Abstract
Umbilical cord mesenchymal stem cells (UC-MSCs) show properties similar to bone marrow mesenchymal stem cells (BM-MSCs), although controversial data exist regarding their osteogenic potential. We prepared clinical-grade UC-MSCs from Wharton's Jelly and we investigated if UC-MSCs could be used as substitutes for BM-MSCs in muscoloskeletal regeneration as a more readily available and functional source of MSCs. UC-MSCs were loaded onto scaffolds and implanted subcutaneously (ectopically) and in critical-sized calvarial defects (orthotopically) in mice. For live cell-tracking experiments, UC-MSCs were first transduced with the luciferase gene. Angiogenic properties of UC-MSCs were tested using the mouse metatarsal angiogenesis assay. Cell secretomes were screened for the presence of various cytokines using an array assay. Analysis of implanted scaffolds showed that UC-MSCs, contrary to BM-MSCs, remained detectable in the implants for 3 weeks at most and did not induce bone formation in an ectopic location. Instead, they induced a significant increase of blood vessel ingrowth. In agreement with these observations, UC-MSC-conditioned medium presented a distinct and stronger proinflammatory/chemotactic cytokine profile than BM-MSCs and a significantly enhanced angiogenic activity. When UC-MSCs were orthotopically transplanted in a calvarial defect, they promoted increased bone formation as well as BM-MSCs. However, at variance with BM-MSCs, the new bone was deposited through the activity of stimulated host cells, highlighting the importance of the microenvironment on determining cell commitment and response. Therefore, we propose, as therapy for bone lesions, the use of allogeneic UC-MSCs by not depositing bone matrix directly, but acting through the activation of endogenous repair mechanisms.
Introduction
Bone marrow mesenchymal stem cells (BM-MSCs) are considered the gold standard cell population in bone engineering and regenerative medicine applications [1]. The implantation of these cells combined with ceramic-based biomaterials in bone defects gives the best results in terms of bone formation both in animal models [2,3] and in patients [4]. Although BM-MSCs are a feasible and autologous source of MSCs, their painful and invasive collection method has limited their use. Moreover, BM-MSCs are adult stem cells with lower proliferation capacity compared with other MSCs originating from fetal tissues [5]. The umbilical cord (UC) can be a source of highly proliferating fetal MSCs [6,7] that are more easily collectable than BM-MSCs. UC-MSCs are isolated from Wharton's Jelly, representing a noncontroversial source of fetal stem cells [8]. Because of their fetal origin, nowadays, the use of UC-MSCs in an allogeneic system is almost the only application for them. UC-MSCs have great immunomodulation capacity [9]; they act on the innate immunity by inhibiting the maturation and the activation of dendritic cells [10], the activation of the CD56dim NK subpopulation, and NK cytotoxicity [11]. Moreover, UC-MSCs have an influence on adaptive immunity by blocking T cell proliferation [9,10] and modulating B cell proliferation and differentiation [12,13]. Thus, UC-MSCs have become an appealing cell source for treatment of diseases where inflammation plays a crucial role such as graft-versus-host disease [14] (www.clinicaltrials.gov; Identifier: NCT02032446). Indeed, fetal MSCs have been shown to possess stronger immunomodulation properties and lower immunogenicity than adult MSCs [15,16]. In view of a rapid increase in the use of UC-MSCs in the clinic, protocols of cell isolation and expansion under good manufacturing practice (GMP) guidelines are being developed.
In this context, we prepared clinical-grade UC-MSCs [6], and we investigated if UC-MSCs could be used as substitutes for BM-MSCs in musculoskeletal regeneration. UC-MSCs clearly display typical MSC features as defined by the International Society for Cellular Therapy [17]. Both UC-MSCs and BM-MSCs are plastic adherent and show similar cell surface marker expression. Interestingly, the only major difference is their differentiation potential [6]. UC-MSCs have a very weak osteogenic [18–20], chondrogenic [21], and adipogenic [19,20] differentiation capacity compared with BM-MSCs. The reasons for these differences need to be further clarified, but it has been postulated that the reduced differentiation potential of UC-MSCs may depend on their position within the UC tissue they are isolated from [22]. As the cells in the human UC stroma are not uniformly distributed, it may be hypothesized that, depending on the isolation technique used, slightly different types of primitive cells with unequal differentiation capabilities can be obtained [6]. It must also be taken into consideration that whereas BM-MSCs may be more committed to osteogenesis because of their natural density in the bone and in maintaining the self-renewal capacity of hematopoietic stem cells, UC-MSCs having a fetal origin might require stronger inductive stimuli or more time to differentiate into a specific tissue [23,24].
Increasing evidence has also recently suggested a predominant paracrine role of MSCs in promoting tissue regeneration due to their secretome enriched in proinflammatory, chemotactic, and angiogenic factors rather than due to their direct replacement of affected cells at the site of injury [25]. Additionally, since vascularization is a crucial aspect in the tissue regeneration process, this inherent characteristic of MSCs, particularly of UC-MSCs, would be interesting to explore.
Hence, to better clarify the potential of UC-MSCs in regenerative medicine and to compare them with BM-MSCs in an in vivo osteogenic environment, we wanted to answer the following questions:
1. Can an osteogenic environment, whether ectopic or orthotopic, influence the osteogenic regenerative capacity of UC-MSCs expanded according to a clinical-grade protocol?
2. Are the regenerative effects of implanted UC-MSCs attributed to their direct engraftment or could their paracrine/trophic effects be responsible?
3. Can UC-MSCs mediate a therapeutic angiogenic response, thus leading to enhanced bone regeneration?
Materials and Methods
Cell isolation and culture
Human UC-MSC culture
Human UCs were collected from pregnant women after cesarean sections. Informed written consent was obtained. The UC processing was performed in accordance with a GMP-compliant protocol for the isolation and expansion of UC-MSCs [6]. Briefly, the UC was cut into segments, which were split open to expose the inner surface. The UC segments were transferred to an expansion medium, consisting of alpha-minimum essential medium with GlutaMAX™ (Invitrogen) enriched with 5% human platelet lysate (PL) obtained from healthy donors, 2 IU/mL Na-heparin (Epsoclar; Hospira s.r.l.), and 100 μg/mL streptomycin (Sigma Chemical Co.), and minced. Minced pieces were incubated in the expansion medium at 37°C and 5% CO2 for 1 week to allow the cells to adhere, and then they were removed. At 80% confluence, the cells were detached by treatment with Tryple (Invitrogen) and passaged. All experiments were performed using at least three different primary cultures at either P1 or P2.
Human BM-MSC culture
Human BM-MSCs were obtained from iliac crest marrow aspirates of healthy bone marrow transplant donors after informed consent. The BM-MSCs were cultured in complete culture medium consisting of Coon's modified Ham's F-12 medium containing 2 mM glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin (Sigma Chemical Co.), and 10% fetal bovine serum (FBS; Invitrogen) in the presence of 1 ng/mL human recombinant fibroblast growth factor-2 (FGF-2; Peprotech). At 80% confluence, the cells were detached by treatment with 0.05% trypsin −0.01% ethylenediaminetetraacetic acid (EDTA; Sigma Chemical Co.) and passaged. All experiments were performed using at least three different primary cultures at either P1 or P2.
Conditioned medium preparation
The conditioned medium (CM) was collected from 80% confluent cultures of either UC-MSCs or BM-MSCs in 10-cm tissue culture dishes. Each culture dish received 7 mL of serum-free media for 48 h. The CM was collected by centrifugation at 440 g for 5 min and by a second centrifugation at 1,750 g for 3 min, both centrifugations were at 4°C [26].
