl]), CYP3A4 (Hs00430021_ml), CYP3A7 (Hs00426361_ml), NOS3 (nitric oxide synthase 3, or eNOS [Hs01574659_ml]), CDH5 (cadherin 5, type 2, or CD144 [Hs00901463_ml]), KDR (kinase insert domain receptor, or CD309 [Hs00911700_ml]), PDGFRB (platelet-derived growth factor receptor, beta polypeptide, or CD140b [Hs01019589_ml]), PECAMI (platelet/endothelial cell adhesion molecule 1, or CD31 [Hs00169777_ml]), VWF (von Willebrand factor [Hs00169795_ml]), SMPD4 (sphingomyelin phosphodiesterase 4, or neutral sphingomyelinase-3 [Hs04187047_gl]), and ACTB (actin, beta, or beta-actin [HS99999903_ml]) using the ddCt method. Beta-actin served as a housekeeping gene for internal normalization, and negative polymerase chain reaction (PCR) controls included no template (water). Total human fetal liver RNA from freshly isolated day 0 cells was used as a relative quantitative normalizer.
Flow cytometry
Surface marker expression of cells from the initial (day 0) and cultured (day 4) suspensions was investigated using flow cytometry. For each condition, 1 million cells were incubated with blocking buffer containing 20% FcR block (Miltenyi Biotec, Auburn, CA), 0.5% BSA, and 2 mM EDTA (Sigma-Aldrich) in Dulbecco's phosphate buffered saline without calcium and magnesium (Invitrogen, Carlsbad, CA), and then labeled with monoclonal antibodies directly conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), AlexaFluor 647 (AF647), AlexaFluor 488 (AF488), or V450. The monoclonal antibodies (all from BD Biosciences) were as follows: lin1-FITC, CD31-PE, CD34-AF700, CD309-AF647, and CD144-V450. Nonspecific binding with isotype-matched controls (Becton Dickinson, Bedford, MA) was used to establish gating. Analysis was performed using a FACSAria II flow cytometer (BD Biosciences) and the FlowJo software version 9.5.2 (Tree Star, Ashland, OR). Compensation beads (BD Biosciences) were used to compensate for fluorochrome spectral overlap. Negative controls included nonstained cells and isotype control stained cells. Isotype controls were mouse IgG1 PE, AF700, AF647, and V450 (all from Becton Dickinson).
Immunohistochemistry
At the end of the culture period, cells from within the cell compartment of the bioreactors were removed, embedded in O.C.T. compound (Sakura, Torrance, CA), frozen, and then cut into 10 μm sections. The frozen sections were fixed with either 4% paraformaldehyde or a 1:1 mixture of acetone and methanol. Sections were blocked with 10% goat serum (Sigma-Aldrich) and 1% FcR blocking reagent (Miltenyi Biotec) in phosphate buffered saline and subsequently stained with diamidinophenylindole dihydrochloride (Sigma-Aldrich) for cell nuclei, rabbit anti-CD31 (Abcam), mouse anti-vWF (Santa Cruz, Dallas, TX), and mouse anti-CD235a (Dako Cytomation, Glostrup, Denmark) primary antibodies and either AlexaFluor 555-conjugated goat anti-rabbit or AlexaFluor 488-conjugated goat anti-mouse secondary antibodies (Invitrogen). Sections of cells were analyzed by confocal microscopy (Fluoview 1000; Olympus, Center Valley, PA). Human adult and fetal liver tissue were used as positive controls and were treated the same as the cell samples.
Statistical analysis
Data are given as mean±standard error of the mean from five experiments. Significant differences were evaluated using the Student's t-test. ANCOVA was used to determine the significance of production or consumption rates of various metabolic parameters. A value of p<0.05 was considered statistically significant for all analyses.
Results
Cell viability
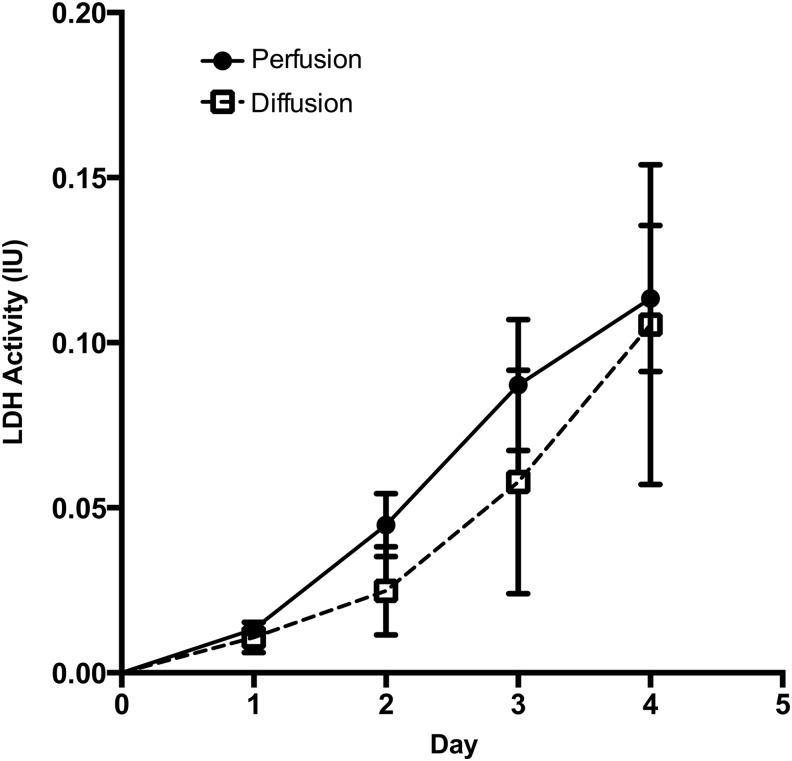
The average viability of freshly isolated cells was 90.10% as determined by Trypan Blue exclusion. During culture of the cells in bioreactors, cell viability was determined indirectly by analyzing daily culture medium samples for LDH activity (Fig. 2), an indicator of cell membrane integrity. LDH activity was not significantly different between perfusion and diffusion conditions and the cumulative LDH released for both conditions was rather low (<1 IU). The average viability of harvested cell fractions, as determined by Trypan Blue exclusion, was 89.90% for perfusion and 88.60% for diffusion conditions. Together, these results indicate that the bioreactor microenvironment was well suited for the culture of fetal liver cells.
