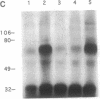
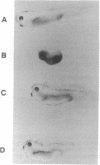
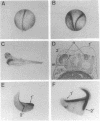
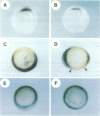
Abstract
Bone morphogenetic proteins (BMPs), which are members of the transforming growth factor beta (TGF-beta) superfamily, have been implicated in bone formation and the regulation of early development. To better understand the roles of BMPs in Xenopus laevis embryogenesis, we have cloned a cDNA coding for a serine/threonine kinase receptor that binds BMP-2 and BMP-4. To analyze its function, we attempted to block the BMP signaling pathway in Xenopus embryos by using a dominant-negative mutant of the BMP receptor. When the mutant receptor lacking the putative serine/threonine kinase domain was expressed in ventral blastomeres of Xenopus embryos, these blastomeres were respecified to dorsal mesoderm, eventually resulting in the formation of a secondary body axis. These findings suggest that endogenous BMP-2 and BMP-4 are involved in the dorsal-ventral specification in the embryo and that ventral fate requires induction rather than resulting from an absence of dorsal specification.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asashima M., Nakano H., Uchiyama H., Sugino H., Nakamura T., Eto Y., Ejima D., Nishimatsu S., Ueno N., Kinoshita K. Presence of activin (erythroid differentiation factor) in unfertilized eggs and blastulae of Xenopus laevis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6511–6514. doi: 10.1073/pnas.88.15.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand T., MacLellan W. R., Schneider M. D. A dominant-negative receptor for type beta transforming growth factors created by deletion of the kinase domain. J Biol Chem. 1993 Jun 5;268(16):11500–11503. [PubMed] [Google Scholar]
- Cho K. W., Blumberg B., Steinbeisser H., De Robertis E. M. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991 Dec 20;67(6):1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
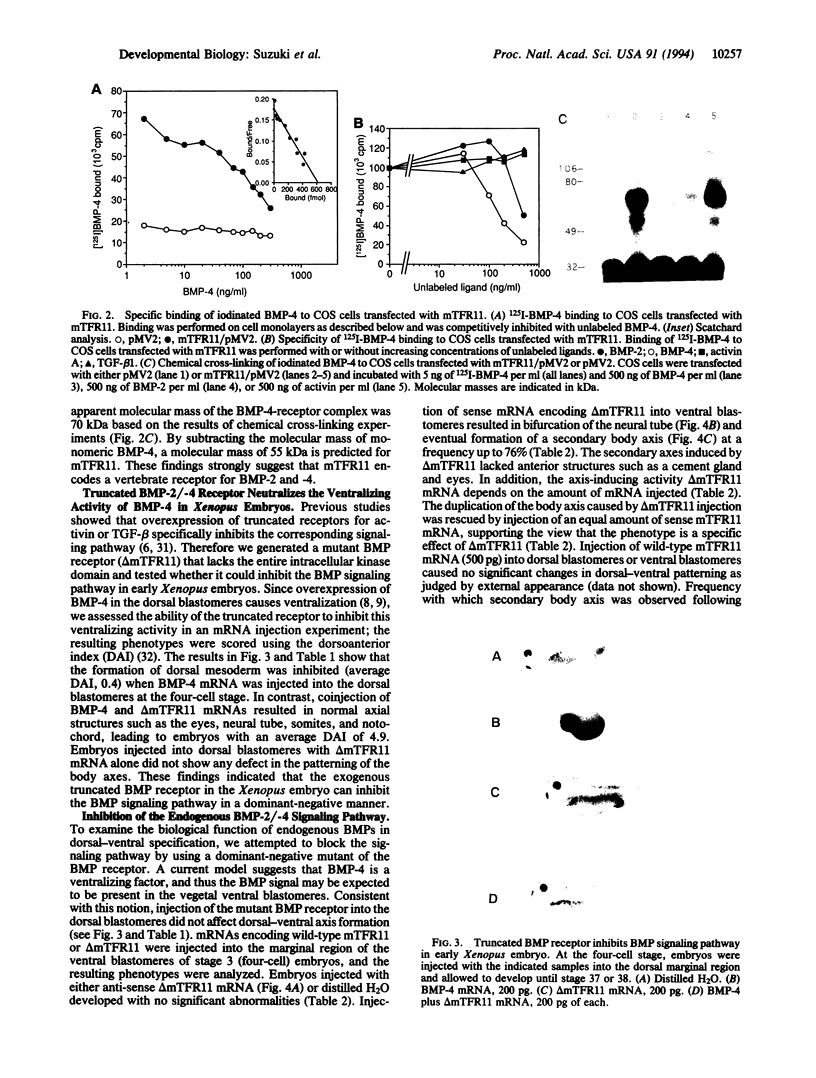
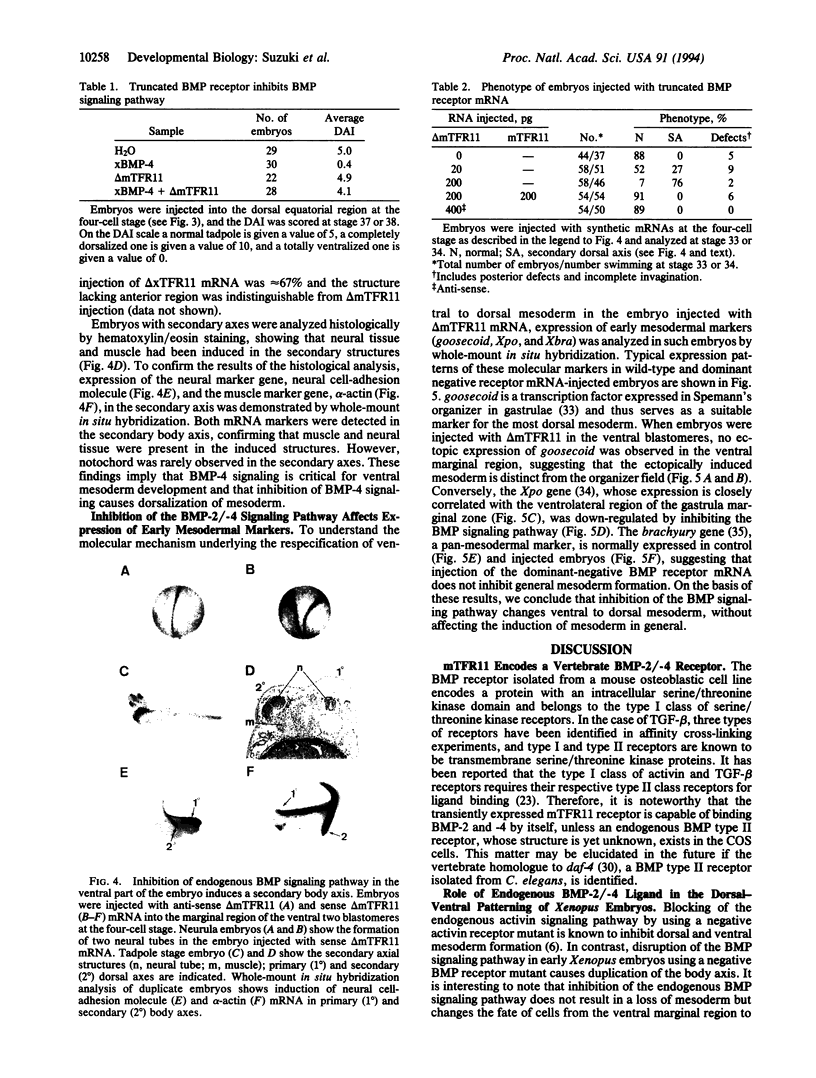
- Dale L., Howes G., Price B. M., Smith J. C. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992 Jun;115(2):573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- Ebner R., Chen R. H., Lawler S., Zioncheck T., Derynck R. Determination of type I receptor specificity by the type II receptors for TGF-beta or activin. Science. 1993 Nov 5;262(5135):900–902. doi: 10.1126/science.8235612. [DOI] [PubMed] [Google Scholar]
