Abstract
A series of pyrazolo[1,5-a]pyridine-3-carboxamide derivatives were designed and synthesized as new anti-Mycobacterium tuberculosis (Mtb) agents. The compounds exhibit promising in vitro potency with nanomolar MIC values against the drug susceptive H37Rv strain and a panel of clinically isolated multidrug-resistant Mtb (MDR-TB) strains. One of the representative compounds (5k) significantly reduces the bacterial burden in an autoluminescent H37Ra infected mouse model, suggesting its promising potential to be a lead compound for future antitubercular drug discovery.
Keywords: Antitubercular agent; H37Rv; pyrazolo[1,5-a]pyridine; structure−activity relationship; tuberculosis
Tuberculosis (TB) remains one of the world’s deadliest pandemic diseases with over 9.0 million new cases and 1.5 million deaths estimated by the World Health Organization (WHO) in 2013.1 Despite the forty-year success of the inexpensive quadruple-drug therapy [a combination of isoniazid (INH), rifampicin (RIF), pyrazinamide, and ethambutol], development and dissemination of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Mycobacterium tuberculosis (Mtb) strains, together with coinfection with Human Immunodeficiency Virus (HIV), have intensified an urgent need for new anti-TB drug discovery.2,3 Encouragingly, for the first time since 1970s, an ATP synthase inhibitor bedaquiline4 (1, also known as TMC207) was approved by the US FDA as a novel active ingredient of combinational therapies for clinical management of adult patients with MDR pulmonary TB in 2012.5 However, the drug possesses serious adverse effects such as cardiac arrhythmias5 and displayed higher death rates than that of the placebo group in a clinical investigation,6 which may limit its wide application in clinical practice. Several other anti-TB molecules were also identified with different modes of action.2 For instance, a bicyclic nitroimidazofuran pro-drug PA-824 (2) was reported to kill both replicating and hypoxic nonreplicating Mtb through a Ddn-mediated activation and has been advanced to phase II clinical trial.7,8 Imidazo[1,2-a]pyridine amide (IPA)9−13 analogues [e.g., Q203 (3)9,10 and compound 4(11)] were also discovered to demonstrate strong inhibitory potencies against a panel of drug-susceptible and drug-resistant Mtb strains by targeting the QcrB subunit of the menaquinol cytochrome c oxidoreductase (bc1 complex), which is a critical component of mycobacterial energy metabolism.14 Clinical outcomes of the compounds, particularly their capability against MDR and XDR Mtb strains in patients, are eagerly awaited. However, given the fact of only one singular FDA approval in 40 years and the high attrition rate of drug development, it is still highly valuable to identify new molecules with alternative scaffolds as effective anti-TB drug candidates.
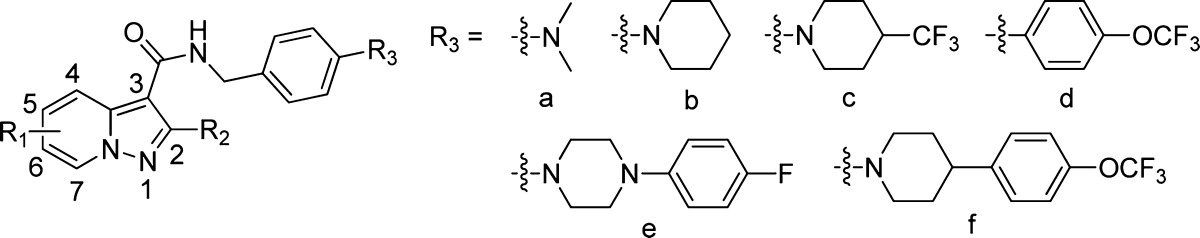
Pyrazolo[1,5-a]pyridine moiety is a drug-like scaffold that is frequently observed in FDA approved or clinically investigating drugs including antiallergic agent Ibudilast,15 platelet aggregation inhibitor KC-764,16 dopamine D4 antagonist FAUC213,17 etc. (Supporting Information). It shares highly similar 3-dimensional conformation and electronic property to that of imidazo[1,2-a]pyridine, which is the pharmaceutical core of Q203 (3) and compound 4. Therefore, a series of pyrazolo[1,5-a]pyridine-3-carboxamide derivatives (5a–5v) were designed as new anti-TB agents by using a scaffold hopping strategy in which 2,5-dimethyl groups were introduced at first because of the synthetic feasibility and keeping the geometric similarity to that of compound 4 (Figure 1).