FIG. 2.
Cell viability. Daily medium samples drawn from the bioreactor cultures were analyzed for lactate dehydrogenase (LDH) activity as an indirect measurement of cell viability. Data are given as means±standard error of the mean (SEM) for five biological repeats.
Flow cytometry of cell populations isolated from the fetal liver
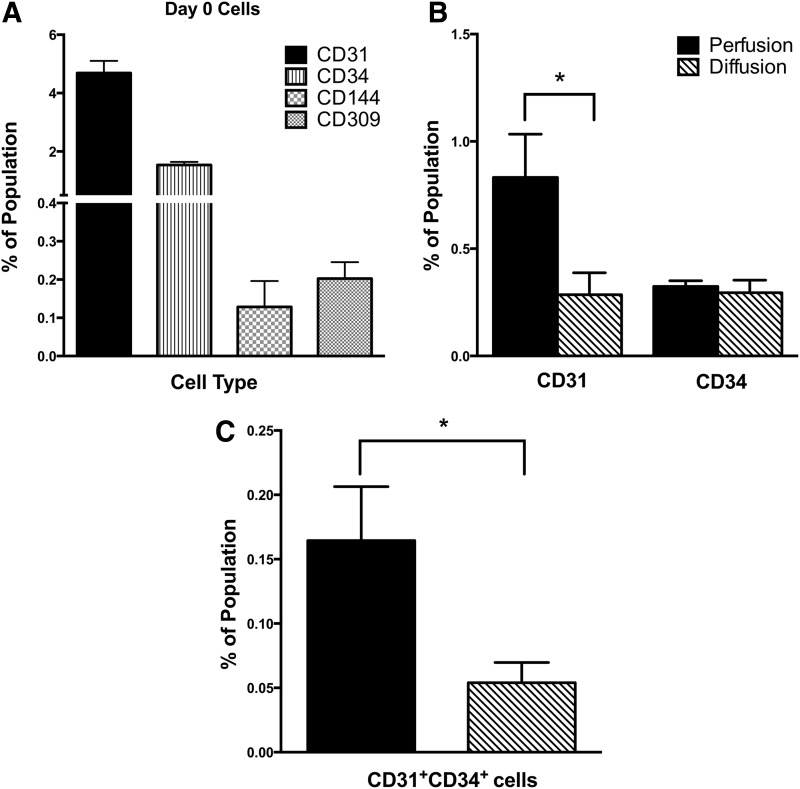
Flow cytometry performed on freshly isolated fetal liver cell suspensions (Fig. 3A) revealed that mature endothelial (CD31+), endothelial progenitor (CD144+, CD309+, CD31+CD34+), and hematopoietic (CD34+) populations were present on day 0. On average, the total fetal liver suspensions contained 4.68%±0.41% CD31+, 0.12%±0.06% CD144+, 0.20%±0.04% CD309+, 0.82%±0.10% CD31+CD34+, and 1.54%±0.10% CD34+ cells. Such populations could give rise to cells of endothelial and hematopoietic lineages during 3D bioreactor culture.
FIG. 3.
Fluorescence-activated cell sorting. Human fetal liver cells were analyzed before bioreactor culture on day 0 (A) and after 4 days of culture (B, C) in bioreactors configured to provide perfusion (dark bars) and diffusion (patterned bars) conditions during culture. Cell populations were analyzed for mature endothelial cells (CD31+), endothelial progenitors (CD309+, CD144+, CD31+CD34+), and hematopoietic cells (CD34+). Data are given as means±SEM for five biological repeats (*p<0.05).
Surface marker expression of cultured fetal liver cells
The cell surface expression patterns of various markers were analyzed using flow cytometric analysis on cells harvested from perfusion and diffusion bioreactors. After 4 days of culture, the expression of all of the markers was decreased relative to the day 0 cells. In particular, no CD309+ or CD144+ cells could be detected in the harvested cell fractions. Despite not being able to resolve CD309 or CD144 populations in these fractions, a significantly higher (p<0.05) percentage of cells in the perfusion condition were CD31+CD34+ (Fig. 3C), indicating that an endothelial progenitor-like population was maintained to a greater extent during culture in bioreactors with medium recirculation. Additionally, a significant (p<0.05) increase in the percentage of total CD31+ cells was observed in the perfusion condition (0.83%±0.20%) compared with the diffusion condition (0.28%±0.10%) (Fig. 3B). Three-dimensional perfusion conditions appear to better support the maintenance of both mature and progenitor endothelial cell populations when compared with diffusion conditions.