- Estevez M., Attisano L., Wrana J. L., Albert P. S., Massagué J., Riddle D. L. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993 Oct 14;365(6447):644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- Frolik C. A., Wakefield L. M., Smith D. M., Sporn M. B. Characterization of a membrane receptor for transforming growth factor-beta in normal rat kidney fibroblasts. J Biol Chem. 1984 Sep 10;259(17):10995–11000. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Harland R. M. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Melton D. A. A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature. 1992 Oct 15;359(6396):609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Lapan P. M., Wright C. V., Hogan B. L. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992 Jun;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Kao K. R., Elinson R. P. The entire mesodermal mantle behaves as Spemann's organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol. 1988 May;127(1):64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley D. M. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994 Jan;8(2):133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M. What do BMPs do in mammals? Clues from the mouse short-ear mutation. Trends Genet. 1994 Jan;10(1):16–21. doi: 10.1016/0168-9525(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Kondo M., Tashiro K., Fujii G., Asano M., Miyoshi R., Yamada R., Muramatsu M., Shiokawa K. Activin receptor mRNA is expressed early in Xenopus embryogenesis and the level of the expression affects the body axis formation. Biochem Biophys Res Commun. 1991 Dec 16;181(2):684–690. doi: 10.1016/0006-291x(91)91245-8. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler S., Candia A. F., Ebner R., Shum L., Lopez A. R., Moses H. L., Wright C. V., Derynck R. The murine type II TGF-beta receptor has a coincident embryonic expression and binding preference for TGF-beta 1. Development. 1994 Jan;120(1):165–175. doi: 10.1242/dev.120.1.165. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991 Jun 14;65(6):973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W., Kintner C. R. Cloning of a second type of activin receptor and functional characterization in Xenopus embryos. Science. 1992 Mar 27;255(5052):1702–1705. doi: 10.1126/science.1313188. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Christian J. L. Competence modifiers synergize with growth factors during mesoderm induction and patterning in Xenopus. Cell. 1992 Nov 27;71(5):709–712. doi: 10.1016/0092-8674(92)90545-n. [DOI] [PubMed] [Google Scholar]
- Nishimatsu S., Suzuki A., Shoda A., Murakami K., Ueno N. Genes for bone morphogenetic proteins are differentially transcribed in early amphibian embryos. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1487–1495. doi: 10.1016/s0006-291x(05)81574-8. [DOI] [PubMed] [Google Scholar]