Figure 1.
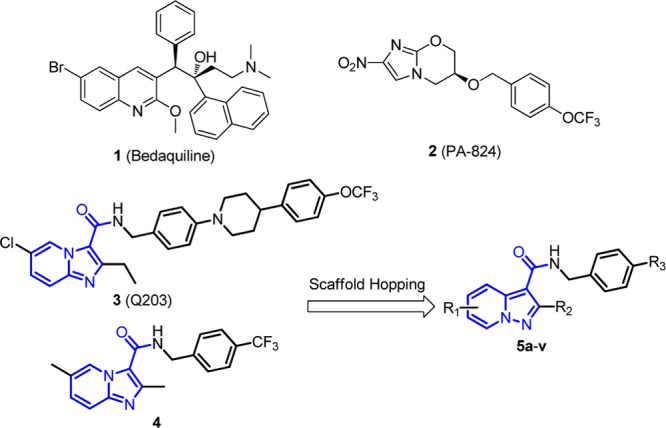
Representatives of the antitubercular agents and the new designed molecules.
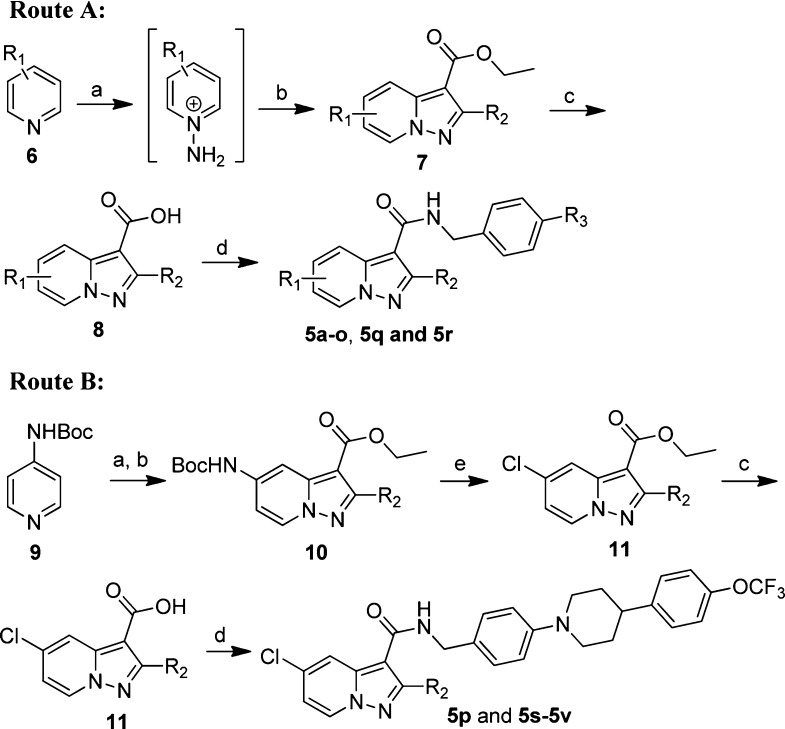
Compounds 5a–5v were readily synthesized using a straightforward amidation of pyrazolo[1,5-a]pyridine-3-carboxylic acids 8 or 11 with different primary amines (Scheme 1). Briefly, an N-amination of substituted pyridines 6 or 9 with O-(2,4-dinitrophenyl)hydroxylamine (DNPH) or O-mesitylenesulfonylhydroxylamine (MSH), followed by a 1,3-bipolar cycloaddition with substituted ethyl propiolate, produced the pivotal intermediate pyrazolo[1,5-a]pyridine-3-carboxylates 7 or 10.18 Compounds 7 were hydrolyzed to yield the carboxylic acids 8, which were coupled with different commercially available or self-prepared amines (Supporting Information) to produce the designed compounds 5a–5o, 5q, and 5r with good yields (Route A). While due to the difficulty of direct N-amination of halogen-substituted pyridine, additional removal of Boc protecting group and diazotization reaction of compounds 10 were needed to convert NHBoc group to chlorine substitution for the synthesis of compounds 5p and 5s–5v (Route B).
Scheme 1. Synthesis of Compounds 5a–5v.