Platelet lysate preparation
Whole blood was collected from voluntary donors following current procedures for blood donation. Blood units were screened for the absence of infectious agents in compliance with national regulatory requirements. Buffy coats (BC) were obtained by centrifugation of whole blood donations. Five BC units were pooled, platelet-rich plasma (PRP) separated by light-spin centrifugation, and the platelets concentrated by a second heavy-spin centrifugation. PRP was resuspended in homologous plasma and stored at −30°C. The standard PRP unit had a platelet concentration of 1–2×106/μL. PL was obtained by subjecting PRP to three cycles of freezing and thawing. The PL was aliquoted and stored at −20°C.
Ectopic bone formation assay
All experimental animal procedures were evaluated and approved by the IRCCS AOU San Martino-IST Ethics Committee for animal experimentation (CSEA) and communicated to the Italian Ministry of Health in accordance with article 7 of the D.lgs 27/01/1992 n.116/92.
The in vivo analysis was based on an established model of ectopic bone formation [27]. Briefly, we prepared the implantable constructs by seeding 2.5×106 UC-MSCs onto ceramic scaffolds (Skelite; 4×4×4 mm cubes of 33% hydroxyapatite and 67% silicon-stabilized tricalcium phosphate, Si-TCP) and embedding the scaffolds in a fibrin glue (Tissucol; Baxter). Moreover, nonseeded empty Skelite and Skelite seeded with 2.5×106 BM-MSC constructs were used as negative and positive control groups, respectively (n=4).
Scaffolds were implanted subcutaneously on the dorsal surface of immunocompromised mice (CD-1 Nu/Nu; Charles River). The implants were recovered at 60 days and histologically analyzed.
Histological analysis
Harvested samples were fixed in 3.7% paraformaldehyde, decalcified in EDTA 10%, and paraffin embedded. Sections were cut at 4 μm thickness and stained with hematoxylin and eosin (H&E) and Masson's trichrome (MTC) (Masson's trichrome special staining kit; Bio-Optica) stains. Moreover, chromogenic in situ hybridization (Zytovision kit) to detect human ALU repeat sequences was performed following the manufacturer's instructions. Images of the sections were acquired using a phase-contrast Axiovert 200M microscope (Zeiss). Polarized light images were acquired using a phase-contrast Axiophot microscope (Zeiss).
The number of blood vessels in the ectopic implants (n=3) was manually counted in multiple, representative MTC-stained sections for UC-MSCs and BM-MSC-seeded and empty scaffolds (six per condition per experiment). The bone surface area in the orthotopic implants (n=3) was manually measured in multiple representative H&E-stained sections for all groups. Three sections per condition per experiment were analyzed. The ratio between the neoformed bone area and total defect area was calculated for each section.
To perform the histologic quantification analysis, ImageJ (NIH) software was used.
In vivo live cell-tracking analysis
To perform live cell-tracking experiments, UC-MSCs and BM-MSCs were transduced using a replication-incompetent amphotropic bicistronic retrovirus encoding the firefly luciferase gene (Luc) [28]. Retroviral vectors and infection protocols were performed as previously described [29]. Luc-infected UC-MSCs and BM-MSCs were selected by adding G418 (Gibco) at 0.5 mg/mL to the medium for 10 days. MSC-transduced cells (2.5×106) were seeded onto Skelite scaffolds and implanted as reported above.
The in vivo bioluminescence reaction induced by the substrate luciferin (150 mg/kg; D-Luciferin Firefly Xenogen) injections in mice was detected using the IVIS® Imaging System LUMINA II (PerkinElmer) (n=3 in BM-MSC implants and n=6 in UC-MSC implants). Mice were imaged for 5 min after 1, 7, 21, and 30 days postimplantation.
Metatarsal angiogenesis assay
The metatarsal angiogenesis assay was performed as previously described [30]. Briefly, metatarsals of 17-day-old, wild-type mouse embryos (FVB/N; Charles River) were transferred to culture dishes to allow their attachment for 72 h. Bones were then cultured for 13 days with either UC-MSC CM or BM-MSC CM, both implemented with 10% FBS. Fresh complete medium containing 10% FBS was used as the experiment control. After 13 days of culture, bones were fixed and stained for PECAM-1/CD31. Quantification of the number of CD31 positively stained pixels and the outgrowth area from bone were performed using ImageJ (NIH) software (n=3).
Proteomic identification and bioinformatics analysis
Cytokine screening of UC-MSC secretome was performed on CM using a human cytokine array (Panel A; R&D Systems) following the manufacturer's instructions. The cytokines identified in the CM of UC-MSCs were uploaded into the web-based DAVID platform (www.david.abcc.ncifcrf.gov). The protein list was analyzed by clusterization into Gene Ontology (GO) biological processes.
Assessment of bone formation in the mouse calvarial defect
Discs of nanohydroxyapatite/poly (ester urethane) (nHA/PU) with a diameter of 5 mm and height of 2 mm were provided by the AO Research Institute (Davos, Switzerland). These scaffolds have been shown to support orthotopic bone formation in a rabbit model [2]. Immediately before surgery, 2.5×106 UC-MSCs were loaded onto the scaffolds and embedded in a fibrin glue (Tissucol; Baxter). Moreover, nonseeded empty nHA/PU discs and discs seeded with 2.5×106 BM-MSC constructs were used as negative and positive control groups, respectively (n=3).
The constructs were implanted in 5-mm critical-size defects in the calvaria of immunocompromised mice (CD-1 Nu/Nu; Charles River).
The critical-sized calvarial defects were made as previously described in the literature [31]. Mice were anesthetized with ketamine (80–100 mg/kg) and xylazine (5–10 mg/kg). A 5-mm defect was prepared in the center of the parietal bones of each mouse calvarium using Mectron Piezosurgery® instruments (Mectron s.p.a.). The construct was then carefully placed and the flap was repositioned and sutured using 4.0 vicryl (Ethicon) suture material. The implants were recovered 90 days postoperatively, decalcified, and then histologically analyzed.
Statistical analysis
The results are expressed as mean and standard error of the mean. Statistical significance was determined using the ordinary one-way ANOVA and the value of P<0.05 was considered to be statistically significant. Statistical analysis was performed by Graphpad software.
Results
UC-MSC behavior in an ectopic bone formation model
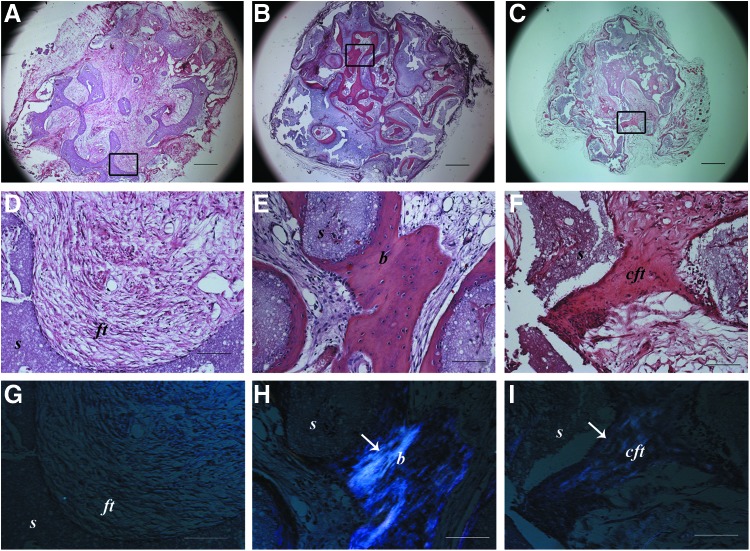
Histological assessment of the tissues formed within the ectopically implanted scaffolds revealed the formation of mature bone in the pores of the scaffolds seeded with BM-MSCs (Fig. 1E), whereas UC-MSC-seeded constructs showed a compact fibrous tissue ascribable to an immature bone-like structure (Fig. 1F). In the nonseeded implants, a loose connective fibrous tissue was observed (Fig. 1D). Polarized light examination confirmed the presence of highly organized collagen fibers in the BM-MSC-seeded implants (Fig. 1H) compared with the weaker signal observable in the UC-MSC-seeded scaffolds (Fig. 1I). In the nonseeded scaffold samples, no polarized light signal was detected (Fig. 1G).