Liver-specific protein secretion
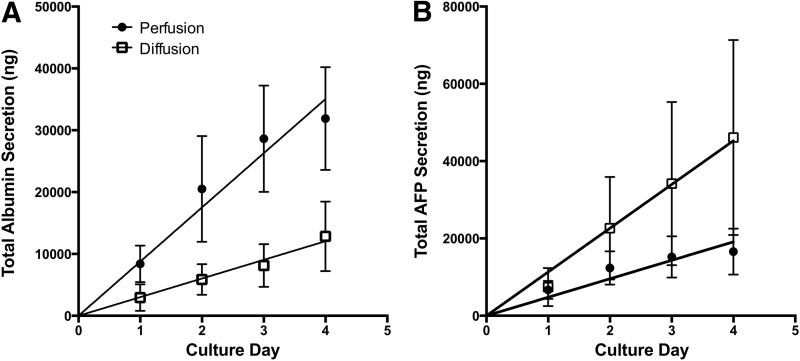
To investigate the effect of perfusion versus diffusion 3D microenvironments on liver-specific protein secretion, ELISAs were performed for albumin (Fig. 4A) and AFP (Fig. 4B), secreted by mature and fetal liver cells, respectively. Both culture conditions displayed steady albumin production, with the perfusion condition giving a significantly increased rate of secretion (p<0.0001) as determined by ANCOVA analysis of the linear regressions of the data. In contrast, AFP production appeared to plateau for the perfusion condition after 4 days, while steadily increasing in the diffusion bioreactor (p<0.01). The continued increase in albumin secretion coupled with the decrease in AFP secretion suggests that bioreactors with medium perfusion conditions promote differentiation of the fetal liver cells.
FIG. 4.
Albumin and alpha-fetoprotein (AFP) secretion. Daily medium samples from bioreactor cultures of human fetal liver cells exposed to perfusion (dark circles) and diffusion (squares) conditions in 3D bioreactors were analyzed using enzyme-linked immunosorbent assays for the levels of (A) albumin and (B) AFP secreted by the cells. Data are given as means±SEM for five biological repeats. Solid lines represent linear regressions of the data sets used for ANCOVA analysis.
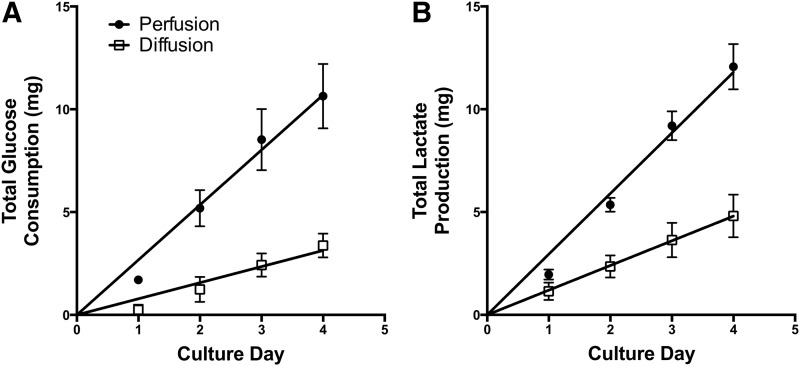
Metabolic activities in culture
Medium samples drawn from the recirculation stream of the bioreactors were analyzed daily throughout the 4 day culture period for concentrations of glucose and lactate as indicators of cellular metabolism. Figure 5A shows cumulative glucose consumption for the experimental conditions. In both conditions cells consumed glucose over the 4-day culture period with the perfusion condition showing a significantly increased rate of glucose consumption (p<0.0001). Lactate production rates correlated well with glucose consumption and displayed a similar trend, with the production rate of lactate (Fig. 5B) being much greater in the perfusion condition compared with the diffusion condition (p<0.0001). Overall, the metabolic activities of the cells cultured in bioreactors exposed to perfusion conditions were significantly increased relative to the diffusion condition.
FIG. 5.
Glucose and lactate metabolism. Human fetal liver cells in bioreactors configured to provide perfusion (dark circles) and diffusion (squares) conditions were monitored during culture for (A) glucose consumption and (B) lactate production. Data are given as means±SEM for five biological repeats. Solid lines represent linear regressions of the data sets used for ANCOVA analysis.
Additionally, the daily medium samples collected from the recirculation stream were analyzed for a variety of bloodgas parameters, including pH, pCO2, pO2, and [HCO3−] to ensure that physiological medium conditions were maintained in the bioreactors. The perfusion and diffusion bioreactors were supplied with oxygen from an external laboratory supply and the pH of the systems was regulated by the action of HEPES buffer in the culture medium. Both the perfusion and diffusion bioreactor systems experienced similar physiological pH conditions during the entire culture period (data not shown).