Reagents and conditions: (a) DNPH, MeCN, 40 °C, 18 h for all compounds expect 5q, or MSH, DCM, 0 °C, 2 h for compound 5q; (b) K2CO3, DMF, rt, 18 h, 32–57%; (c) NaOH, EtOH, H2O, 60 °C, 100%; (d) amines, EDCI, HOBt, Et3N, DMF, 80 °C, overnight, 40–87%; (e) (i) TFA, DCM, rt, 2 h, 100%; (ii) CuCl, con. HCl, NaNO2 (aq, 0.4 M), 0–80 °C, 45 min, 46–86%.
Anti-TB activities of compounds 5a–5v were preliminarily screened by using a cost-efficient in vitro assay against a selectable marker-free avirulent autoluminescent H37Ra Mtb strain in which the bacteria growth was conveniently monitored by the bioluminescence intensity without adding any substrates.19,20 The minimum inhibitory concentration (MIC) values of the active compounds were further determined by a well-established microplate alamar blue assay (MABA) against H37Rv.21 The MICs are interpolated values obtained by using an in-house curve-fitting program. The VERO cell growth inhibition was also tested using the Cell Counting Kit-8 (CCK-8) assay to assess the compounds’ potential cytotoxicity. All of the three reference compounds (i.e., INH, RIF, and Q203) displayed comparable MIC values to the reported data,10 supporting the reliability of our screening conditions (Table 1).
Table 1. In Vitro Antitubercular Activity of Compounds 5a–5v against the Mtb Strains H37Rv and H37Ra and VERO Cellular Toxicity.
| MIC [nM (μg/mL)] |
IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| compd | R1 | R2 | R3 | H37Rva | H37Rab | VEROc |
| 5a | 5-Me | Me | -CF3 | 69.1 (0.024d) | 287.9 (0.1) | >100 |
| 5b | 5-Me | Me | a | 86.8 (0.028) | 310.2 (0.1) | >100 |
| 5c | 5-Me | Me | b | 16.6 (0.006) | 27.6–82.8 (0.01–0.03) | >100 |
| 5d | 5-Me | Me | c | 13.9 (0.006) | 2.3 (0.001) | >100 |
| 5e | 5-Me | Me | d | 13.7 (0.006) | 6.8 (0.003) | 50.74 |
| 5f | 5-Me | Me | e | 13.1 (0.006) | 6.6 (0.003) | >100 |
| 5g | 5-Me | Me | f | 7.7 (0.004) | 5.7 (0.003) | >100 |
| 5h | 4-Me | Me | f | 1148 (0.60) | >19136 (>10.0) | n.d.e |
| 5i | 6-Me | Me | f | 13.4 (0.007) | 574.1 (0.3) | >100 |
| 5j | 7-Me | Me | f | 114.8 (0.06) | 5741 (3.0) | n.d. |
| 5k | 5-OMe | Me | f | 11.1 (0.006) | 5.6 (0.003) | >100 |
| 5l | H | Me | f | 9.8 (0.005) | 589.9 (0.3) | >100 |
| 5m | 5-Et | Me | f | 11.2 (0.006) | 18.6–55.9 (0.01–0.03) | >100 |
| 5n | 5-i-Pr | Me | f | 47.2 (0.026) | 1816–5448 (1.0–3.0) | n.d. |
| 5o | 5-t-butyl | Me | f | >1771 (>1.0) | >17710 (>10) | n.d. |
| 5p | 5-Cl | Me | f | 7.4 (0.004) | 18.4 (0.01) | >100 |
| 5q | 5-CF3 | Me | f | 52.0 (0.03) | 1734 (1.0) | >100 |
| 5r | 5-phenyl | Me | f | 478.9 (0.28) | >17105 (>10) | n.d. |
| 5s | 5-Cl | H | f | 358.7 (0.20) | 18905 (10.0) | n.d. |
| 5t | 5-Cl | Et | f | 10.8 (0.006) | 53.8 (0.03) | >100 |
| 5u | 5-Cl | cyclo-Pr | f | 17.6 (0.01) | 175.7 (0.1) | >100 |
| 5v | 5-Cl | phenyl | f | 1547 (0.94) | >16528 (>10) | n.d. |
| 3 | 10.8 (0.006) | 5.4 (0.003) | >100 | |||
| INH | 2989 (0.41) | 729 (0.10) | n.d. | |||
| RIF | 36.4 (0.03) | 3.64 (0.003) | n.d. | |||
Anti-TB activity assays against H37Rv were performed using MABA.