- Sato S. M., Sargent T. D. Localized and inducible expression of Xenopus-posterior (Xpo), a novel gene active in early frog embryos, encoding a protein with a 'CCHC' finger domain. Development. 1991 Jul;112(3):747–753. doi: 10.1242/dev.112.3.747. [DOI] [PubMed] [Google Scholar]
- Shoda A., Murakami K., Ueno N. Biologically active BMP-2 in early Xenopus laevis embryos. Biochem Biophys Res Commun. 1994 Feb 15;198(3):1267–1274. doi: 10.1006/bbrc.1994.1179. [DOI] [PubMed] [Google Scholar]
- Sive H. L. The frog prince-ss: a molecular formula for dorsoventral patterning in Xenopus. Genes Dev. 1993 Jan;7(1):1–12. doi: 10.1101/gad.7.1.1. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Green J. B., Weigel D., Herrmann B. G. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991 Oct 4;67(1):79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Knecht A. K., Wu M., Harland R. M. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993 Feb 11;361(6412):547–549. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Nagai T., Nishimatsu S., Sugino H., Eto Y., Shibai H., Murakami K., Ueno N. Autoinduction of activin genes in early Xenopus embryos. Biochem J. 1994 Mar 1;298(Pt 2):275–280. doi: 10.1042/bj2980275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Nishimatsu S., Shoda A., Takebayashi K., Murakami K., Ueno N. Biochemical properties of amphibian bone morphogenetic protein-4 expressed in CHO cells. Biochem J. 1993 Apr 15;291(Pt 2):413–417. doi: 10.1042/bj2910413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Shioda N., Maeda T., Tada M., Ueno N. A mouse TGF-beta type I receptor that requires type II receptor for ligand binding. Biochem Biophys Res Commun. 1994 Feb 15;198(3):1063–1069. doi: 10.1006/bbrc.1994.1151. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Ohse C., Wang E. A., Wozney J. M., Yamashita K. Bone morphogenetic protein-2 stimulates alkaline phosphatase activity and collagen synthesis in cultured osteoblastic cells, MC3T3-E1. Biochem Biophys Res Commun. 1991 Jan 15;174(1):96–101. doi: 10.1016/0006-291x(91)90490-x. [DOI] [PubMed] [Google Scholar]
- Thies R. S., Bauduy M., Ashton B. A., Kurtzberg L., Wozney J. M., Rosen V. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992 Mar;130(3):1318–1324. doi: 10.1210/endo.130.3.1311236. [DOI] [PubMed] [Google Scholar]
- Thomsen G., Woolf T., Whitman M., Sokol S., Vaughan J., Vale W., Melton D. A. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990 Nov 2;63(3):485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Yamashita H., Ichijo H., Franzén P., Laiho M., Miyazono K., Heldin C. H. Characterization of type I receptors for transforming growth factor-beta and activin. Science. 1994 Apr 1;264(5155):101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]