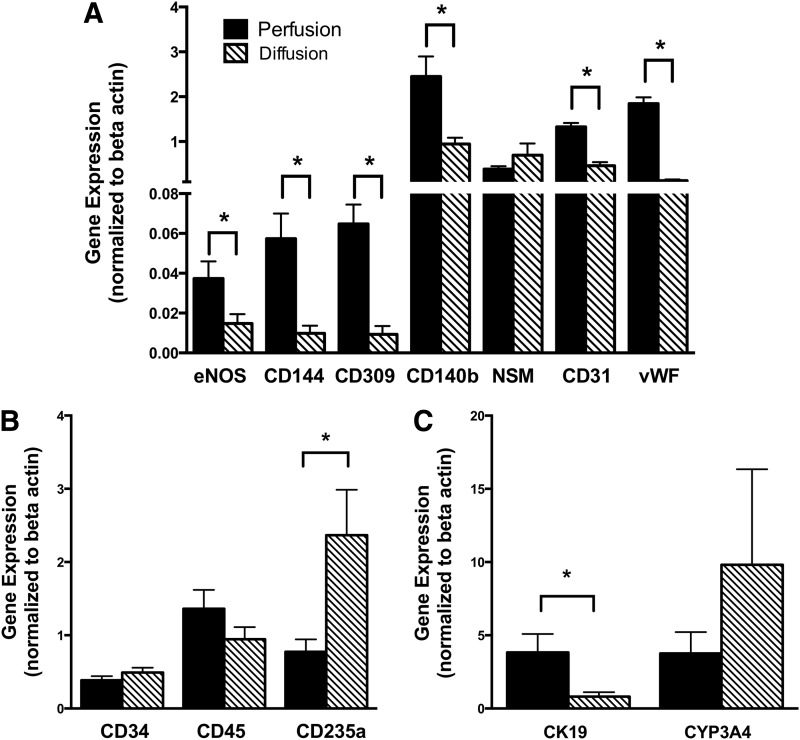
Endothelial gene expression
A number of endothelial, hematopoietic, and liver-specific genes were analyzed using real-time-PCR to determine any differences in gene expression between fetal liver cells exposed to perfusion and diffusion conditions in the bioreactor (Fig. 6A–C). Expression of known shear-sensitive genes, such as eNOS28,29 and CD140b30,31 was increased significantly in cells harvested from reactors exposed to perfusion conditions (p<0.05 for both). Neutral sphingomyelinase (NSM), which is localized on endothelial cell caveolae, responds in a transient fashion to shear stress.32 As expected, due to its known transient response, no differences were observed between the gene expressions of NSM in the experimental conditions after 4 days of culture. In addition, genes for mature endothelial markers such as vWF (p<0.0001) and CD31 (p<0.0001) as well as endothelial progenitors such as CD144 (p<0.05) and CD309 (p<0.05) were all significantly increased in the perfusion microenvironment, suggesting that the physical forces generated by constant medium recirculation affected endothelial differentiation.
FIG. 6.
Gene expression. Human fetal liver cells were analyzed after 4 days of culture in bioreactors configured to provide perfusion (dark bars) and diffusion (patterned bars) conditions during culture. Gene expression was normalized to day 0 freshly isolated human fetal liver cells, with beta-actin as a housekeeping gene. Harvested cells from the two conditions were analyzed for gene expression of (A) endothelial related genes: endothelial nitric oxide synthase (eNOS), CD144, CD309, CD140b, neutral sphingomyelinase (NSM), CD31, and von Willebrand factor (vWF), (B) hematopoietic genes: CD34, CD45, and CD235a, and (C) liver-related genes: CYP3A4 and CK19. Data are given as means±SEM for five biological repeats (*p<0.05).
Hematopoietic gene expression
Hematopoietic gene expression (Fig. 6B) was largely unaffected by the type of bioreactor culture condition, with both CD34 and CD45 genes displaying no significant differences. Interestingly, the expression of CD235a, a mature red blood cell marker, displayed a marked difference between perfusion and diffusion culture conditions (p<0.05). During fetal development hematopoiesis occurs in the fetal liver and gradually shifts into the bone marrow during the second trimester.33,34 The observed decrease in CD235a expression for the perfusion condition suggests decreased hematopoietic activity and maturation of the cells when compared with the diffusion condition.
Hepatic gene expression
Genes specific to mature and fetal liver function (Fig. 6C) were compared between the two conditions to investigate hepatic differentiation. CYP3A4 is the primary cytochrome P450 (CYP) of mature hepatocytes whereas CYP3A7 is associated with immature fetal parenchymal cells. CYP3A7 is replaced by CYP3A4 during postnatal development.35,36 In both perfusion and diffusion conditions CYP3A7 expression was not detected after 4 days of culture whereas CYP3A4 expression was upregulated relative to day 0 cells, although no significant difference was noted between experimental conditions (p=0.39). Expression of the bile duct epithelial marker CK19 was improved under perfusion conditions and gave a five-fold increase in expression relative to diffusion conditions after 4 days in culture (p<0.05).