Anti-TB activity assays against H37Ra were performed using the autoluminescent assay.
VERO: African green monkey kidney cell line. The cell growth inhibition was evaluated using the CCK-8 assay.
The activity data in the brackets are reported in μg/mL.
Not determined. Values are means of two or more independent experiments, and the variation is <20%.
We were pleased to find the first designed molecule 5a, which carries an identical p-trifluoromethylbenzyl group to that in compound 4, exhibited strong antimycobacterial activity against Mtb strain H37Rv and the avirulent Mtb strain H37Ra with MIC values of 69.1 and 287.9 nM, respectively. Further investigation revealed the 4-CF3 group could be replaced by a dimethylamino moiety (5b) without obviously affecting the anti-TB potency. When a 1-piperidinyl (5c) or 4-(trifluoromethyl)piperidin-1-yl (5d) was introduced at the R3 position, the anti-H37Rv activity was improved 5–6-fold. The investigation also suggested that R3 position is well tolerated with large hydrophobic substituent. For instance, trifluoromethoxyphenyl (5e) and 4-(4-fluorophenyl)piperazin-1-yl (5f) derivatives displayed comparable anti-TB activities to that of 5d. Encouragingly, compound 5g, which harbors the identical lipophilic tail to that of Q203, demonstrated the strongest antimycobacterial activity against both H37Rv and H37Ra Mtb strains, with MIC values of 7.7 and 5.7 nM, respectively. Moreover, compound 5g did not display obvious cytotoxicity with an IC50 value of >100 μM in a VERO cell growth inhibition assay, supporting it as a new promising starting point for further structure–activity relationship study.
We first investigated the potential impact of R1 substituent on the anti-TB activity by altering the substituted position of a methyl group on the pyridine ring (5g–5j). It was shown that the 5-position is optimal for substitution (5g). When the methyl group was merged to 4-, 6-, or 7-position, the resulting compounds (5h, 5i, and 5j) were 2–149-fold less potent against Mtb H37Rv strain. Further investigation also revealed that the 5-methyl group (5g) could be replaced by a methoxyl (5k), ethyl (5m), or chloride (5p) moiety without obviously affecting the anti-TB potency. However, a large hydrophobic substituent at this position is detrimental. For instance, 5-isopropyl (5n) and 5-phenyl (5r) displayed MIC values of 47.4 and 478.9 nM against Mtb H37Rv strain, which are 6.2- and 62.2-fold less potent than 5g, respectively. Interestingly, a removal of the 5-substitutent group (5l) barely affected the suppressing function against H37Rv strain. The impact of the R2 substituent was also investigated. It was clear that this position was well tolerated by a small hydrophobic group such as a methyl (5g), ethyl (5t), or cyclopropyl (5u) moiety. However, when it was unsubstituted (5s) or phenyl substituted (5v), the resulting compounds were significantly less potent with MIC values of 358.7 and 1547 nM against H37Rv strain, respectively.
The antimycobacterial activities of compounds 5f, 5g, 5k, and 5t were further validated by determining their MIC, IC50, and IC90 values against the replication of a fluorescent reporter strain of Mtb H37Rv in liquid medium under aerobic conditions (Table 2).22 It was shown that compounds 5f and 5k displayed comparable antitubercular effects against H37Rv to that of RIF, whereas compounds 5g and 5t were moderately less potent. The IC50 and IC90 values of compound 5k are 2.5 and 6.7 nM, respectively.
Table 2. In Vitro MIC, IC50, and IC90 Values of Compounds 5f, 5g, 5k, and 5t against Fluorescent Reporter Strain of Mtb H37Rv.