FIG. 1.
Histological images of ectopic implants after 2 months and stained with hematoxylin and eosin (A–F) and analyzed by polarized light (G–I). Black rectangles indicate the areas from which higher magnification images where taken. (A, D, G) noncell-seeded empty scaffold; (B, E, H) bone marrow mesenchymal stem cell (BM-MSC)-seeded scaffolds; (C, F, I) umbilical cord mesenchymal stem cell (UC-MSC)-seeded scaffolds. s, scaffold; ft, fibrous tissue; b, bone; cft, compact fibrous tissue; (↑), polarized light signal. Scale bars=600 μm (A–C) and 100 μm (D–I). (n=4 for both cell-seeded and not seeded scaffold groups). Color images available online at www.liebertpub.com/scd
Low-magnification micrographs (Fig. 1A–C) showed that the bone tissue in BM-MSC-seeded scaffolds was formed in most of the area of the implant (Fig. 1B), whereas in UC-MSC-seeded scaffolds, the immature bone-like matrix was sparsely present (Fig. 1C) with the majority of the area filled with a loose connective tissue similar to that seen completely filling the nonseeded scaffolds (Fig. 1A).
UC-MSC angiogenic effect in vivo
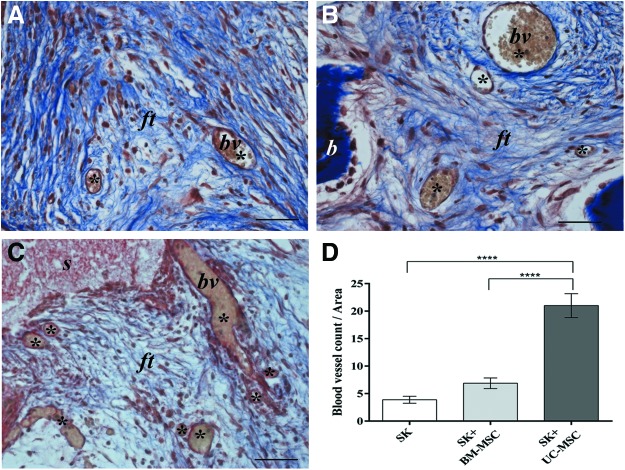
Although only a minimal amount of immature bone-like matrix could be detected in the UC-MSC-seeded scaffolds, an accurate analysis of these implants revealed an abundance of blood vessels (Fig. 2C) compared with the BM-MSC-seeded (Fig. 2B) and empty implants (Fig. 2A). The characteristic gold staining of the MTC stain revealed the presence of red blood cells in most of the vessel-like structures (Fig. 2A–C). Quantitative analysis confirmed the presence of a significantly higher number of blood vessels in UC-MSC implants than in either BM-MSC-seeded (P<0.0001) or empty scaffolds (P<0.0001) (Fig. 2D).
FIG. 2.
Histological images of ectopic implants recovered after 2 months and stained with Masson's trichrome showing a high number of blood vessel-like structures in the UC-MSC-seeded scaffold (C) compared with the BM-MSC-seeded scaffold (B) and empty scaffold (A). bv, blood vessel; ft, fibrous tissue; b, bone; (*), blood vessel count. Scale bar=100 μm. (n=4 for both cell-seeded and not seeded scaffold groups). In (D) a quantification of the number of blood vessels present in 2-month implants of the empty scaffold (SK) and BM-MSC and UC-MSC-seeded scaffolds (SK+BM-MSC and SK+UC-MSC) is presented. (****P<0.0001). Color images available online at www.liebertpub.com/scd
In situ hybridization analysis showed that there was no positive signal for human ALU repeat sequences detected in any area of the 2-month UC-MSC implants, including the fibroblasts forming the compact fibrous tissue and the endothelial cells lining the vessel structures. Therefore, based on this observation, we classified the immature bone-like structure (Fig. 3E, F) and the neoformed vessels of murine origin (Fig. 3G, H). On the contrary, the mature bone formed in BM-MSC implants was undoubtedly of human origin. Cells derived from implanted human BM-MSCs were evident both in the newly formed bone as osteoblasts and osteocytes and in the fibrous tissue as fibroblast-like cells (Fig. 3C, D). As expected, no signal for ALU sequences was detected in the nonseeded implants (Fig. 3A, B).
FIG. 3.
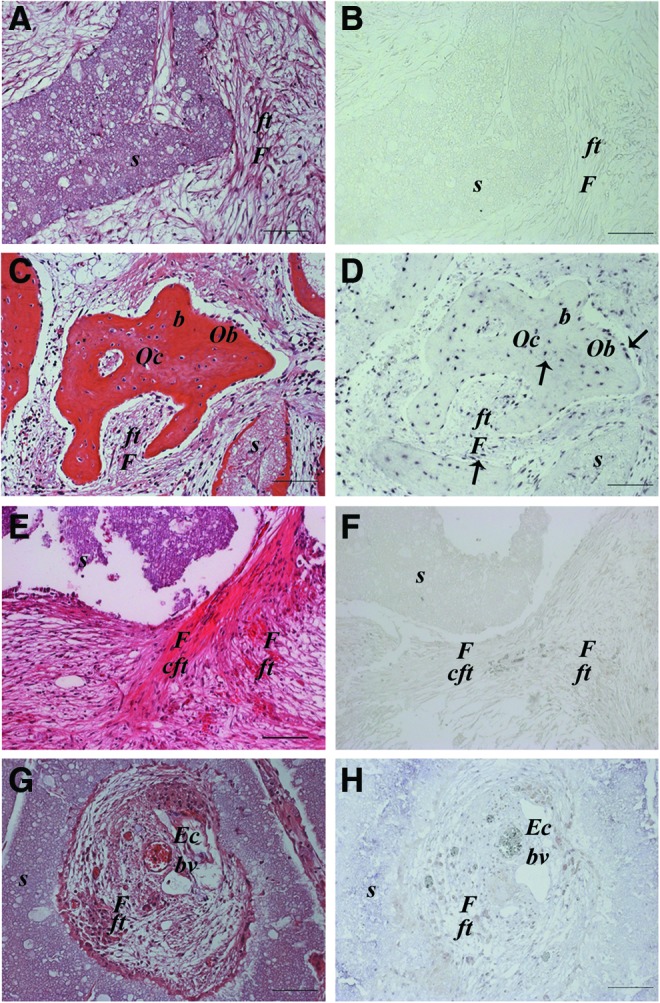
