Abstract
Background
Chronic megacolon is a rare disease of the colonic motor function characterized by a permanent increase in colonic diameter.
Methods
We reviewed electronic medical records of all patients diagnosed with chronic megacolon from 1999 to 2014 at Mayo Clinic. Our aim was to summarize clinical and motility features, including colonic compliance and tone measured by colonic barostat-controlled 10cm long infinitely compliant balloon. Colonic compliance curves were compared to healthy (40) and disease (47) control groups.
Results
Among 24 identified patients, the mean maximal colonic diameter on abdominal radiograph was 12.7±0.8cm. The cause of megacolon was idiopathic in 16/24, and secondary in 8/24. A relatively high prevalence (10/24) of comorbid pelvic floor dyssynergia was identified. At the time of this report, 16 patients had undergone colectomy. In general, megacolon presented high fasting colonic volume at relatively low pressures (16-20mmHg), suggesting high colonic compliance; similarly, volumes at operating pressures that ensured apposition of the balloon to the colonic wall suggested low colonic tone. Median balloon volume at 44mmHg distension was 584mL (IQR 556.5-600) in patients with megacolon compared to 251mL (212-281) in healthy, 240mL (207-286) in functional constipation and 241mL (210.8-277.5) in diarrhea-predominant irritable bowel syndrome controls. Colon's tonic response to feeding was generally intact, and there was frequently maintained phasic contractile response to feeding.
Conclusions
Chronic megacolon is a severe colonic dysmotility, manifesting radiologically with increased colonic diameter; it can be proven by measuring colonic compliance, and typically requires colectomy because of failed medical therapy.
Keywords: idiopathic, compliance, tone, manometry, constipation, barostat
Introduction
Chronic megacolon is characterized by a permanently enlarged diameter of the colon due to a chronic process. This abnormal diameter reflects colonic distension and manifests clinically as a chronic disorder of colonic motility, often with intractable constipation, abdominal pain and bloating. Chronic megacolon is caused by diseases of intestinal smooth muscle cells and the enteric and extrinsic nervous systems. However, the connective tissue component of the colonic wall may also be relevant in the context of alterations of tone and increased colonic diameter. Malfunctions of any of these components may thus result in megacolon.
The pathogenesis of chronic megacolon has been attributed to an ‘idiopathic’ disorder or a heterogeneous group of secondary causes. Examples of myopathic disorders include Duchenne's and myotonic muscular dystrophy [1,2], visceral myopathy, as well as degenerative leiomyopathy, a rare condition described mainly in African children [3]. Disorders of the enteric nervous system causing megacolon may result from deficient migration of neural crest cells to the developing colon, as in congenital disorders such as Hirschsprung's disease [4], hypoganglionosis and intestinal ganglioneuromatosis [5,6], spinal cord dysraphism [possibly causing extrinsic denervation [7]]. Other acquired disorders causing megacolon include a reduction in the number of interstitial cells of Cajal [ICC [8,9]] or reduction in enteric nerves, which may be idiopathic [9] or related to Chagas disease [10]. Some diseases, such as Parkinson's disease, may cause either extrinsic denervation or intrinsic neuropathy, resulting in megacolon [11].
There have been studies of colonic motility in patients with chronic colonic pseudo-obstruction [12], though it is unclear whether the reports included patients with chronically dilated colon. Those reports document, in those with intestinal pseudo-obstruction with colonic involvement and constipation, discrete motor abnormalities, such as absence of high amplitude colonic contractions, and failure to increase phasic contractions in postprandial period (considered consistent with neuropathy) or complete absence of contractions (suggestive of myopathy). However, no systematic analyses of colonic compliance or tone are available to date.
The aims of this study were to appraise the demographics and clinical features, associated medical conditions, and colonic tone, and compliance among a cohort of adult patients with chronic megacolon diagnosed at Mayo Clinic in Rochester, Minnesota over a period of 15 years.
Materials and Methods
Search Strategy for Identifying Adults with Chronic Megacolon
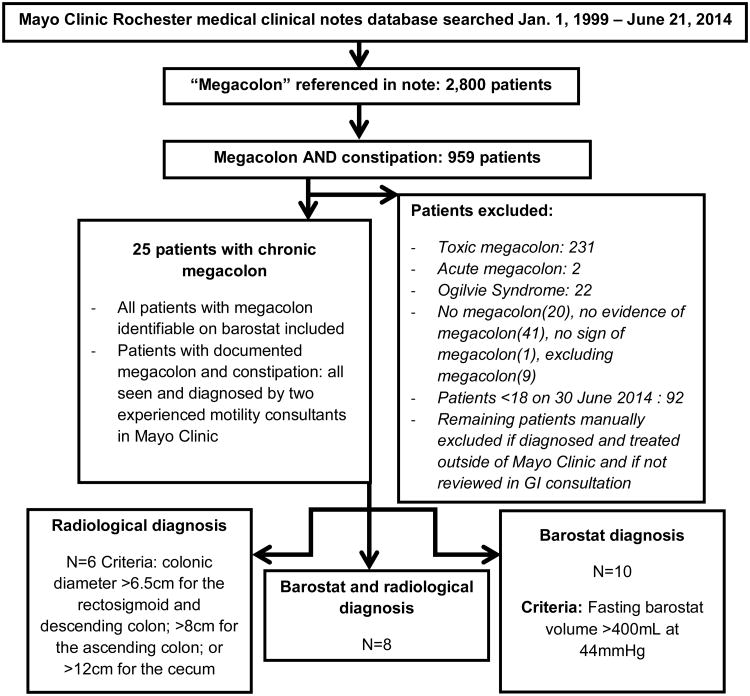
The proprietary Medical Clinical Notes Search Tool available at Mayo Clinic was used to identify adult patients with chronic megacolon through their electronic medical records (Figure 1). A total of 2,800 patient records were identified with the word “megacolon” in their clinical records, with 959 identified with both “megacolon” and “constipation” mentioned in their records. Patients who were under 18 years of age, those who underwent surgery for chronic megacolon prior to referral to Mayo Clinic, or those with “toxic megacolon”, “acute megacolon” or “Ogilvie Syndrome (also defined in the medical records as acute colonic pseudoobstruction)” were excluded; this resulted in identification of 24 patients with chronic megacolon.