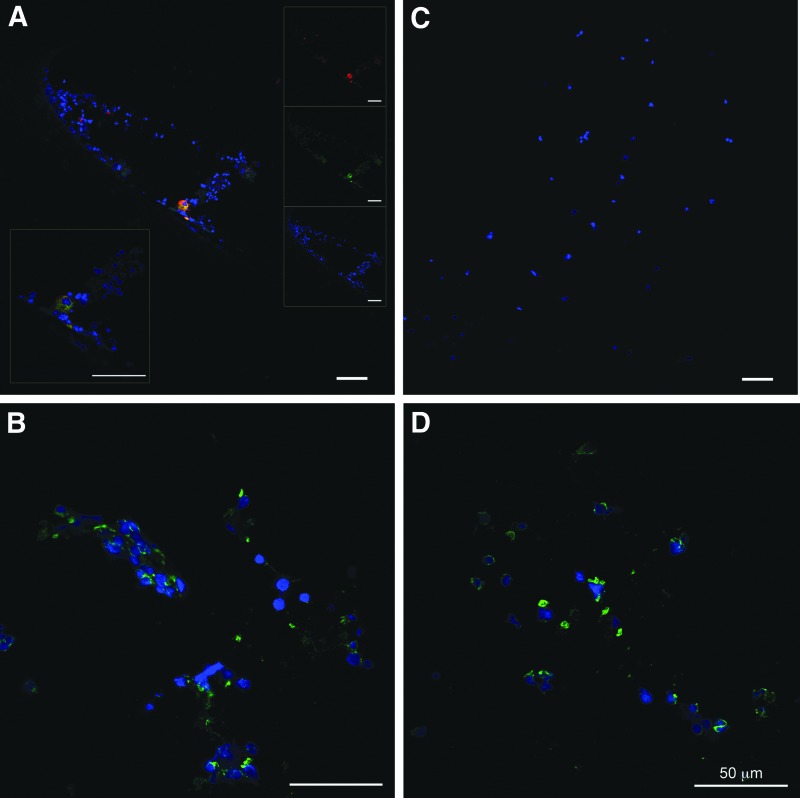
Immunohistochemistry
Sections of harvested cells from the two experimental conditions were examined for the presence of endothelial and hematopoietic markers. The endothelial markers CD31 and vWF were present on sections of human fetal liver cells from the perfusion condition (Fig. 7A), but no cells expressing such markers were observed in sections from the diffusion condition (Fig. 7C). Cells harvested from both perfusion and diffusion reactors stained positively for CD235a, an erythrocyte marker (Fig. 7B, D).
FIG. 7.
Immunohistochemistry. Human fetal liver cells were analyzed after 4 days of culture in bioreactors with perfusion (A, B) and diffusion (C, D) conditions. Cells were stained for CD31 (red) (A, C) and (green) for vWF (A, C), or CD235a (B, D). All sections were stained with diamidinophenylindole dihyrochloride for cell nuclei (blue). Insets in (A) display higher magnification detail (lower left) and individual color channels for the image (right). All scale bars=50 μm.
Discussion
The culture of liver cells in dynamic 3D conditions is advantageous compared with traditional static two-dimensional (2D) dish cultures, especially since the natural architecture of the liver can be more readily replicated in such an environment. Many basic static models exist that begin to recreate the liver in a 3D in vitro setting, such as the use of collagen sandwich culture of hepatocytes4–6 and cocultures of parenchymal and nonparenchymal cells.37,38 Various 3D systems have been developed to provide clinical bioartificial liver support, implementing cell lines, porcine, or human adult liver cells (for review, see Zhao et al.39). Another very important factor that influences cell differentiation and is necessary to create in vivo-like structures is vascularization.40,41 Salerno et al. employed a culture model that combined primary human hepatocytes with human umbilical vein cells (HUVECs) on PEEK-WC/PU scaffolds and generated tubule structures while improving albumin synthesis and drug biotransformation.42 In addition, HUVECs have been cocultured with human fetal liver progenitors in 3D fibrin gels,43 resulting in the formation of vascular structures and improving proliferation of the liver progenitors. HUVECs have also been cultured with mesenchymal precursor cells,44 resulting in the formation of stable vascular networks. While these studies are useful for understanding vascular network formation, they are limited since coculture with HUVECs does not truly represent the in vivo cell populations.
In contrast to the adult liver cells, fetal liver cells are an attractive option for regenerative medicine research due to their better availability and quality, higher proliferation capacity, and differentiation potential,45 and the fact that they have been shown to contain multiple progenitor cell types, including endothelial progenitors.13 Previous studies have demonstrated that the culture of mouse and rat-derived fetal liver cells on 3D scaffolds improved hepatic function relative to monolayer cultures.46–48 Additionally, mouse fetal liver cells have been cultured in rotating wall bioreactors,49 giving increased albumin secretion promoting the formation of bile duct structures. The bioreactor technology used in this study was previously used to culture mouse fetal liver cells8 for periods of 3–5 weeks, resulting in neo-tissue formation, albumin secretion, and cytochrome P450 induction. In the literature, no other perfusion bioreactor-based culture models have utilized human fetal liver cells. Static 3D cultures of human fetal liver cells were described,43,50 and bioreactor or 3D cultures of either fetal porcine,51,52 rat,47 or murine8,46,53,54 liver cells were developed. Xiong et al.43 demonstrated enhanced proliferation and albumin secretion of the human fetal livers when cocultured with HUVECs in 3D fibrin gels. In our study we used total human fetal liver cells cultured in a bioreactor configured to provide three-dimensionality and perfusion as an in vivo-like model to investigate hepatic and endothelial differentiation. Previous experiments using our bioreactor technology have demonstrated support of the differentiation of human fetal liver cells embedded in hyaluronan hydrogels9 as well as the maturation of human fetal hepatocytes10 when compared with traditional static 2D Petri dish culture, but have not investigated specifically the effect of medium flow on cell differentiation. These bioreactors provide constant medium flow, which mimics the natural perfusion in the liver and improves mass transfer compared with static cultures, leading to significantly increased rates of glucose consumption and lactate production for reactors configured to provide perfusion conditions. Huang et al. have demonstrated that HepG2 cells seeded on PCL porous scaffolds exposed to flow gave higher cell numbers, increased glucose consumption, and albumin production compared with cultures without flow.55 Shear stress from fluid flow is also well known to affect the morphology and gene expression of endothelial cells,24 increasing the expression of genes such as CD309 (KDR),56,57 CD31,57 eNOS,28 and PDGF-b.30 In our study a flow rate of 15 mL/min from medium recirculation significantly increased expression of a number of endothelial related genes, including CD31, CD309, CD144, vWF, eNOS, and CD140b relative to the reactors that received only slow diffusive feed at 2 mL/h.