| compd | MIC (nM) | IC50 (nM) | IC90 (nM) |
|---|---|---|---|
| 5f | 6.3 | 2.5 | 6.5 |
| 5g | 11.0 | 5.1 | 12.0 |
| 5k | 5.6 | 2.5 | 5.7 |
| 5t | 129 | 31.3 | 150 |
| RIF | 6.5 | 4.1 | 6.9 |
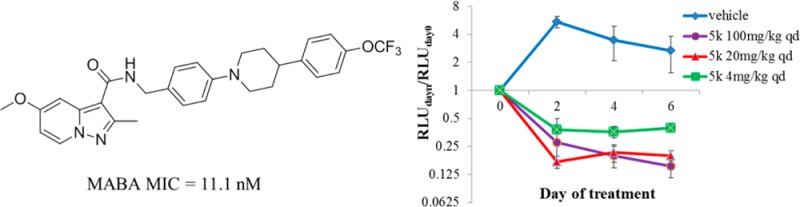
Encouraged by their strong potencies against the drug-sensitive Mtb H37Rv strain, compounds 5g, 5k, 5p and 5t were further evaluated against a panel of clinical isolated 3495, P71, 9804, 4768, P163 MDR strains23 by using an autoluminescent assay.19 It was shown that all of the compounds displayed excellent potencies against the resistant Mtb strains with MIC values ranged from 11.1 to 1914 nM (Table 3). Particularly, compound 5k exhibited superior inhibition against five resistant strains with similar MIC values (11.1–223 nM) to that of the wild-type Mtb H37Rv, suggesting its promising potential for both drug-sensitive and resistant Mtb strains.
Table 3. Antitubercular Activity of Compounds 5g, 5k, 5p, and 5t against Drug-Resistant Clinical Mtb Isolates.
| MIC (nM)b |
|||||
|---|---|---|---|---|---|
| strains | resistancea | 5g | 5k | 5p | 5t |
| 3495 | HRSZ | 1914 | 22.3–223 | n.d.c | n.d. |
| P71 | HRZ | <383 | 11.1–223 | <184 | 71.8–1795 |
| 9804 | HRZ | <383 | <11.1 | <184 | <35.9 |
| 4768 | EHRSZ | <383 | 11.1–223 | <184 | 35.9–71.8 |
| P163 | EHSZ | <383 | 11.1–223 | <184 | <35.9 |
H, isoniazid; R, rifampicin; S, streptomycin; E, ethambutol; Z, pyrazinamide.
Values are means of two independent experiments, and the variation is <20%.
Not determined.
Given its promising antimycobacterial activity against both drug-susceptible and drug-resistant Mtb strains, the in vivo antitubercular efficacy of 5k was evaluated using a modified real-time monitoring noninvasive mouse model infected with the selectable marker-free autoluminescent Mtb strain H37Ra.19,20 The animals were infected with log-phase autoluminescent Mtb H37Ra via intravenous injection (2 × 106 CFU per mouse) and then were repeatedly administrated with agent 5k once daily via oral gavage for 6 consecutive days. The bacterial burden was measured by monitoring the bioluminescence intensity (relative light unit, RLU) from the same batch of live mice every other day. As shown in Figure 2, compound 5k exhibited dose-dependent in vivo antitubercular activity and was well tolerated in all of the tested groups with no mortality (data not shown) observed during treatment. Administration with CMC-Na (vehicle) permitted a nearly 2.8-fold increase in RLU over 6 days; whereas compound 5k with the three tested doses prevented any increase over baseline, with RLUdayn/RLUday0 ratios of 0.40, 0.19, and 0.15 in 4, 20, and 100 mg/kg/day treated groups, respectively. Especially, with 100 mg/kg/day, compound 5k exhibited a sustained bactericidal activity against Mtb H37Ra, resulting in a 3.6-fold, 5.0-fold, and 6.5-fold decrease in RLU from baseline on day 2, day 4, and day 6, respectively. These results strongly suggest the promising potential of compound 5k to serve as a lead compound for further anti-TB drug discovery.
Figure 2.

Mean RLU count (±SD) assessed every other day in live, anesthetized mice and normalized to the baseline RLU value. Days post-initial treatment (x-axis) is plotted against the corresponding RLUdayn/RLUday0 ratio (y-axis). Blue, vehicle; purple, 5k 100 mg/kg qd; red, 5k 20 mg/kg qd; green, 5k 4 mg/kg qd.
In summary, a series of pyrazolo[1,5-a]pyridine-3-carboxamide derivatives were designed as new antitubercular agents by using a scaffold hopping strategy. The compounds exhibit excellent in vitro inhibitory activities with low nanomolar MIC values against both drug-sensitive Mtb strain H37Rv and drug-resistant clinical isolates. One of the most promising compounds, 5k, displayed significant bacterial burden reduction in the Mtb H37Ra infected mouse model. This compound may serve as a new promising lead compound for further antitubercular drug discovery.
Supporting Information Available
Experimental procedures for the synthesis of 5a–5v and self-prepared amines, 1H NMR and 13C NMR for final compounds, and details of in vitro and in vivo assays. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00176.