Figure 1. Search Strategy to Identify Adults with Chronic Megacolon.
Mayo Clinic Institutional Review Board approved this study for patients who had consented to the use of their medical records for research.
Data Extraction
The data extracted for this study included demographics, clinical features, family history, colon imaging, colonic barostat-manometry data, anorectal manometry data, treatment for megacolon, and follow-up information.
Diagnostic Criteria
All patients had at least 1 criterion for chronic megacolon based on imaging studies or intraluminal assessment of colonic motor function. Criteria for diagnosing megacolon on imaging studies were: colonic diameter greater than 6.5cm at the pelvic brim as proposed previously [13], greater than 8cm in the ascending colon, or greater than 12cm in the cecum [14]. Among 7 patients in whom imaging studies were unavailable, a fasting volume of a 10cm colonic segment >400mL at 44mmHg distension were included as megacolon. This cut-off value was determined by prior work conducted by the same research team, based on studies of 47 patients with diarrhea-predominant irritable bowel syndrome (IBS-D) and 40 healthy volunteers [15,16]; a volume of 400mL at 44mmHg distension had never been observed in these control groups.
Participants
In the study period between January 1, 1999 and June 30, 2014, we identified 24 patients diagnosed with chronic megacolon by one of two (MC and AEB) experienced gastroenterologists with expertise in motility disorders. Family history was recorded as part of the evaluation by the staff gastroenterologists, rather than geneticists.
Measurement of Colonic Motility and Tone
Colonic motility and tone were analyzed in the 10 patients with suspected megacolon in whom there was no radiological documentation of megacolon in order to appraise the utility of these measurements. Colonic motor functions were measured using intracolonic 6-lumen manometry (5cm apart) and a 10cm long infinitely compliant balloon (Hefty Baggies; Mobil Chemical, Pittsford, NY) in which infused air was maintained at constant pressures by means of an electronic rigid piston barostat (Engineering Department; Mayo Clinic, Rochester, MN). This was performed as described in detail previously [17].
Before the colonic motility study, all medications with potential effects on colonic motility were discontinued for at least 48 hours. The colon was cleansed with 2-5L of polyethylene glycol 3350 and electrolyte solution (Golytely®; Abbott Laboratories, Chicago, IL). Patients fasted overnight. The next morning, a colonic manometric-barostat assembly was positioned in the left colon by colonoscopy without sedation [17].
The study commenced after a 30-minute equilibration period. Colonic motor activity was measured as described previously (30). After a conditioning distension, the balloon was inflated from 0-44mmHg in 4-mmHg steps at 30-second intervals to measure colonic compliance. Thereafter, we measured, in order, contractile responses to a meal (1000kcal; 35% carbohydrate, 53% fat, and 12% protein) and to neostigmine (1mg intravenously) with the polyethylene balloon clamped at baseline pressure and colonic compliance with 0-44mmHg distension as performed during the fasting, baseline period.
Colonic motor activity was quantified using established approaches. Only data recorded by the barostat are presented in this paper because phasic pressure activity recorded by manometry is less reliable when the colonic diameter exceeds 5.6cm [18], although there is evidence that high resolution fiber-optic manometry is capable of recording non-occluding reductions in diameter, provided that they occurred with sufficiently viscous content in silico or in the rabbit colon [19]. The phasic contraction measurements in our studies were not conducted in the presence of viscous colonic content, since we cleansed the colon of stool with the bowel preparation and removed any residual fluid at the time of colonic tube placement. Hence, we assessed the phasic pressure activity qualitatively, not with the same quantitative goals used in measurements of colonic compliance and tone. This approach was also reinforced by the experience of other groups that were unable to classify specific manometric findings as reflective of myopathic or neuropathic abnormalities in patients with colonic motility disorders [20]. The phasic contractile activity on manometric recordings served mostly to evaluate phasic volume events measured by barostat.
Data from 47 IBS-D patients, 46 functional constipation/constipation-predominant IBS (IBS-C) patients, and 40 healthy volunteers [15,16] acquired by the same method in our laboratories were used as control in order to appraise the colonic motility measurements in the patients with chronic megacolon.
Statistical Analysis
Data are generally presented as median (interquartile range, IQR). Analysis of variance on ranks (Kruskal Wallis test) was used to compare measurements of colonic tone and compliance in the patients with megacolon and in controls with IBS-D, functional constipation/IBS-C and healthy controls.
Results
Demographics
Figure 1 shows the search strategy and identification of patients with chronic megacolon, based on the electronic medical record. Fifteen patients (62.5%) were women. Patients reported that their symptoms began in childhood (46%) or in adulthood (54%). The age at diagnosis of megacolon was 43.1±4.5 (SEM) years. In patients in whom the symptoms began in adulthood, the age at diagnosis was 45.8±5 years. Forty-two percent of patients had a first generation family member with chronic constipation. Among the patients with megacolon, 54.5% reported that other members of their family had reported chronic constipation since childhood, whereas 30.8% of the patients reported other family members with constipation presenting in adulthood.