The increased flow rate in the perfusion reactors not only provided mechanical cues to the endothelial cells, but also influenced differentiated functions of the fetal parenchymal hepatic cells (hepatoblasts), that is, the secretion of liver-specific proteins. AFP is the major protein produced by fetal liver cells. Production of this protein is terminated postnatally and albumin becomes the major secreted protein for differentiated adult liver cells (hepatocytes).58 As such, fetal liver cells would be expected to secrete AFP, followed by albumin as they begin to differentiate toward more mature liver cells. In this study fetal liver cells that were cultured in bioreactors configured to provide perfusion conditions gave a significantly increased rate of production of albumin compared with cells cultured under diffusive flow after only 4 days. Albumin secretion by cells in the liver is regulated by a feedback mechanism59 and the increased flow rate in the perfusion reactors likely increases albumin production and aids in the maturation of the cells. Because of this feedback mechanism, the sole use of albumin as a marker for differentiation is somewhat limited. Thus, we also investigated the expression of cell type-specific genes and secretion of AFP. Correspondingly, cells in the perfusion reactors also ceased producing AFP after 4 days, whereas the cells in the diffusion reactors continued to secrete AFP into the medium stream, further supporting that these cells were increasingly differentiated under flow conditions. Yet, when compared with perfusion bioreactor cultures of adult human liver cells,60,61 the per cell secretion of albumin of fetal liver cells even in perfusion conditions is considerably low, being about 1/10th to 1/100th of adult cells. This implies additional measures to induce further differentiation of fetal liver-derived cells toward mature hepatocytes if fetal liver-derived cells are to be considered as an alternative source for in vitro applications such as pharmacological screening or extracorporeal liver support. We did not presume, however, that cells from fetal livers of gestational ages between 16 and 20 weeks are capable to mature within 4 days of culture toward mature hepatocytes based on their early developmental stage; longer culture periods mimicking the in vivo development are expected to be required. Human fetal liver cell 3D cocultures with HUVECs43 demonstrated a considerable increase in albumin secretion not before 8 days of culture, with maximum rates being beyond 20 days of culture. A previous study from us,10 which compared human fetal liver cells in perfusion bioreactor culture with those in static Petri dish culture after 14 days, showed a significant strong increase in CYP3A4 gene and protein expression, mature hepatocyte surface marker ASGPR expression by fluorescence-activated cell sorting (FACS), and a more mature cell morphology, including bile ducts in histology.
Conclusively, in the present study we implemented multicompartment hollow fiber membrane-based 3D bioreactors configured to provide either perfusion or diffusive mass exchange and demonstrated that fetal liver cells exposed to medium recirculation of 15 mL/min were metabolically more active, displayed increased expression of a number of endothelial and liver related genes, increased FACS percentages of mature endothelial cells, and became increasingly differentiated toward mature liver cells after 4 days of culture when compared with diffusion conditions. The combination of differentiation and the increase in expression of endothelial genes indicate that these bioreactors could be used as organotypical models for tissue engineering research.
Acknowledgments
The work presented in this study was supported by a grant from the National Institutes of Health (NIH R01 HL108631). The authors thank the staff of Allegheny Reproductive Health Clinic for their enthusiasm and diligence in supporting the project.
Disclosure Statement
J.C.G. has shares in the company StemCell Systems that provided the bioreactors. No other competing financial interests exist.
References
- 1.UNOS, United Network for Organ Sharing. (2013). www.unos.org
- 2.Evans V.J., Earle W.R., Wilson E.P., Waltz H.K., and Mackey C.J. The growth in vitro of massive cultures of liver cells. J Nat Cancer Inst 12, 1245, 1952 [PubMed] [Google Scholar]
- 3.Gerschenson L.E., and Casanello D. Metabolism of rat liver cells cultured in suspension: insulin and glucagon effects on glycogen level. Biochem Biophys Res Commun 33, 584, 1968 [DOI] [PubMed] [Google Scholar]
- 4.Dunn J.C., Yarmush M.L., Koebe H.G., and Tompkins R.G. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J 3, 174, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Dunn J.C., Tompkins R.G., and Yarmush M.L. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog 7, 237, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Dunn J.C., Tompkins R.G., and Yarmush M.L. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol 116, 1043, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlach J.C., Mutig K., Sauer I.M., Schrade P., Efimova E., Mieder T., Naumann G., Grunwald A., Pless G., Mas A., Bachmann S., Neuhaus P., and Zeilinger K. Use of primary human liver cells originating from discarded grafts in a bioreactor for liver support therapy and the prospects of culturing adult liver stem cells in bioreactors: a morphologic study. Transplantation 76, 781, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Monga S.P., Hout M.S., Baun M.J., Micsenyi A., Muller P., Tummalapalli L., Ranade A.R., Luo J.H., Strom S.C., and Gerlach J.C. Mouse fetal liver cells in artificial capillary beds in three-dimensional four-compartment bioreactors. Am J Pathol 167, 1279, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmelzer E., Triolo F., Turner M.E., Thompson R.L., Zeilinger K., Reid L.M., Gridelli B., and Gerlach J.C. Three-dimensional perfusion bioreactor culture supports differentiation of human fetal liver cells. Tissue Eng Part A 16, 