Histological images of ectopic implants recovered after 2 months and stained with hematoxylin and eosin (A, C, E, G) and analyzed by in situ hybridization for the human ALU repeat sequence (B, D, F, H). Empty scaffold (SK) (A, B); BM-MSC-seeded scaffold osteocytes and osteoblasts of human origin are present. Moreover, human cells are detectable and also found in the fibrous tissue as fibroblasts (C, D); UC-MSC-seeded scaffold. No human cells present in the compact fibrous tissue (E, F) and in the blood vessel endothelium (G, H). s, scaffold; F, fibroblast; ft, fibrous tissue; cft, compact fibrous tissue; Oc, osteocyte; Ob, osteoblast; b, bone; Ec, endothelial cell; bv, blood vessel; (↑), human cell. Scale bar=100 μm. (n=4). Color images available online at www.liebertpub.com/scd
In vivo permanence of UC-MSCs
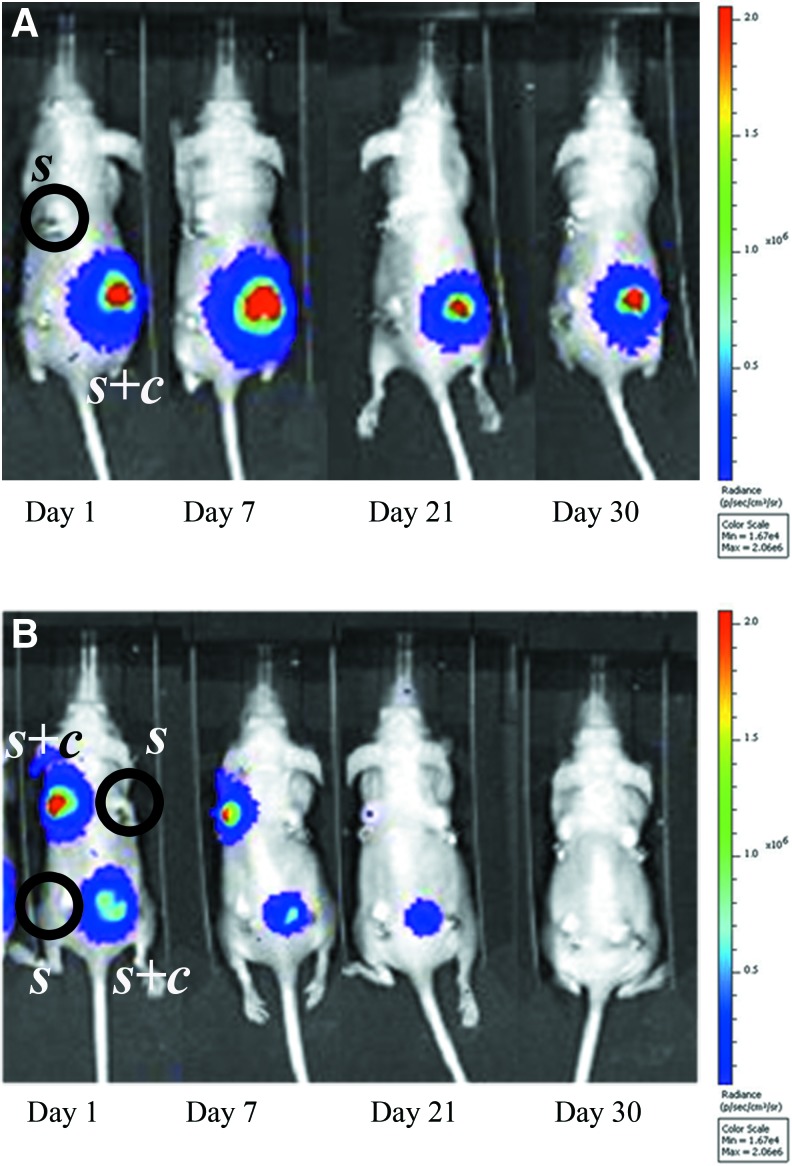
In light of the previous evidence, we performed live cell-tracking analysis by detecting the light signal produced by transduced MSCs. The bioluminescent signal produced by UC-MSCs drastically decreased over time and completely disappeared by 1 month (Fig. 4B). On the contrary, the BM-MSC bioluminescent signal remained detectable throughout the entire testing period (Fig. 4A).
FIG. 4.
In vivo bioluminescence imaging, at different time points after surgery, of mice ectopically implanted with scaffolds seeded with cells (s+c) or not seeded (s). A decreasing signal is noticeable for UC-MSC-seeded scaffolds (B), whereas the signal for the BM-MSC-seeded scaffold remains stable throughout the whole observation time (A). No signal is detected in empty scaffolds (A, B). (n=6 for both UC-MSC-seeded and not seeded scaffold groups, and n=4 for the BM-MSC-seeded scaffold group). Color images available online at www.liebertpub.com/scd
UC-MSC secretome induces angiogenesis in vitro
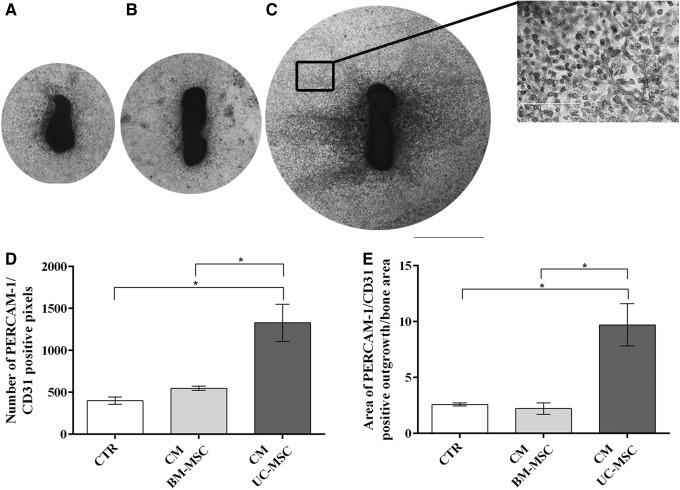
In light of the previous data, we investigated whether the UC-MSC secretome (CM) alone could induce angiogenesis in a functional in vitro assay. Indeed, a widespread sprouting of blood vessels from fetal bone was induced by UC-MSC CM (Fig. 5C). On the contrary, BM-MSC CM showed a poor effect on inducing the formation of new blood vessels and behaved similar to the fresh medium controls (CTR) (Fig. 5A, B). UC-MSC CM significantly enhanced the number of PECAM/CD31-stained cells (vs. CTR P<0.05; vs. CM BM-MSC P<0.05) (Fig. 5D) as well as the area of vessel outgrowth when compared with the other conditions (vs. CTR P<0.05; vs. CM BM-MSC P<0.05) (Fig. 5E).
FIG. 5.
Blood vessel sprouting in explants of mouse fetal metatarsal bones maintained in different culture conditions. Vessels were stained with antibodies against PECAM-1/CD31. Explants were cultured in the presence of (A) control medium (CTR); (B) BM-MSC-conditioned medium (BM-MSC CM); and (C) UC-MSC-conditioned medium (UC-MSC CM). Scale bar=1,000 μm (A–C and C insert). Quantification of the number of PECAM-1/CD31-positive pixels and the area of vessel outgrowth are shown in (D, E), respectively. (*P<0.05) (n=3 for both cell CM and control medium).
UC-MSC secretome is rich in proinflammatory, proangiogenic, and chemotactic cytokines
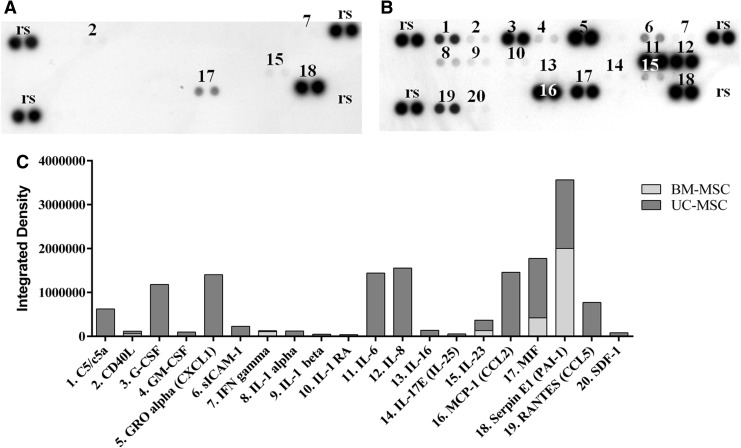
We addressed the possible paracrine effects of UC-MSCs by investigating their secretome. Numerous cytokines were secreted by UC-MSCs (Fig. 6B). Most of the ones present in a high amount (IL-6, IL-8, CCL2, PAI-1, CXCL1, MIF, and G-CSF) (Fig. 6C) are known to be involved in the modulation of the inflammatory process. These cytokines were not secreted by BM-MSCs or their level in the CM was much lower than in UC-MSC CM. PAI-1 was the only cytokine secreted in a relatively significant amount by BM-MSCs in the same conditions (Fig. 6A, C).