Associated Conditions
Eight of 24 patients (33.3%) had another condition known to be associated with megacolon. Five patients had a condition associated with intrinsic or extrinsic neural involvement, namely extrinsic denervation, chemotherapy-induced neuropathy, congenital hindgut dysgenesis, and multiple endocrine neoplasia type IIB with ganglioneuromatosis. Three patients had a systemic disorder affecting connective tissue, one each with scleroderma, Ehlers-Danlos Syndrome and transthyretin amyloidosis. No patients had a skeletal muscle myopathy.
Symptoms and Plain Abdominal Radiology
The principal symptoms reported by patients were abdominal pain (83%), bloating (96%) and abdominal distention (96%) (Table 1). The median number of bowel movements passed per week was 1.5 (IQR 1-2.5).
Table 1.
Symptoms and signs at time of presentation to Mayo Clinic [number (percentage) unless otherwise stated]. Evidence of rectal evacuation disorder was based on abnormal balloon expulsion and/or abnormal anal manometry.
| Clinical and Radiological Features | |
|---|---|
| Bowel movements (BM) | |
| Average number BM/week | 1.5 (range 1-2.5) |
| Straining >25% of the time | 5/24 (21%) |
| Sense of incomplete evacuation | 5/24 (21%) |
| Digital maneuvers to evacuate BM | 1/24 (4%) |
| Abdominal pain (%) | 20/24 (84%) |
| Bloating | 23/24 (96%) |
| Abdominal distension | 23/24 (96%) |
| Evidence of rectal evacuation disorder | |
| Balloon expulsion requiring >200g weight | 8/24 (33%) |
| Abnormal resting anal sphincter pressure | 12/24 (50%) |
| Treatments received prior to evaluation | |
| Fiber supplementation | 3/24 (13%) |
| Osmotic laxative | 12/24 (50%) |
| Prokinetic | 10/24 (42%) |
| Enemas | 8/24 (33%) |
| Maximum diameter (cm) of colon on radiological examination | |
| Mean | 12.74 ± 0.79 (SEM) |
| Median | 12 (IQR10.8-13) |
The largest colonic diameter in any segment of the colon in each of the 15 individuals who had abdominal radiographs on record was 12.7+ 0.8cm (mean ± SEM).
Anorectal Testing
Twelve patients underwent anorectal testing, including anorectal manometry and a balloon expulsion test, because a digital rectal examination suggested a rectal evacuation disorder. The balloon expulsion test (which was regarded as the primary evidence of pelvic floor dyssynergia) was abnormal (>200g required to expel the balloon) in 8 patients, confirming the concomitant diagnosis of pelvic floor dyssynergia. In the other patients, there was also high resting anal sphincter pressure (>90mmHg).
Colonic Motility Testing
Fasting colonic tone and compliance
The characteristic feature of chronic megacolon is an excessively high fasting volume (suggesting low tone) of the infinitely compliant intra-colonic balloon at operating pressures (defined as pressures at which the infinitely compliant balloon is able to identify respiratory variation, typically 8-16mmHg) (Figure 2).
Figure 2.
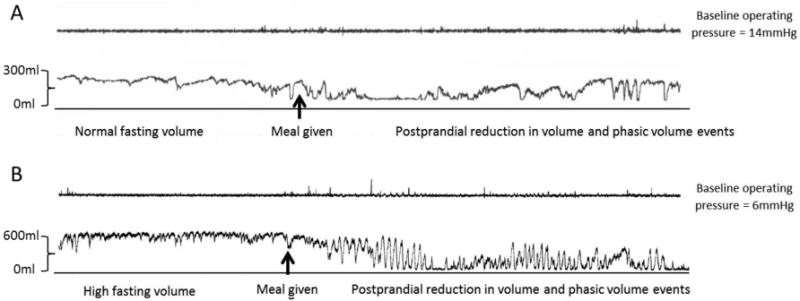
Phasic and tonic contractile activity measured under constant pressure conditions in the colon (operating pressure 6mmHg) of patients with a) slow transit constipation and b) chronic megacolon. Note the large colonic volume (indicating low tone) during fasting and the persistence of phasic contractile activity despite the low colonic tone after the ingestion of a 1000kcal liquid nutrient meal.
In addition, colonic compliance is markedly increased in megacolon (Figure 3). The colonic pressure-volume relationships are characterized by a marked increase in volume with a relatively small increase in the intra-balloon pressure (e.g. from 12 to 20mmHg) (Figure 3). Even at 8mmHg, the colonic balloon volume was significantly higher in patients with chronic megacolon (165mL, IQR 85-301) than in healthy controls (30mL, IQR 28-32), IBS-D (34mL, IQR 32-36) and functional constipation/IBS-C (28.5mL, IQR 10.8-51) (p=0.001 overall by Kruskal Wallis test and p<0.05 for megacolon compared to the three control groups, corrected for multiple comparisons) (Figures 3 and 4).
Figure 3.