Author Contributions
⊥ These authors contributed equally to this work.
We thank financial support from the Key Program of the Chinese Academy of Sciences (KJZD-EW-L02), Youth Innovation Promotion Association of Chinese Academy of Sciences and Scientific, the Chinese Academy of Sciences ‘One Hundred Talents Program’ (Category A, to T.Z.), Technological Innovation Program of Foshan City (2013HK100212), and the Open Project Grant (2014SKLRD-O06) from the State Key Lab of Respiratory Disease, Guangzhou Medical University. In addition, this work was supported by National Institutes of Health and the National Institute of Allergy and Infectious Diseases, Contract No. HHSN272201100009I.
The authors declare no competing financial interest.
Supplementary Material
References
- World Health Organization. Global Tuberculosis Control WHO Report 2014; WHO/HTM/TB/2014.08; 2014.
- Zumla A.; Nahid P.; Cole S. T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug. Discovery 2013, 12 (5), 388–404. [DOI] [PubMed] [Google Scholar]
- Zumla A. I.; Gillespie S. H.; Hoelscher M.; Philips P. P.; Cole S. T.; Abubakar I.; McHugh T. D.; Schito M.; Maeurer M.; Nunn A. J. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect. Dis. 2014, 14 (4), 327–340. [DOI] [PubMed] [Google Scholar]
- Andries K.; Verhasselt P.; Guillemont J.; Gohlmann H. W.; Neefs J. M.; Winkler H.; Van Gestel J.; Timmerman P.; Zhu M.; Lee E.; Williams P.; de Chaffoy D.; Huitric E.; Hoffner S.; Cambau E.; Truffot-Pernot C.; Lounis N.; Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 307 (5707), 223–227. [DOI] [PubMed] [Google Scholar]
- Cohen J. Infectious disease. Approval of novel TB drug celebrated--with restraint. Science 2013, 339 (6116), 130. [DOI] [PubMed] [Google Scholar]
- Diacon A. H.; Pym A.; Grobusch M. P.; de los Rios J. M.; Gotuzzo E.; Vasilyeva I.; Leimane V.; Andries K.; Bakare N.; De Marez T.; Haxaire-Theeuwes M.; Lounis N.; Meyvisch P.; De Paepe E.; van Heeswijk R. P. G.; Dannemann B. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N. Engl. J. Med. 2014, 371 (8), 723–732. [DOI] [PubMed] [Google Scholar]
- Stover C. K.; Warrener P.; VanDevanter D. R.; Sherman D. R.; Arain T. M.; Langhorne M. H.; Anderson S. W.; Towell J. A.; Yuan Y.; McMurray D. N.; Kreiswirth B. N.; Barry C. E.; Baker W. R. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 2000, 405 (6789), 962–966. [DOI] [PubMed] [Google Scholar]
- Singh R.; Manjunatha U.; Boshoff H. I.; Ha Y. H.; Niyomrattanakit P.; Ledwidge R.; Dowd C. S.; Lee I. Y.; Kim P.; Zhang L.; Kang S.; Keller T. H.; Jiricek J.; Barry C. E. 3rd PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 2008, 322 (5906), 1392–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethe K.; Bifani P.; Jang J.; Kang S.; Park S.; Ahn S.; Jiricek J.; Jung J.; Jeon H. K.; Cechetto J.; Christophe T.; Lee H.; Kempf M.; Jackson M.; Lenaerts A. J.; Pham H.; Jones V.; Seo M. J.; Kim Y. M.; Seo M.; Seo J. J.; Park D.; Ko Y.; Choi I.; Kim R.; Kim S. Y.; Lim S.; Yim S.-A.; Nam J.; Kang H.; Kwon H.; Oh C.-T.; Cho Y.; Jang Y.; Kim J.; Chua A.; Tan B. H.; Nanjundappa M. B.; Rao S. P. S.; Barnes W. S.; Wintjens R.; Walker J. R.; Alonso S.; Lee S.; Kim J.; Oh S.; Oh T.; Nehrbass U.; Han S.-J.; No Z.; Lee J.; Brodin P.; Cho S.-N.; Nam K.; Kim J. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat. Med. 2013, 19 (9), 1157–1160. [DOI] [PubMed] [Google Scholar]