FIG. 6.
Cytokine array membrane assay of MSC CM; (A) BM-MSC CM; (B) UC-MSC CM; (C) quantification of the positive pixels for each identified cytokine in BM-MSC and UC-MSC CM. rs, reference spots represent an internal control.
Bioinformatic analysis performed in GO gave information about the biological processes involving the screened cytokines. The prevalent biological functions recorded concerned the activation of angiogenesis, cell signaling, cell proliferation, chemotaxis, tissue repair/regeneration, modulation of leukocytes, and inflammation. Each of these biological processes involved two to nine of the screened cytokines.
The first four biological processes that emerged by the analysis are strictly related to generic cytokine roles; therefore, we excluded the first four GO term scores (gray rows) from the evaluation (Table 1).
Table 1.
Bioinformatics Analysis in Gene Ontology of the Screened Cytokines for Umbilical Cord Mesenchymal Stem Cell-Conditioned Medium
| GO term | Cytokine involved | Count | % | P-value | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immune system process | MIF | IL-8 | IL-6 | IL-25 | IL-23A | IL-1B | IL-IA | IL-1RA | IFNG | C5 | CSF2 | CXCL1 | CCL5 | CCL2 | CD40L | 15 | 94 | 1,00E-15 | |
| Response to wounding | PAI-1 | MIF | IL-8 | IL-6 | IL-25 | IL-23A | IL-1B | IL-IA | IL-1RA | C5 | CXCL1 | CCL5 | CCL2 | CD40L | 14 | 88 | 2,50E-17 | ||
| Defense response | MIF | IL-8 | IL-6 | IL-25 | IL-23A | IL-1B | IL-IA | IL-1RA | IFNG | C5 | CXCL1 | CCL5 | CCL2 | CD40L | 14 | 88 | 1,70E-16 | ||
| Inflammatory response | MIF | IL-8 | IL-6 | IL-25 | IL-23A | IL-1B | IL-IA | IL-1RA | C5 | CXCL1 | CCL5 | CCL2 | CD40L | 13 | 81 | 7,70E-18 | |||
| Regulation of cell proliferation | PAI-1 | IL-8 | IL-6 | IL-1B | IL-IA | IFNG | CSF2 | CXCL1 | CCL2 | 9 | 56 | 4,10E-07 | |||||||
| Locomotory behavior | IL-8 | IL-6 | IL-1B | IFNG | C5 | CXCL1 | CCL5 | CCL2 | 8 | 50 | 5,40E-09 | ||||||||
| Negative regulation of apoptosis | MIF | IL-6 | IL-1B | IL-1A | CSF2 | CCL2 | CD40L | 7 | 44 | 9,90E-07 | |||||||||
| Cell chemotaxis | IL-8 | IL-6 | IL-1B | IFNG | CCL5 | CCL2 | 6 | 38 | 3,60E-10 | ||||||||||
| Localization of cell | IL-8 | IL-6 | IL-1B | IFNG | CCL5 | CCL2 | 6 | 38 | 1,20E-05 | ||||||||||
| Regulation of secretion | IL-6 | IL-1B | IL-1A | IFNG | CD40L | 5 | 31 | 4,90E-05 | |||||||||||
| Cell activation | IL-8 | IL-6 | IL-23A | CSF2 | CD40L | 5 | 31 | 1,90E-04 | |||||||||||
| Positive regulation of cell communication | IL-6 | IL-1B | IFNG | CSF2 | CCL2 | 5 | 31 | 3,20E-04 | |||||||||||
| Regulation of angiogenesis | PAI-1 | IL-6 | IL-1B | IL-1A | 4 | 25 | 3,70E-05 | ||||||||||||
| Acute inflammatory response | IL-6 | IL-1B | IL-1A | C5 | 4 | 25 | 1,40E-04 | ||||||||||||
| Regulation of vascular endothelial growth factor production | IL-6 | IL-1B | IL-1A | 3 | 19 | 3,80E-05 | |||||||||||||
| Acute-phase response | IL-6 | IL-1B | IL-1A | 3 | 19 | 8,00E-04 | |||||||||||||
| Blood coagulation | PAI-1 | IL-6 | CD40L | 3 | 19 | 5,10E-03 | |||||||||||||
| Leukocyte-mediated immunity | IL-6 | C5 | CD40L | 3 | 19 | 3,70E-03 | |||||||||||||
| Chronic inflammatory response | IL-1B | CCL5 | 2 | 13 | 6,40E-03 | ||||||||||||||
| Platelet activation | IL-6 | CD40L | 2 | 13 | 3,30E-02 | ||||||||||||||
| Regeneration | PAI-1 | CCL2 | 2 | 13 | 7,10E-02 | ||||||||||||||
Biological processes identified by the cytokine clusterization (activation of angiogenesis, cell signaling, cell proliferation, chemotaxis, tissue repair/regeneration, modulation of leukocytes, and inflammation) involved from two to nine of the selected cytokines. The first four GO term scores (gray rows) were excluded.
GO, gene ontology.
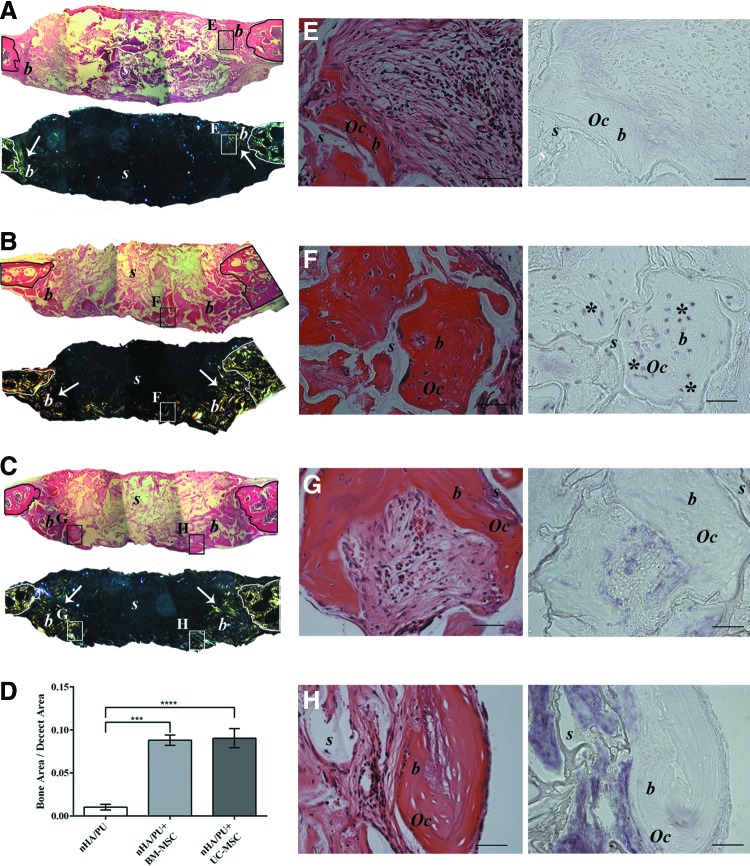
UC-MSC-induced bone formation by the host cells in an orthotopic mouse model
Histological evaluation of the 90-day recovered orthotopic implants showed that none of the bone defects from any of the three groups had completely closed (Fig. 7A–C). However, abundant, newly formed mature bone tissue was observed in the defects that received BM-MSC-seeded scaffolds (Fig. 7B). Nevertheless, a good amount of mature bone was also observed in the defects filled with UC-MSC-seeded scaffolds (Fig. 7C). In the defects that received noncell-seeded scaffolds, only a small amount of new bone tissue was noted (Fig. 7A, D). An analysis using polarized light highlighted the presence of abundant bone tissue with highly organized fibers in BM-MSC-seeded implants (Fig. 7B). In UC-MSC-seeded implants, the polarized light revealed less extended and less defined fibers (Fig. 7C). A negligible signal was detected in nonseeded implants (Fig. 7A). There were no significant differences in the amounts of new bone filled between BM-MSC and UC-MSC groups (P=0.97). However, it was significantly higher in both groups with respect to the nonseeded controls (CTR vs. UC-MSC P<0.0001; CTR vs. BM-MSC P<0.001) (Fig. 7D).