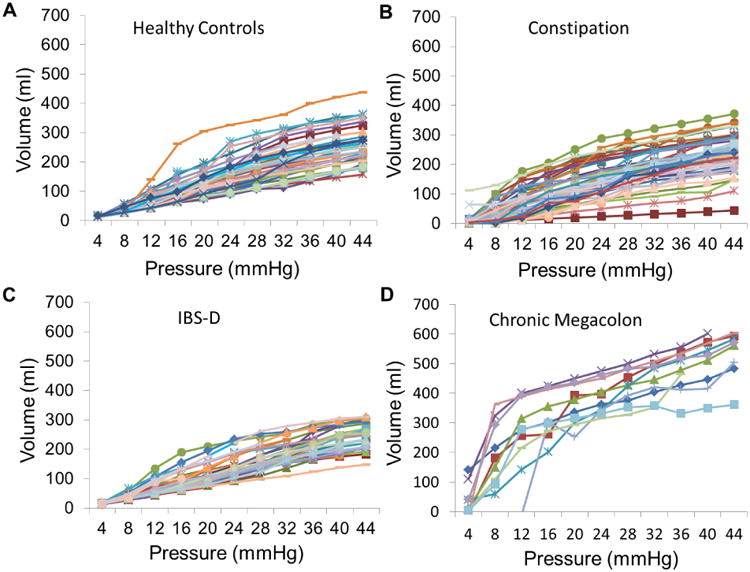
Colonic compliance in (A) healthy controls, (B) functional constipation/constipation-predominant irritable bowel syndrome and (C) diarrhea-predominant irritable bowel syndrome (IBS-D) control groups; and (D) patients with chronic megacolon. Note the markedly increased volume of the intracolonic balloon (10cm long) in patients with megacolon compared to controls. Note also the marked increase in intraballoon volume (>300mL) at 16mmHg distension in all except one patient with megacolon, which is observed in only one healthy control and in none of the IBS-D patients.
Figure 4.
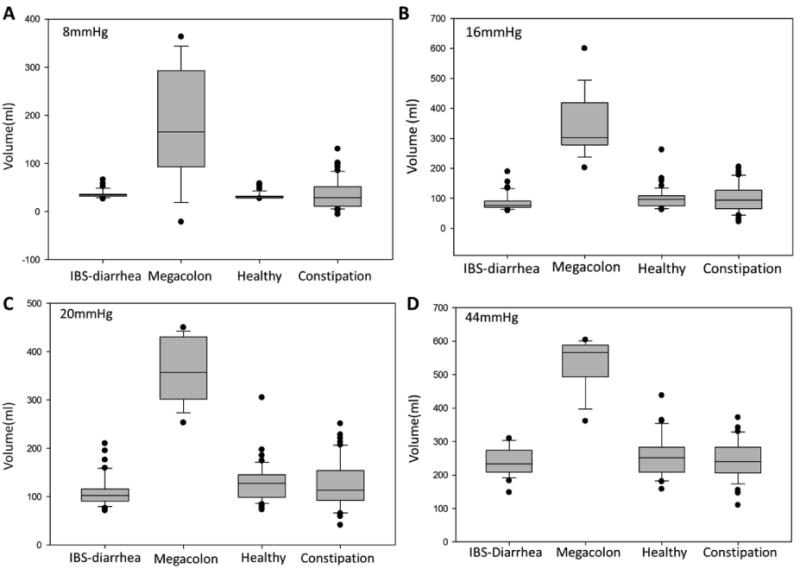
Volume in 10cm long colonic barostat balloon at 8, 16, 20 and 44mHg distension pressures (overall p value <0.001 for each pressure by Kruskal-Wallis test and megacolon is significantly different when compared to each group, p<0.05 by Dunn's method).
A colonic balloon volume greater than 300mL at a pressure of 20mmHg is virtually diagnostic of chronic megacolon (Figure 4). At 44mmHg, the median balloon volume was 584mL (IQR 556.5-600) in the patients with megacolon compared to 251mL (IQR 212-281) in healthy controls, 241mL (210.8-277.5) in IBS-D controls and 240mL (IQR 207-286) in IBS-C controls (p<0.0001 overall by Kruskal Wallis test and p<0.05 for megacolon compared to the three control groups, corrected for multiple comparisons).
Postprandial colonic motor activity
The postprandial response in patients with megacolon was associated with frequent and profound phasic contractile responses, based on the phasic volume contractions of >100mL, as observed in 7/10 patients (70%).
The average postprandial balloon volume measured at constant pressure (Figure 2) during the first postprandial hour was expressed as a percent reduction of barostat balloon volume from preprandial volume. The average postprandial volume change was not significantly lower in patients with megacolon [27.6% (14.2-39.1)] compared to healthy controls [36.5% (23.8-50.5)] and patients with IBS-D [29.8% (19.4-40.8)]. However, it is important to note that the balloon volume measured postprandially is significantly impacted by the large phasic volume reductions observed regularly in the postprandial period, as shown by the example in Figure 2; these short duration volume reductions are consistent with phasic colonic contractions.
Effect of neostigmine on colonic motility
Eight patients with megacolon received neostigmine (1mg intravenously) and the compliance curve was repeated.
At 16mmHg distension, the balloon volume was 169.8 mL (median, IQR 81-273.5) after neostigmine, compared to the balloon volume recorded at the same pressure prior to administration of the meal or medication [290 mL (228-390), p=0.06].
At 44mmHg distension, the corresponding balloon volumes were 499.5mL (median, IQR 343-591) after neostigmine, compared to 577mL (500-598) prior to the meal or medication (p=0.10). This suggests that there is still a tonic response to neostigmine, but the drug does not restore normal colonic tone.
Management
Four of the 24 patients were initially treated with oral pyridostigmine at a typical dose of 60mg three times daily. Three of these 4 patients eventually underwent a colectomy. Fifteen of 24 patients (62.5%) had a subtotal colectomy with ileorectal anastomosis. One patient underwent colectomy with terminal ileostomy and closure of rectal stump. The medical records documented the outcome of colectomy with ileorectal anastomosis for 10 patients as follows: in 7 patients, symptoms resolved after colectomy, except for single documented episodes of subacute intestinal obstruction that was attributed to adhesions and resolved spontaneously in 2 patients; the remaining 3 patients continued to experience symptoms of constipation after surgery. Two of these 3 patients had pelvic floor dysfunction causing an evacuation disorder and underwent pelvic floor retraining with biofeedback.
Six of the 16 patients underwent surgery at other institutions and no detailed outcome data are available.