2007, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Ring A., Gerlach J., Peters G., Pazin B.J., Minervini C.F., Turner M.E., Thompson R.L., Triolo F., Gridelli B., and Miki T. Hepatic maturation of human fetal hepatocytes in four-compartment three-dimensional perfusion culture. Tissue Eng Part C Methods 16, 835, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Gerlach J.C., Hout M., Edsbagge J., Bjorquist P., Lubberstedt M., Miki T., Stachelscheid H., Schmelzer E., Schatten G., and Zeilinger K. Dynamic 3D culture promotes spontaneous embryonic stem cell differentiation in vitro. Tissue Eng Part C Methods 16, 115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherqui S., Kurian S.M., Schussler O., Hewel J.A., Yates J.R., 3rd, and Salomon D.R. Isolation and angiogenesis by endothelial progenitors in the fetal liver. Stem Cells 24, 44, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Nava S., Westgren M., Jaksch M., Tibell A., Broome U., Ericzon B.G., and Sumitran-Holgersson S. Characterization of cells in the developing human liver. Differentiation 73, 249, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Vollmar B., and Menger M.D. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev 89, 1269, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Oda M., Yokomori H., and Han J.Y. Regulatory mechanisms of hepatic microcirculation. Clin Hemorheol Microcirc 29, 167, 2003 [PubMed] [Google Scholar]
- 16.Moriyasu F., Nishida O., Ban N., Nakamura T., Sakai M., Miyake T., and Uchino H. “Congestion index” of the portal vein. AJR Am J Roentgenol 146, 735, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Cosar S., Oktar S.O., Cosar B., Yucel C., and Ozdemir H. Doppler and gray-scale ultrasound evaluation of morphological and hemodynamic changes in liver vascualture in alcoholic patients. Eur J Radiol 54, 393, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Torok E., Pollok J.M., Ma P.X., Vogel C., Dandri M., Petersen J., Burda M.R., Kaufmann P.M., Kluth D., and Rogiers X. Hepatic tissue engineering on 3-dimensional biodegradable polymers within a pulsatile flow bioreactor. Dig Surg 18, 196, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Roy P., Washizu J., Tilles A.W., Yarmush M.L., and Toner M. Effect of flow on the detoxification function of rat hepatocytes in a bioartificial liver reactor. Cell Transplant 10, 609, 2001 [PubMed] [Google Scholar]
- 20.Fiegel H.C., Havers J., Kneser U., Smith M.K., Moeller T., Kluth D., Mooney D.J., Rogiers X., and Kaufmann P.M. Influence of flow conditions and matrix coatings on growth and differentiation of three-dimensionally cultured rat hepatocytes. Tissue Eng 10, 165, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Brooks A.R., Lelkes P.I., and Rubanyi G.M. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics 9, 27, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Davies P.F. Flow-mediated endothelial mechanotransduction. Physiol Rev 75, 519, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topper J.N., and Gimbrone M.A., Jr. Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today 5, 40, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Resnick N., and Gimbrone M.A., Jr. Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J 9, 874, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto K., Sokabe T., Watabe T., Miyazono K., Yamashita J.K., Obi S., Ohura N., Matsushita A., Kamiya A., and Ando J. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol 288, H1915, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto K., Takahashi T., Asahara T., Ohura N., Sokabe T., Kamiya A., and Ando J. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol (1985) 95, 2081, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Schmelzer E., Wauthier E., and Reid L.M. The phenotypes of pluripotent human hepatic progenitors. Stem Cells 24, 1852, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Rizzo V., McIntosh D.P., Oh P., and Schnitzer J.E. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J Biol Chem 273, 34724, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Uematsu M., Ohara Y., Navas J.P., Nishida K., Murphy T.J., Alexander R.W., Nerem R.M., and Harrison D.G. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol 269, C1371, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Resnick N., Collins T., Atkinson W., Bonthron D.T., Dewey C.F., Jr., and Gimbrone M.A., Jr. Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci U S A 90, 4591, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh H.J., Li N.Q., and Frangos J.A. Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol 260, H642, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Czarny M., Liu J., Oh P., and Schnitzer J.E. Transient mechanoactivation of neutral sphingomyelinase in caveolae to generate ceramide. J Biol Chem 278, 4424, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Mikkola H.K., and Orkin S.H. The journey of developing hematopoietic stem cells. Development 133, 3733, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Palis J., and Segel G.B. Developmental biology of erythropoiesis. Blood Rev 12, 106, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Stevens J.C., Hines R.N., Gu C., Koukouritaki S.B., Manro J.R., Tandler P.J., and Zaya M.J. Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther 307, 573, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Lacroix D., Sonnier M., Moncion A., Cheron G., and Cresteil T. Expression of CYP3A in the human liver—evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem 247, 625, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Bader A., Knop E., Kern A., Boker K., Fruhauf N., Crome O., Esselmann H., Pape C., Kempka G., and Sewing K.F. 3-D coculture of hepatic sinusoidal cells with primary hepatocytes-design of an organotypical model. Exp Cell Res 226, 223, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Bhatia S.N., Balis U.J., Yarmush M.L., and Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J 13, 1883, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Zhao L.F., Pan X.P., and Li L.J. Key challenges to the development of extracorporeal bioartificial liver support systems. Hepatobiliary Pancreat Dis Int 11, 243, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Lee J., Cuddihy M.J., and Kotov N.A. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev 14, 61, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Novosel E.C., Kleinhans C., and Kluger P.J. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev 63, 300, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Salerno S., Campana C., Morelli S., Drioli E., and De Bartolo L. Human hepatocytes and endothelial cells in organotypic membrane systems. Biomaterials 32, 8848, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Xiong A., Austin T.W., Lagasse E., Uchida N., Tamaki S., Bordier B.B., Weissman I.L., Glenn J.S., and Millan M.T. Isolation of human fetal liver progenitors and their enhanced proliferation by three-dimensional coculture with endothelial cells. Tissue Eng Part A 14, 995, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koike N., Fukumura D., Gralla O., Au P., Schechner J.S., and Jain R.K. Tissue engineering: creation of long-lasting blood vessels. Nature 428, 138, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Fiegel H.C., Kneser U., Kluth D., Metzger R., Till H., and Rolle U. Development of hepatic tissue engineering. Pediatr Surg Int 25, 667, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Koyama T., Ehashi T., Ohshima N., and Miyoshi H. Efficient proliferation and maturation of fetal liver cells in three-dimensional culture by stimulation of oncostatin M, epidermal growth factor, and dimethyl sulfoxide. Tissue Eng Part A 15, 1099, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Hanada S., Kojima N., and Sakai Y. Soluble factor-dependent in vitro growth and maturation of rat fetal liver cells in a three-dimensional culture system. Tissue Eng Part A 14, 149, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Miyoshi H., Ehashi T., Ema H., Hsu H.C., Nakauchi H., and Ohshima N. Long-term culture of fetal liver cells using a three-dimensional porous polymer substrate. ASAIO J 46, 397, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Ishikawa M., Sekine K., Okamura A., Zheng Y.W., Ueno Y., Koike N., Tanaka J., and Taniguchi H. Reconstitution of hepatic tissue architectures from fetal liver cells obtained from a three-dimensional culture with a rotating wall vessel bioreactor. J Biosci Bioeng 111, 711, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Hanada S., Kayano H., Jiang J., Kojima N., Miyajima A., Sakoda A., and Sakai Y. Enhanced in vitro maturation of subcultivated fetal human hepatocytes in three dimensional culture using poly-L-lactic acid scaffolds in the presence of oncostatin M. Int J Artif Organs 26, 943, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Ehashi T., Ohshima N., and Miyoshi H. Three-dimensional culture of porcine fetal liver cells for a bioartificial liver. J Biomed Mater Res A 77, 90, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Huang H., Hanada S., Kojima N., and Sakai Y. Enhanced functional maturation of fetal porcine hepatocytes in three-dimensional poly-L-lactic acid scaffolds: a culture condition suitable for engineered liver tissues in large-scale animal studies. Cell Transplant 15, 799, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Ehashi T., Koyama T., Ookawa K., Ohshima N., and Miyoshi H. Effects of oncostatin M on secretion of vascular endothelial growth factor and reconstruction of liver-like structure by fetal liver cells in monolayer and three-dimensional cultures. J Biomed Mater Res A 82, 73, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Minagawa K., Koyama T., and Miyoshi H. Stimulating effects of fibroblast growth factors on hepatic function of fetal liver cells synergistically with oncostatin M in three-dimensional culture. J Biosci Bioeng 107, 307, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Huang H., Oizumi S., Kojima N., Niino T., and Sakai Y. Avidin-biotin binding-based cell seeding and perfusion culture of liver-derived cells in a porous scaffold with a three-dimensional interconnected flow-channel network. Biomaterials 28, 3815, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Staalesen T., Risberg B., and Mattsson E. The kinase insert domain-containing receptor (KDR) is regulated by shear stress. Scand Cardiovasc J 36, 368, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Glen K., Luu N.T., Ross E., Buckley C.D., Rainger G.E., Egginton S., and Nash G.B. Modulation of functional responses of endothelial cells linked to angiogenesis and inflammation by shear stress: differential effects of the mechanotransducer CD31. J Cell Physiol 227, 2710, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Nayak N.C., and Mital I. The dynamics of alpha-fetoprotein and albumin synthesis in human and rat liver during normal ontogeny. Am J Pathol 86, 359, 1977 [PMC free article] [PubMed] [Google Scholar]
- 59.Pietrangelo A., Panduro A., Chowdhury J.R., and Shafritz D.A. Albumin gene expression is down-regulated by albumin or macromolecule infusion in the rat. J Clin Invest 89, 1755, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeilinger K., Schreiter T., Darnell M., Soderdahl T., Lubberstedt M., Dillner B., Knobeloch D., Nussler A.K., Gerlach J.C., and Andersson T.B. Scaling down of a clinical three-dimensional perfusion multicompartment hollow fiber liver bioreactor developed for extracorporeal liver support to an analytical scale device useful for hepatic pharmacological in vitro studies. Tissue Eng Part C Methods 17, 549, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Pless G., Steffen I., Zeilinger K., Sauer I.M., Katenz E., Kehr D.C., Roth S., Mieder T., Schwartlander R., Muller C., Wegner B., Hout M.S., and Gerlach J.C. Evaluation of primary human liver cells in bioreactor cultures for extracorporeal liver support on the basis of urea production. Artif Organs 30, 686, 2006 [DOI] [PubMed] [Google Scholar]