FIG. 7.
Histological images of orthotopic implants in mouse calvaria recovered after 3 months, stained with hematoxylin and eosin, and analyzed by polarized light at low magnification (A–C) and stained with hematoxylin and eosin and analyzed by in situ hybridization for human ALU repeat sequences at high magnification (E–H). Black rectangles indicate the areas from which higher magnification images where taken. (A, E) Empty scaffold (PU-HA); (B, F) BM-MSC-seeded scaffold (PU-HA+BM-MSC); (C, G, H) UC-MSC-seeded scaffold (PU-HA+UC-MSC). (n=3 for both cell-seeded and not seeded scaffold groups.) A quantification of the new bone formed in each condition determinated by measuring the surface area of new bone is shown in (D). s, scaffold; b, bone; (↑), polarized light signal; Oc, osteocyte; (*), human cell. Scale bar=1,000 and 50 μm for (A–C) and (E–H), respectively. ****(mHA/PU vs mHA/PU+UC-MSC P<0.0001 and vs mHA/PU+BM-MSC P<0.001). Color images available online at www.liebertpub.com/scd
To determine the origin of the regenerated bone, in situ hybridization analysis of human ALU sequences was performed. Similar to what was demonstrated in the ectopic implants, in the BM-MSC-seeded scaffolds, osteocytes of human origin were clearly detectable (Fig. 7F), indicating that the seeded MSCs remained in the scaffold for 3 months and successfully differentiated into viable osteoblasts that could lay down new bone matrix. On the contrary, we could not detect any signal for human ALU sequences in osteocytes within the newly formed bone in the UC-MSC implants, neither in the center (Fig. 7H) nor at the periphery (Fig. 7G) of the defect. This confirmed the murine origin of the regenerated tissue and again that UC-MSCs did not physically remain in the scaffolds. As expected, no signal was detected in the nonseeded implants (Fig. 7E).
Discussion
Despite several articles that have reported similar phenotypic characteristics for UC-MSCs and BM-MSCs, controversial data exist on their respective roles in the body's physiological tissue environments and their fate after in vitro expansion and in vivo implantation. In this work, we were mainly concerned with the osteogenic potential of in vitro-expanded UC-MSCs compared with BM-MSCs [30–32].
To obtain the cells required for the comparison of the osteogenic potential of the two MSC populations, we relied on culture protocols authorized for use in cell therapy applications (www.clinicaltrials.gov, Identifier: NCT02032446) [4]. In view of the increasing use of UC-MSCs for clinical therapy, it has become crucial to adhere to GMP guidelines for the ex vivo manipulation and expansion of these cells. Regulatory requirements strongly recommend avoiding any animal-derived medium component as FBS for cell expansion [33,34]. In this context, a GMP-compliant method was PL as a substitute for FBS. While other isolation and expansion protocols of UC-MSCs were proposed [33,35], the method we adopted allows a minimal manipulation of the UC, thus avoiding the possible initial selection of cell subpopulations [6] yielding the isolation of high-grade MSCs [36].
On the contrary, the classical expansion protocols for BM-MSCs, accepted for clinical use by the regulatory agencies [4], still employ FBS with FGF-2 as the medium additional supplement, which provides excellent results [37,38]. However, with regard to the present study, it should be noted that no major changes were observed in the phenotype characteristics and properties of BM-MSCs expanded in the presence of PL or FBS [34,36,39].
Although UC-MSCs are considered MSCs since they fulfill classical criteria [17], they do not appear as efficient as BM-MSCs for ectopic bone formation. UC-MSCs failed to deposit a well-developed bone structure, being limited to the promotion of the deposition of a dense collagen matrix. Similar results were obtained also by other research groups [32,40]. Furthermore, preinduction of UC-MSCs by osteogenic differentiation media, or BMP-2, did not significantly enhance their ossification potential in vivo [32,41]. The bone lack was even not influenced by the in vivo stay time (data not shown) or by the ceramic scaffold itself [42].
On the contrary, ectopically implanted BM-MSCs underwent osteogenic differentiation and deposited a true bone tissue. Interestingly, when orthotopically implanted in the mouse calvaria, both MSC populations promoted the formation of new bone; however, while bone was directly deposited by BM-MSCs, UC-MSCs did not directly deposit a bone matrix and instead triggered the recruitment of osteogenic host cells.
We showed by in situ hybridization and by flow cytometry (data not shown) that UC-MSCs remained in vivo at the implantation site only for a brief time. This observation is consistent with other published data where implanted human amniotic fluid stem cells were detected only for a few days within the implants [43]. Similarly, implanted UC-MSCs remained detectable in a skin lesion model only up to 11 days [44]. However, others have reported that ectopically implanted UC-MSCs could be detected in newly formed bone tissue after 8 weeks of implantation [45].
At variance with UC-MSCs, it is widely accepted that BM-MSCs are able to directly engraft, differentiate, and deposit new bone [40], although only 10% of the adult implanted BM-MSCs remain in the ectopic implants after 14 days [46].
Recent studies suggest that the benefits of MSC transplantation may be associated with a paracrine modulatory effect and not only with a direct engraftment potential [43,47]. In particular, fetal MSCs have gained much attention as tools capable of activating the endogenous repair mechanisms of the host by furnishing a burst of paracrine factors [44,46]. Such an effect has been demonstrated despite their rapid clearance from the site of implantation and confirms that their physical existence at the site throughout the entire time required for repair to take place is not necessary to ensure complete healing. Furthermore, it has been shown that the secretome of these cells alone is capable of bringing about the same response [23,48]. The degree of stemness or level of lineage commitment of an MSC population appeared to directly influence this phenomenon. UC-MSCs, expressing low levels of typical embryonic stem cell (ESC) markers, such as POUF1, Nanog, Sox-2, and Lin28 [49], show a gene profile closer to ESCs [50], whereas BM-MSCs express an osteogenic gene profile [24]. We observed that UC-MSC paracrine activity played a major role in the in vivo angiogenic effect elicited by implanted cells. The origin of the blood vessels was murine and no human cells differentiated into endothelial cells, indicating an indirect angiogenic effect rather than a direct differentiation of the cells. This was also confirmed by the fact that the UC-MSC secretome (CM) alone was capable of significantly inducing angiogenesis [49,51]. This effect was mediated only by UC-MSCs, excluding the possible role of endothelial progenitor contaminants as previously demonstrated [6].
Indeed, UC-MSCs have a secretome enriched with growth factors and cytokines related to angiogenesis, tissue repair, and wound healing processes when compared with BM-MSCs [52]. Many growth factors linked with angiogenesis were identified in the UC-MSC secretome, such as VEGF-D, PDGF-AA, TGF-β2, b-FGF, and HGF [53]. Interestingly, we also found that UC-MSCs secreted proinflammatory (CD40L, C5, and IL-6), proangiogenic (IL-6 and PAI-1), and chemotactic (CCL2, CCL5, IL-6, and IL-8) cytokines in high quantities [49,54]. IL-6 was recently associated with MSC pluripotency and immunoprivilege. The combination of BM-MSCs and IL-6 was shown to be much more effective in attenuating liver fibrosis than BM-MSCs alone [55]. These results suggest that UC-MSCs alone could be a more appropriate candidate for cell therapy since these cells secrete higher IL-6 levels [56].