Discussion
This paper assessed the clinical features and colonic motor functions in patients with chronic megacolon. Only 24 patients with documented chronic megacolon were identified in our tertiary referral practice, which suggests that the condition is rare. The salient features of dysfunctions of the tone of the colon include higher colonic compliance, reduced colonic tone, and large phasic reductions in colonic volume after a meal. Moreover, 7 of 10 patients in whom outcome data were available reported that their symptoms of constipation had resolved after colectomy.
Chronic megacolon can be diagnosed by imaging studies using the criteria of colonic diameter greater than 6.5cm at the pelvic brim [13], greater than 8cm in the ascending colon, or greater than 12cm in the cecum [14]. However, the sensitivity of these measurements has never been formally tested. Here, we describe that, in patients with clinical suspicion of chronic megacolon or when the imaging studies are equivocal, colonic motility testing should be considered to confirm the reduced colonic compliance and tone. Hence, chronic megacolon can be diagnosed by recording volume in a 10cm long balloon of over 300mL at a pressure of 20mmHg, and over 400mL at a pressure of 44mmHg. Additionally, our study suggests that intra-colonic motility testing may be beneficial to study response to treatment (i.e. intravenous neostigmine as a strong colonic prokinetic) and to select patients for treatment with anticholinesterase treatment (with oral pyridostigmine) which can be useful as a bridge to or to delay surgery.
The following disturbances have been documented in the literature in idiopathic chronic megacolon: a relative increase in non-adrenergic, non-cholinergic (NANC) inhibitory neuronal input to smooth muscle with a concomitant relative decrease in excitatory cholinergic and NANC nerves [21] or a reduction in ICCs [22]. Connective tissue disorders predisposing to megacolon have been less well characterized. Desmosis coli, characterized by atrophy in the tendinous fibrous net supporting the muscularis propria, has been postulated to cause idiopathic megacolon and was documented in a large proportion of patients with idiopathic megacolon and chronic constipation [23-25]. Thus, it has been postulated that disruption of the connective tissue network in the muscularis propria results in impairment of the coordinated movements of the longitudinal and circular muscle layers [26,27]. Indeed, megacolon has been observed in patients with certain systemic connective tissue disorders, including Ehlers-Danlos Syndrome [EDS [28-31]], amyloidosis [32] and scleroderma [33,34].
One-third of patients had a condition that is known to be associated with reduced colonic tone. Consistent with a previous study [16], there was no clinically identifiable explanation for extrinsic or enteric nerve dysfunction in a majority of patients, suggesting that the primary abnormality may be a loss of enteric nerves and/or ICCs [8, 9]. We did not systematically evaluate patients for joint hypermobility syndrome, which is associated with functional gastrointestinal disorders, particularly constipation [35-37]. This association suggests that a defect in collagen synthesis may predispose to both conditions. This hypothesis is supported by the observation that chronic megacolon is observed in many patients with Ehlers-Danlos Syndrome. Indeed, some forms of joint hypermobility syndrome are now regarded as a mild phenotype of Ehlers-Danlos Syndrome [35]. In the future, we believe that patients presenting with chronic megacolon should be screened for joint hypermobility syndrome and Ehlers-Danlos Syndrome. Unfortunately, no definitive biomarker or genetic test for these conditions is currently available, and therefore these conditions are usually identified by clinical phenotype.
Ten of 24 patients (42%) with megacolon also had evidence of pelvic floor dysfunction. This raises the question as to whether megacolon is an additional manifestation of a systemic neuromuscular disorder, an acquired response to colonic atony, or is partly a response to the outlet obstruction. Rectal distension, as may occur in pelvic floor dysfunction, inhibits colonic tone through a negative feedback mechanism mediated by a viscerovisceral reflex [38]. Patients with constipation due to pelvic floor dysfunction also have an impaired colonic contractile response to a meal; this response improves after biofeedback therapy [39]. The presence of pelvic floor dysfunction is relevant in selecting the sequence of treatments for megacolon. Without correcting pelvic floor dysfunction, subtotal colectomy with ileorectal anastomosis is less likely to relieve symptoms of slow transit constipation [40-42]. This appeared to be the case in two patients in this cohort, both of whom required pelvic floor therapy with biofeedback after colectomy in order to relieve the constipation. Hence, if there are clinical features of rectal evacuation disorder, it is recommended that pelvic floor function should be investigated and followed with biofeedback training, if necessary, in order to restore normal rectal evacuation before elective colectomy.
In general, treatment of chronic megacolon is similar to that of intractable chronic constipation. In the tertiary care setting, patients generally present after failure of standard therapy for constipation, including osmotic agents and gastrointestinal stimulants (e.g. bisacodyl, senna alkaloids). While there are no controlled studies in chronic megacolon, prokinetic agents such as prucalopride should be considered, based on their efficacy in chronic constipation [43]. A therapeutic trial of the acetylcholinesterase inhibitor, pyridostigmine [44], taken 30 minutes preprandially, may be a reasonable alternative, based on prior experience with constipation associated with autonomic neuropathy [45] and diabetes mellitus [46]. Moreover, an assessment of the colonic contractile response to neostigmine with intracolonic measurement is useful because it predicted the pyridostigmine-induced acceleration of colonic transit [45]. If pyridostigmine is ineffective, as occurred in 4 patients in this series, elective colectomy should be considered in patients without contraindications for surgery. There is no other literature report documenting patient satisfaction following elective colectomy as a treatment for chronic megacolon.
By inhibiting acetylcholinesterase, pyridostigmine increases the level of acetylcholine. The lack of efficacy of pyridostigmine in many patients may be due to several factors including reduced acetylcholine release as a result of loss of enteric nerves, a reduction in other (e.g, non-adrenergic non-cholinergic) neurons [47], and/or severe connective tissue or smooth muscle dysfunction in scleroderma or myopathic disorders (e.g. myotonic dystrophy). Future studies examining the efficacy of pyridostigmine or other colonic prokinetics [such as 5-HT4 agonists prucalopride [48] and YKP10811 [49], or the ghrelin agonist relamorelin [50]] in various subtypes of chronic megacolon are thus needed to better establish their potential therapeutic values and indications.