- Kang S.; Kim R. Y.; Seo M. J.; Lee S.; Kim Y. M.; Seo M.; Seo J. J.; Ko Y.; Choi I.; Jang J.; Nam J.; Park S.; Kang H.; Kim H. J.; Kim J.; Ahn S.; Pethe K.; Nam K.; No Z.; Kim J. Lead optimization of a novel series of imidazo[1,2-a]pyridine amides leading to a clinical candidate (Q203) as a multi- and extensively-drug-resistant anti-tuberculosis agent. J. Med. Chem. 2014, 57 (12), 5293–5305. [DOI] [PubMed] [Google Scholar]
- Abrahams K. A.; Cox J. A. G.; Spivey V. L.; Loman N. J.; Pallen M. J.; Constantinidou C.; Fernández R.; Alemparte C.; Remuiñán M. J.; Barros D.; Ballell L.; Besra G. S. Identification of novel imidazo[1,2-a]pyridine inhibitors targeting M. tuberculosis QcrB. PLoS One 2012, 7 (12), e52951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraski G. C.; Markley L. D.; Hipskind P. A.; Boshoff H.; Cho S.; Franzblau S. G.; Miller M. J. Advent of imidazo[1,2-a]pyridine-3-carboxamides with potent multi- and extended drug resistant antituberculosis activity. ACS Med. Chem. Lett. 2011, 2 (6), 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraski G. C.; Markley L. D.; Cramer J.; Hipskind P. A.; Boshoff H.; Bailey M.; Alling T.; Ollinger J.; Parish T.; Miller M. J. Advancement of imidazo[1,2-]pyridines with improved pharmacokinetics and nanomolar activity vs Mycobacterium tuberculosis. ACS Med. Chem. Lett. 2013, 4 (7), 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bald D.; Koul A. Respiratory ATP synthesis: the new generation of mycobacterial drug targets?. FEMS Microbiol. Lett. 2010, 308 (1), 1–7. [DOI] [PubMed] [Google Scholar]
- Nishino K.; Ohkubo H.; Ohashi M.; Hara S.; Kito J.; Irikura T. KC-404: a potential anti-allergic agent with antagonistic action against slow reacting substance of anaphylaxis. Jpn. J. Pharmacol. 1983, 33 (2), 267–278. [DOI] [PubMed] [Google Scholar]
- Awano K.; Suzue S.; Segawa M. Synthesis of 3-substituted pyrazolo[1,5-a]pyridine derivatives with inhibitory activity on platelet aggregation. I. Chem. Pharm. Bull. 1986, 34 (7), 2828–2832. [DOI] [PubMed] [Google Scholar]
- Lober S.; Hubner H.; Utz W.; Gmeiner P. Rationally based efficacy tuning of selective dopamine d4 receptor ligands leading to the complete antagonist 2-[4-(4-chlorophenyl)piperazin-1-ylmethyl]pyrazolo[1,5-a]pyridine (FAUC 213). J. Med. Chem. 2001, 44 (17), 2691–2694. [DOI] [PubMed] [Google Scholar]
- D. Kendall J. Synthesis and reactions of pyrazolo[1,5-a]pyridines and related heterocycles. Curr. Org. Chem. 2011, 15 (14), 2481–2518. [Google Scholar]
- Yang F.; Njire M. M.; Liu J.; Wu T.; Wang B.; Liu T.; Cao Y.; Liu Z.; Wan J.; Tu Z.; Tan Y.; Tan S.; Zhang T. Engineering more stable, selectable marker-free autoluminescent mycobacteria by one step. PLoS One 2015, 10 (3), e0119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Li S. Y.; Nuermberger E. L. Autoluminescent Mycobacterium tuberculosis for rapid, real-time, non-invasive assessment of drug and vaccine efficacy. PLoS One 2012, 7 (1), e29774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L.; Franzblau S. G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997, 41 (5), 1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollinger J.; Bailey M. A.; Moraski G. C.; Casey A.; Florio S.; Alling T.; Miller M. J.; Parish T. A dual read-out assay to evaluate the potency of compounds active against Mycobacterium tuberculosis. PLoS One 2013, 8 (4), e60531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.; Hu Z.; Zhang T.; Cai X.; Kuang H.; Liu Y.; Chen J.; Yang F.; Zhang K.; Tan S.; Zhao Y. Role of pncA and rpsA gene sequencing in detection of pyrazinamide resistance in Mycobacterium tuberculosis isolates from southern China. J. Clin. Microbiol. 2014, 52 (1), 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.