Recent studies have highlighted the essential roles of proresolving M2 macrophages in tissue repair and regeneration [57]. We showed that UC-MSC secretome was enriched in M2 inducers, GM-CSF [58], CCL2, and IL-6 [59], thus suggesting a crucial role of the UC-MSC secretome in activating M2 proresolving macrophages, thereby modulating the wound healing process [26].
Despite their poor osteogenic capability when implanted ectopically, UC-MSCs were able to sustain the formation of new bone matrix in an orthotopic model in an amount comparable with that of implanted BM-MSCs [30,31]. It remains unclear if the in vivo paracrine effects of UC-MSCs are merely mediated by the UC-MSC secretome itself or also by an intercellular cross talk between the exogenous stem cells and the host endogenous progenitors; a new concept recently hypothesized in the literature [25].
In summary, the therapeutic potential of UC-MSCs appears to be vast and diverse and we are just beginning to discover their true potential. Although additional experiments are required to investigate further UC-MSC secretome-mediated angiogenesis in vivo, it can already be deduced from our study that UC-MSCs could have an effective potential use as an angiogenic tool. This is in addition to the immunomodulating role of the UC-MSC secretome making these cells, and/or their secretome, attractive therapeutic candidates for treatment of chronic wounds such as diabetic ulcers or nonhealing fractures.
Acknowledgments
The authors would like to thank Dr. Michele Cilli for the surgical assistance and Dr. Mauro Alini for providing the poly (ester urethane) scaffold. This work was partially supported by funds from Regione Liguria, Italy (POR-FESR 2007–2013).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mauney JR, Ph D, Volloch V. and Kaplan DL. (2005). Role of adult mesenchymal stem cells in bone tissue-engineering applications: current status and future prospects. Tissue Eng 11:787–802 [DOI] [PubMed] [Google Scholar]
- 2.El Backly RM, Zaky SH, Muraglia A, Tonachini L, Brun F, Canciani B, Chiapale D, Santolini F, Cancedda R. and Mastrogiacomo M. (2013). A platelet-rich plasma-based membrane as a periosteal substitute with enhanced osteogenic and angiogenic properties: a new concept for bone repair. Tissue Eng Part A 19:152–165 [DOI] [PubMed] [Google Scholar]
- 3.Giannoni P, Mastrogiacomo M, Alini M, Pearce SG, Corsi A, Santolini F, Muraglia A, Bianco P. and Cancedda R. (2008). Regeneration of large bone defects in sheep using bone marrow stromal cells. J Tissue Eng Regen Med 2:253–262 [DOI] [PubMed] [Google Scholar]
- 4.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E. and Marcacci M. (2001). Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344:385–386 [DOI] [PubMed] [Google Scholar]
- 5.Hass R, Kasper C, Böhm S. and Jacobs R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capelli C, Gotti E, Morigi M, Rota C, Weng L, Dazzi F, Spinelli O, Cazzaniga G, Trezzi R, et al. (2011). Minimally manipulated whole human umbilical cord is a rich source of clinical-grade human mesenchymal stromal cells expanded in human platelet lysate. Cytotherapy 13:786–801 [DOI] [PubMed] [Google Scholar]
- 7.Abu Kasim NH, Govindasamy V, Gnanasegaran N, Musa S, Pradeep PJ, Srijaya TC. and Aziz ZA. (2012). Unique molecular signatures influencing the biological function and fate of post-natal stem cells isolated from different sources. J Tissue Eng Regen Med [Epub ahead of print]; DOI: 10.1002/term.1663 [DOI] [PubMed] [Google Scholar]
- 8.El Omar R, Beroud J, Stoltz J-F, Menu P, Velot E. and Decot V. (2014). Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies?. Tissue Eng Part B Rev 20:523–544 [DOI] [PubMed] [Google Scholar]
- 9.Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D. and McIntosh KR. (2008). Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells 26:2865–2874 [DOI] [PubMed] [Google Scholar]
- 10.Tipnis S, Viswanathan C. and Majumdar AS. (2010). Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol 88:795–806 [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro A, Laranjeira P, Mendes S, Velada I, Leite C, Andrade P, Santos F, Henriques A, Grãos M, et al. (2013). Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther 4:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Che N, Li X, Zhou S, Liu R, Shi D, Lu L. and Sun L. (2012). Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell Immunol 274:46–53 [DOI] [PubMed] [Google Scholar]
- 13.Ji YR, Yang ZX, Han Z-B, Meng L, Liang L, Feng XM, Yang SG, Chi Y, Chen DD, Wang YW. and Han ZC. (2012). Mesenchymal stem cells support proliferation and terminal differentiation of B cells. Cell Physiol Biochem 30:1526–1537 [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Cao Y, Li X, Xu L, Wang Z, Liu P, Yan P, Liu Z, Wang J, et al. (2014). Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplastic anemia: successful engraftment and mild GVHD. Stem Cell Res 12:132–138 [DOI] [PubMed] [Google Scholar]
- 15.Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, Phan TT, Volk H-D, Reichenspurner H, Robbins RC. and Schrepfer S. (2011). Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant 20:655–667 [DOI] [PubMed] [Google Scholar]
- 16.Introna M, Lucchini G, Dander E, Galimberti S, Rovelli A, Balduzzi A, Longoni D, Pavan F, Masciocchi F, et al. (2014). Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant 20:375–381 [DOI] [PubMed] [Google Scholar]
- 17.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 18.Majore I, Moretti P, Stahl F, Hass R. and Kasper C. (2011). Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev 7:17–31 [DOI] [PubMed] [Google Scholar]
- 19.Girdlestone J, Limbani VA, Cutler AJ. and Navarrete C V. (2009). Efficient expansion of mesenchymal stromal cells from umbilical cord under low serum conditions. Cytotherapy 11:738–748 [DOI] [PubMed] [Google Scholar]
- 20.Suzdal'tseva YG, Burunova VV, Vakhrushev IV, Yarygin VN. and Yarygin KN. (2007). Capability of human mesenchymal cells isolated from different sources to differentiation into tissues of mesodermal origin. Bull Exp Biol Med 143:114–121 [DOI] [PubMed] [Google Scholar]
- 21.Bosch J, Houben AP, Radke TF, Stapelkamp D, Bünemann E, Balan P, Buchheiser A, Liedtke S. and Kögler G. (2012). Distinct differentiation potential of “MSC”. derived from cord blood and umbilical cord: are cord-derived cells true mesenchymal stromal cells?. Stem Cells Dev 21:1977–1988 [DOI] [PubMed] [Google Scholar]
- 22.Troyer DL. and Weiss ML. (2008). Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells 26:591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider RK, Puellen A, Kramann R, Raupach K, Bornemann J, Knuechel R, Pérez-Bouza A. and Neuss S. (2010). The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials 31:467–480 [DOI] [PubMed] [Google Scholar]
- 24.Hsieh J. and Fu Y. (2010). Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton's jelly of umbilical cord. Stem Cells Dev 19:1896–1910 [DOI] [PubMed] [Google Scholar]
- 25.Bollini S, Gentili C, Tasso R. and Cancedda R. (2013). The regenerative role of the fetal and adult stem cell secretome. J Clin Med 2:302–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shohara R, Yamamoto A, Takikawa S, Iwase A, Hibi H, Kikkawa F. and Ueda M. (2012). Mesenchymal stromal cells of human umbilical cord Wharton's jelly accelerate wound healing by paracrine mechanisms. Cytotherapy 14:1171–1181 [DOI] [PubMed] [Google Scholar]