Cats, particularly middle-aged male cats, provide an animal model of chronic megacolon that appears to be due to colonic smooth muscle dysfunction. Some cats may become refractory to laxative or prokinetic therapies and may progress through recurrent constipation to obstipation and megacolon. These cats eventually require colectomy and have a generally favorable prognosis for recovery following colectomy [51].
This is the largest series of adult patients with chronic megacolon reported to date. It is a retrospective review of patients referred to a tertiary center. However, it is probable that most patients with symptomatic chronic megacolon reach specialized gastroenterology centers, given the rarity of this condition and the challenges in its clinical management. Thus, these patients are most likely representative of patients with chronic megacolon, although milder phenotypes may remain undiagnosed. The uniformity of the data regarding colonic compliance reassures us that the observations are likely generalizable. There are clearly limitations on the clinical observations in such a retrospective study of medical records; thus, about half of the patients had acquired megacolon, whereas the other half had megacolon since childhood, possibly reflecting congenital abnormalities.
In summary, our study demonstrates that two-thirds of patients with megacolon have an “idiopathic” disorder. Assessments of colonic motor function with intracolonic measurement of colonic compliance and tone are useful, especially when the abdominal radiographs have not identified unequivocal dilatation or the chronic nature, to confirm the diagnosis of chronic megacolon and also to assess the response to a meal and/or pharmacological stimulus. Many patients with chronic megacolon appear to do well after a subtotal colectomy.
Table 2. Management of megacolon after evaluation.
| Management | Number (percentage) |
|---|---|
| Pyridostigmine | 4/24 (17%) |
| Progression to colectomy post-pyridostigmine trial | 3/4 (75%) |
| Colectomy | 16/24 (7%) |
| - with ileorectal anastomosis | 15/16 (94%) |
| - with ileostomy | 1/16 (6%) |
| Outcome of Colectomy | |
| Alleviation of symptoms | 7/16 (44.4%) |
| Symptoms not alleviated | 3/16 (19.9%) |
| Unknown | 6/16 (38.8%) |
Key Messages.
Chronic megacolon is a severe colonic dysmotility and can be diagnosed by measuring colonic compliance (>300mL at 20mmHg distension or >400mL at 44mmHg distension of a 10cm long intracolonic balloon).
Chronic megacolon typically requires colectomy because of failed medical therapy.
Acknowledgments
We thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding: Dr. Camilleri is supported by R01-DK92179 grant from National Institutes of Health.
Footnotes
Disclosures: The authors have no conflicts of interest.
Authors' contributions: Ralph Hurley O'Dwyer: medical student co-investigator; gathered data and reviewed medical records; writing and critical revision of the manuscript
Andrés Acosta: fellow co-investigator; writing and critical revision of the manuscript
Michael Camilleri: study concept and design; analysis and interpretation of data; writing and critical revision of the manuscript; obtained funding
Duane Burton: technical support; study supervision
Irene Busciglio: study coordinator
Adil E. Bharucha: staff physician co-investigator; writing and critical revision of the manuscript
Contributor Information
Andrés Acosta, Email: acostacardenas.andres@mayo.edu.
Duane Burton, Email: burton.duane@mayo.edu.
Irene Busciglio, Email: busciglio.irene@mayo.edu.
Adil E. Bharucha, Email: bharucha.adil@mayo.edu.
References
- 1.Torretta A, Mascagni D, Zeri K, et al. The megacolon in myotonic dystrophy: Case report and review of the literature. Ann Ital Chir. 2000;71:729–733. [PubMed] [Google Scholar]
- 2.Sáinz R, Lanas A, Gomollón F, Moros M. Megacolon in duchenne's disease. Gastroenterology. 1982;83:1155–1156. [PubMed] [Google Scholar]
- 3.Moore S, Schneider J, Kaschala R. Non-familial visceral myopathy: Clinical and pathologic features of degenerative leiomyopathy. Pediatr Surg Int. 2002;18:6–12. doi: 10.1007/s003830200002. [DOI] [PubMed] [Google Scholar]
- 4.Martucciello G. Hirschsprung's disease, one of the most difficult diagnoses in pediatric surgery: A review of the problems from clinical practice to the bench. Eur J Pediatr Surg. 2008;18:140–149. doi: 10.1055/s-2008-1038625. [DOI] [PubMed] [Google Scholar]
- 5.Ibanez C, Fernandez-Gonzalez I. Emergency anesthesia in a woman with mitochondrial neurogastrointestinal encephalopathy. Rev Esp Anestesiol Reanim. 2011;58:585–587. doi: 10.1016/s0034-9356(11)70144-x. [DOI] [PubMed] [Google Scholar]
- 6.Muehlenberg K, Fiedler A, Schaumann I, Muller-Felber W, Wiedmann KH. Intestinal pseudoobstructions and gastric necrosis in mitochondrial myopathy. Dtsch Med Wochenschr. 2002;127:611–615. doi: 10.1055/s-2002-22669. [DOI] [PubMed] [Google Scholar]
- 7.Gattuso JM, Kamm MA. Clinical features of idiopathic megarectum and idiopathic megacolon. Gut. 1997;41:93–99. doi: 10.1136/gut.41.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Park H, Kamm M, Talbot I. Decreased density of interstitial cells of cajal and neuronal cells in patients with slow-transit constipation and acquired megacolon. J Gastroenterol Hepatol. 2005;20:1292–1298. doi: 10.1111/j.1440-1746.2005.03809.x. [DOI] [PubMed] [Google Scholar]
- 9.Wedel T, Spiegler J, Soellner S, et al. Enteric nerves and interstitial cells of cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459–1467. doi: 10.1053/gast.2002.36600. [DOI] [PubMed] [Google Scholar]
- 10.Iantorno G, Bassotti G, Kogan Z, et al. The enteric nervous system in chagasic and idiopathic megacolon. Am J Surg Pathol. 2007:460–468. doi: 10.1097/01.pas.0000213371.79300.a8. [DOI] [PubMed] [Google Scholar]
- 11.Kupsky W, Grimes M, Sweeting J, Bertsch R, Cote L. Parkinson's disease and megacolon: Concentric hyaline inclusions(lewy bodies) in enteric ganglion cells. Neurology. 1987;37:1253–1255. doi: 10.1212/wnl.37.7.1253. [DOI] [PubMed] [Google Scholar]
- 12.Di Lorenzo C, Flores AF, Reddy SN, Snape WJ, Jr, Bazzocchi G, Hyman PE. Colonic manometry in children with chronic intestinal pseudo-obstruction. Gut. 1993;34:803–807. doi: 10.1136/gut.34.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preston DM, Lennard-Jones JE, Thomas BM. Towards a radiologic definition of idiopathic megacolon. Gastrointest Radiol. 1985;10:167–169. doi: 10.1007/BF01893094. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri M. Dysmotility of the small intestinte and colon. In: Yamada T, editor. Textbook of Gastroenterology. Chichester, West Sussex; Hoboken, NJ: Wiley-Blackwell; 2009. pp. 1513–1520. [Google Scholar]
- 15.Esfandyari T, Camilleri M, Busciglio I, Burton D, Baxter K, Zinsmeister AR. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: A randomized, placebo-controlled study. Am J Physiol Gastrointest Liver Physiol. 2007;293:G137–145. doi: 10.1152/ajpgi.00565.2006. [DOI] [PubMed] [Google Scholar]
- 16.Camilleri M, Busciglio I, Acosta A, et al. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am J Gastroenterol. 2014;109:1621–1630. doi: 10.1038/ajg.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravi K, Bharucha AE, Camilleri M, Rhoten D, Bakken T, Zinsmeister AR. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology. 2010;138:89–97. doi: 10.1053/j.gastro.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von der Ohe M, Hanson R, Camilleri M. Comparison of simultaneous recordings of human colonic contractions by manometry and a barostat. Neurogastroenterol Motil. 1994;6:213–222. [Google Scholar]
- 19.Arkwright JW, Dickson A, Maunder SA, et al. The effect of luminal content and rate of occlusion on the interpretation of colonic manometry. Neurogastroenterol Motil. 2013;25:e52–59. doi: 10.1111/nmo.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Berg MM, Di Lorenzo C, Mousa HM, Benninga MA, Boeckxstaens GE, Luquette M. Morphological changes of the enteric nervous system, interstitial cells of cajal, and smooth muscle in children with colonic motility disorders. J Pediatr Gastroenterol Nutr. 2009;48:22–29. doi: 10.1097/MPG.0b013e318173293b. [DOI] [PubMed] [Google Scholar]
- 21.Tomita R, Sakurai K, Fujisaki S, Shibata M. Role of the enteric nervous system in the colon of patients with idiopathic megacolon. Hepatogastroenterol. 2012:2127–2131. doi: 10.5754/hge9659. [DOI] [PubMed] [Google Scholar]
- 22.Faussone-Pellegrini MS, Fociani P, Buffa R, Basilisco G. Loss of interstitial cells and a fibromuscular layer on the luminal side of the colonic circular muscle presenting as megacolon in an adult patient. Gut. 1999;45:775–779. doi: 10.1136/gut.45.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meier-Ruge W. Desmosis of the colon: A working hypothesis of primary chronic constipation. Eur J Pediatric Surg. 1998:299–303. doi: 10.1055/s-2008-1071218. [DOI] [PubMed] [Google Scholar]
- 24.Autschbach F, Gassler N. Idiopathic megacolon. Eur J Gastroenterol Hepatol. 2007:399–400. doi: 10.1097/MEG.0b013e3280116cb8. [DOI] [PubMed] [Google Scholar]
- 25.Meier-Ruge WA, Muller-Lobeck H, Stoss F, Bruder E. The pathogenesis of idiopathic megacolon. Eur J Gastroenterol Hepatol. 2006;18:1209–1215. doi: 10.1097/01.meg.0000236883.13720.c2. [DOI] [PubMed] [Google Scholar]
- 26.Goerrtler K. Der konstruktive bau der menschlichen darmwand. Gegenbauers morphologisches Jahrbuch. 1932:33–58. [Google Scholar]
- 27.Goerrtler K. Der bau der “muscularis mucosae” des menschlichen darmes und ein befund über den bau seiner “muscularis propria”. Gegenbauers morphologisches Jarhbuch. 1949-1951:33–58. [Google Scholar]
- 28.Beighton PH, Murdoch JL, Votteler T. Gastrointestinal complications of the ehlers-danlos syndrome. Gut. 1969;10:1004–1008. doi: 10.1136/gut.10.12.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nardone DA, Reuler JB, Girard DE. Gastrointestinal complications of ehlers-danlos syndrome. N Engl J Med. 1979;300:863–864. doi: 10.1056/nejm197904123001520. [DOI] [PubMed] [Google Scholar]
- 30.Fehlow P, Bernstein K, Tennstedt A, Walther F. Autismus infantum und exzessive aerophagie mit symptomatischem megakolon und ileus bei einem fall von ehlers-danlos syndrom. Pädiatrische Grenzgebiete. 1993;31:259–267. [PubMed] [Google Scholar]