- 27.Muraglia A, Martin I, Cancedda R. and Quarto R. (1998). A nude mouse model for human bone formation in unloaded conditions. Bone 22:131S–134S [DOI] [PubMed] [Google Scholar]
- 28.Ignowski JM. and Schaffer D V. (2004). Kinetic analysis and modeling of firefly luciferase as a quantitative reporter gene in live mammalian cells. Biotechnol Bioeng 86:827–834 [DOI] [PubMed] [Google Scholar]
- 29.Daga A, Muraglia A, Quarto R, Cancedda R. and Corte G. (2002). Enhanced engraftment of EPO-transduced human bone marrow stromal cells transplanted in a 3D matrix in non-conditioned NOD/SCID mice. Gene Ther 9:915–921 [DOI] [PubMed] [Google Scholar]
- 30.Kuiper EJ, Roestenberg P, Ehlken C, Lambert V, van Treslong-de Groot HB, Lyons KM, Agostini H-JT, Rakic J-M, Klaassen I, et al. (2007). Angiogenesis is not impaired in connective tissue growth factor (CTGF) knock-out mice. J Histochem Cytochem 55:1139–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spicer PP, Kretlow JD, Young S, Jansen JA, Kasper FK. and Mikos AG. (2012). Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc 7:1918–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laschke MW, Strohe A, Menger MD, Alini M. and Eglin D. (2010). In vitro and in vivo evaluation of a novel nanosize hydroxyapatite particles/poly(ester-urethane) composite scaffold for bone tissue engineering. Acta Biomater 6:2020–2027 [DOI] [PubMed] [Google Scholar]
- 33.(1997). Opinion of the EMEA on the potential risk associated with medicinal products in relation to bovine spongiform encephalopathy (BSE) (16 April 1996) and report from the Committee for Proprietary Medicinal Products (CPMP) on the'Note for Guidance on minimizing the risk of transmitting animal spongiform encephalopathies via medicinal products' (15 April 1997). Adverse Drug React Toxicol Rev 16:113–121 [PubMed] [Google Scholar]
- 34.Tuschong L, Soenen SL, Blaese RM, Candotti F. and Muul LM. (2002). Immune response to fetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum Gene Ther 13:1605–1610 [DOI] [PubMed] [Google Scholar]
- 35.Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, Hu X, Jiang S, Shi S. and Sun L. (2014). Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther 16:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capelli C, Domenghini M, Borleri G, Bellavita P, Poma R, Carobbio A, Micò C, Rambaldi A, Golay J. and Introna M. (2007). Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone Marrow Transplant 40:785–791 [DOI] [PubMed] [Google Scholar]
- 37.Petsa A, Gargani S, Felesakis A, Grigoriadis N. and Grigoriadis I. (2009). Effectiveness of protocol for the isolation of Wharton's Jelly stem cells in large-scale applications. In Vitro Cell Dev Biol Anim 45:573–576 [DOI] [PubMed] [Google Scholar]
- 38.Majore I, Moretti P, Hass R. and Kasper C. (2009). Identification of subpopulations in mesenchymal stem cell-like cultures from human umbilical cord. Cell Commun Signal 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN. and Papamichail M. (2006). Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 24:462–471 [DOI] [PubMed] [Google Scholar]
- 40.Martin I, Muraglia A, Campanile G, Cancedda R. and Quarto R. (1997). Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology 138:4456–4462 [DOI] [PubMed] [Google Scholar]
- 41.Chevallier N, Anagnostou F, Zilber S, Bodivit G, Maurin S, Barrault A, Bierling P, Hernigou P, Layrolle P. and Rouard H. (2010). Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials 31:270–278 [DOI] [PubMed] [Google Scholar]
- 42.Ohgushi H, Miyake J. and Tateishi T. (2003). Mesenchymal stem cells and bioceramics: strategies to regenerate the skeleton. Novartis Found Symp 249: 118–127; discussion 127–132, 170–174, 239–241 [PubMed] [Google Scholar]
- 43.Mirabella T, Poggi A, Scaranari M, Mogni M, Lituania M, Baldo C, Cancedda R. and Gentili C. (2011). Recruitment of host's progenitor cells to sites of human amniotic fluid stem cells implantation. Biomaterials 32:4218–4227 [DOI] [PubMed] [Google Scholar]
- 44.Sabapathy V, Sundaram B, Vm S, Mankuzhy P. and Kumar S. (2014). Human wharton's jelly mesenchymal stem cells plasticity augments scar-free skin wound healing with hair growth. PLoS One 9:e93726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mueller AA, Forraz N, Gueven S, Atzeni G, Degoul O, Pagnon-Minot A, Hartmann D, Martin I, Scherberich A. and McGuckin C. (2014). Osteoblastic differentiation of Wharton jelly biopsy specimens and their mesenchymal stromal cells after serum-free culture. Plast Reconstr Surg 134:59e–69e [DOI] [PubMed] [Google Scholar]
- 46.Giannoni P, Ph D, Scaglione S, Daga A, Ilengo C, Sci B, Cilli M. and Quarto R. (2010). Short-time survival and engraftment of bone marrow stromal cells in an ectopic model of bone regeneration. Tissue Eng Part A 16:489–499 [DOI] [PubMed] [Google Scholar]
- 47.Prasanna SJ, Gopalakrishnan D, Shankar SR. and Vasandan AB. (2010). Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One 5:e9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ. and Wang C-Y. (2013). The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fong C-Y, Chak L-L, Biswas A, Tan J-H, Gauthaman K, Chan W-K. and Bongso A. (2011). Human Wharton's jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev 7: 1–16 [DOI] [PubMed] [Google Scholar]
- 50.Nagamura-Inoue T. and He H. (2014). Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells 6:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tasso R, Gaetani M, Molino E, Cattaneo A, Monticone M, Bachi A. and Cancedda R. (2012). The role of bFGF on the ability of MSC to activate endogenous regenerative mechanisms in an ectopic bone formation model. Biomaterials 33:2086–2096 [DOI] [PubMed] [Google Scholar]
- 52.Hsieh J-Y, Wang H-W, Chang S-J, Liao K-H, Lee I-H, Lin W-S, Wu C-H, Lin W-Y. and Cheng S-M. (2013). Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One 8:e72604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balasubramanian S, Venugopal P, Sundarraj S, Zakaria Z, Sen Majumdar A. and Ta M. (2012). Comparison of chemokine and receptor gene expression between Wharton's jelly and bone marrow-derived mesenchymal stromal cells. Cytotherapy 14:26–33 [DOI] [PubMed] [Google Scholar]
- 54.Edwards SS, Zavala G, Prieto CP, Elliott M, Martínez S, Egaña JT, Bono MR. and Palma V. (2014). Functional analysis reveals angiogenic potential of human mesenchymal stem cells from Wharton's jelly in dermal regeneration. Angiogenesis 17:851–866 [DOI] [PubMed] [Google Scholar]
- 55.Nasir GA, Mohsin S, Khan M, Shams S, Ali G, Khan SN. and Riazuddin S. (2013). Mesenchymal stem cells and Interleukin-6 attenuate liver fibrosis in mice. J Transl Med 11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amable PR, Teixeira MVT, Carias RBV, Granjeiro JM. and Borojevic R. (2014). Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton's jelly. Stem Cell Res Ther 5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brancato SK. and Albina JE. (2011). Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol 178:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant V, King AE, Faccenda E. and Kelly RW. (2005). PGE/cAMP and GM-CSF synergise to induce a pro-tolerance cytokine profile in monocytic cell lines. Biochem Biophys Res Commun 331:187–193 [DOI] [PubMed] [Google Scholar]
- 59.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C. and Pienta KJ. (2009). CCL2 and interleukin-6 promote survival of human CD11b+peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 284:34342–34354 [DOI] [PMC free article] [PubMed] [Google Scholar]