- 31.Burcharth J, Rosenberg J. Gastrointestinal surgery and related complications in patients with ehlers-danlos syndrome: A systematic review. Dig Surg. 2012;29:349–357. doi: 10.1159/000343738. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Appavu S, Abcavian H. Amyloidosis of the colon. Report of a case and review of the literature. Dis Colon Rectum. 1983;26:541–544. doi: 10.1007/BF02563751. [DOI] [PubMed] [Google Scholar]
- 33.Shamberger RC, Crawford JL, Kirkham SE. Progressive systemic sclerosis resulting in megacolon: A case report. JAMA. 1983;250:1063–1065. [PubMed] [Google Scholar]
- 34.Brandwein M. Megacolon and volvulus complicating systemic sclerosis. Mt Sinai J Med. 1988;55:343–345. [PubMed] [Google Scholar]
- 35.Fikree A, Aziz Q, Grahame R. Joint hypermobility syndrome. Rheum Dis Clin North Am. 2013;39:419–430. doi: 10.1016/j.rdc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Fikree A, Grahame R, Aktar R, et al. A prospective evaluation of undiagnosed joint hypermobility syndrome in patients with gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2014;12:1680–1687.e2. doi: 10.1016/j.cgh.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Zarate N, Farmer AD, Grahame R, et al. Unexplained gastrointestinal symptoms and joint hypermobility: Is connective tissue the missing link? Neurogastroenterol Motil. 2010;22:252–e278. doi: 10.1111/j.1365-2982.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 38.Law N, Bharucha A, Zinsmeister A. Rectal and colonic distention elicit viscerovisceral reflexes in humans. Am J Physiol Gastrointest Liver Physiol. 2002;283:384–389. doi: 10.1152/ajpgi.00359.2001. [DOI] [PubMed] [Google Scholar]
- 39.Mollen RM, Salvioli B, Camilleri M, et al. The effects of biofeedback on rectal sensation and distal colonic motility in patients with disorders of rectal evacuation: Evidence of an inhibitory rectocolonic reflex in humans? Am J Gastroenterol. 1999;94:751–756. doi: 10.1111/j.1572-0241.1999.00947.x. [DOI] [PubMed] [Google Scholar]
- 40.Sohn G, Yu CS, Kim CW, et al. Surgical outcomes after total colectomy with ileorectal anastomosis in patients with medically intractable slow transit constipation. J Korean Soc Coloproctol. 2011;27:180–187. doi: 10.3393/jksc.2011.27.4.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassan I, Pemberton JH, Young-Fadok TM, et al. Ileorectal anastomosis for slow transit constipation: Long-term functional and quality of life results. J Gastrointest Surg. 2006;10:1330–1336. doi: 10.1016/j.gassur.2006.09.006. discussion 1336-1337. [DOI] [PubMed] [Google Scholar]
- 42.Knowles CH, Scott M, Lunniss PJ. Outcome of colectomy for slow transit constipation. Ann Surg. 1999;230:627–638. doi: 10.1097/00000658-199911000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin A, Camilleri M, Kolar G, Erwin P, West CP, Murad MH. Systematic review with meta-analysis: Highly selective 5-ht4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment Pharmacol Ther. 2014;39:239–253. doi: 10.1111/apt.12571. [DOI] [PubMed] [Google Scholar]
- 44.O'Dea CJ, Brookes JH, Wattchow DA. The efficacy of treatment of patients with severe constipation or recurrent pseudo-obstruction with pyridostigmine. Colorect Dis. 2010;12:540–548. doi: 10.1111/j.1463-1318.2009.01838.x. [DOI] [PubMed] [Google Scholar]
- 45.Bharucha AE, Low PA, Camilleri M, Burton D, Gehrking TL, Zinsmeister AR. Pilot study of pyridostigmine in constipated patients with autonomic neuropathy. Clin Auton Res. 2008;18:194–202. doi: 10.1007/s10286-008-0476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bharucha AE, Low P, Camilleri M, et al. A randomised controlled study of the effect of cholinesterase inhibition on colon function in patients with diabetes mellitus and constipation. Gut. 2013;62:708–715. doi: 10.1136/gutjnl-2012-302483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomita R, Sakurai K, Fujisaki S, Shibata M. Role of the enteric nervous system in the colon of patients with idiopathic megacolon. Hepatogastroenterology. 2012;59:2127–2131. doi: 10.5754/hge9659. [DOI] [PubMed] [Google Scholar]
- 48.Bouras E, Camilleri M, Burton D, Thomforde G, McKinzie S, Zinsmeister A. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–360. doi: 10.1053/gast.2001.21166. [DOI] [PubMed] [Google Scholar]
- 49.Shin A, Acosta A, Camilleri M, et al. The serotonin receptor 5-HT4 agonist YKP10811 accelerates intestinal transit and improves bowel functions in patients with functional constipation. Clin Gastroenterol Hepatol. 2014 Aug 19; doi: 10.1016/j.cgh.2014.08.012. Epub ahead of print. [DOI] [Google Scholar]
- 50.Acosta A, Kolar G, Iturrino J, et al. A phase ii, single-center, randomized, double-blind, placebo-controlled, multiple-dose, 2-period, parallel-group study to evaluate the efficacy, safety, and pharmacodynamics of rm 131 administered to patients with chronic constipation. Gastroenterology. 2014;146:S364. [Google Scholar]
- 51.Washabau RJ, Holt D. Pathogenesis, diagnosis, and therapy of feline idiopathic megacolon. Vet Clin North Am Small Anim Pract. 1999;29:589–603. [PubMed] [Google Scholar]