Abstract
“Popcorn workers’ lung” is an obstructive pulmonary disease produced by inhalation of volatile artificial butter flavorings. In rats, inhalation of diacetyl, a major component of butter flavoring, and inhalation of a diacetyl substitute, 2,3-pentanedione, produce similar damage to airway epithelium. The effects of diacetyl and 2,3-pentanedione and mixtures of diacetyl, acetic acid, and acetoin, all components of butter flavoring, on pulmonary function and airway reactivity to methacholine (MCh) were investigated. Lung resistance (RL) and dynamic compliance (Cdyn) were negligibly changed 18 h after a 6-h inhalation exposure to diacetyl or 2,3-pentanedione (100–360 ppm). Reactivity to MCh was not markedly changed after diacetyl, but was modestly decreased after 2,3-pentanedione inhalation. Inhaled diacetyl exerted essentially no effect on reactivity to mucosally applied MCh, but 2,3-pentanedione (320 and 360 ppm) increased reactivity to MCh in the isolated, perfused trachea preparation (IPT). In IPT, diacetyl and 2,3-pentanedione (≥3 mM) applied to the serosal and mucosal surfaces of intact and epithelium-denuded tracheas initiated transient contractions followed by relaxations. Inhaled acetoin (150 ppm) exerted no effect on pulmonary function and airway reactivity in vivo; acetic acid (27 ppm) produced hyperreactivity to MCh; and exposure to diacetyl + acetoin + acetic acid (250 + 150 + 27 ppm) led to a diacetyl-like reduction in reactivity. Data suggest that the effects of 2,3-pentanedione on airway reactivity are greater than those of diacetyl, and that flavorings are airway smooth muscle relaxants and constrictors, thus indicating a complex mechanism.
Employees at microwave popcorn manufacturing factories have developed “popcorn workers’ lung” (Department of Health and Human Services, 2004; Kreiss et al., 2002; Lockey et al., 2009; Schachter, 2002), a lung disease resembling bronchiolitis obliterans (King, 1989), following inhalation of butter flavoring. Sahakian et al. (2008) demonstrated that butter flavoring-exposed workers have an increased risk of occupational asthma and exacerbated asthma symptoms. The flavorings in popcorn also find use in other foods, including caramel, butterscotch, and vanilla flavors and muffin and cake mixes (Day et al., 2011; Harber et al., 2006; Martyny et al., 2008). Employees in a cookie factory developed bronchiolitis obliterans after artificial butter flavoring exposure while preparing the cookie dough (Cavalcanti Zdo et al., 2012). One patient who had worked in a microwave popcorn factory was diagnosed initially with reactive airways disease with a positive methacholine (MCh) challenge test, only to be diagnosed a few years later with bronchiolitis obliterans with no response to a bronchodilator (Zlotolow et al., 2007). Another patient with a positive MCh test eventually acquired bronchiolitis obliterans (Zlotolow et al., 2007). Flavoring inhalation may also pose a respiratory risk to consumers. Upon opening a bag of microwave popcorn, sufficient butter flavoring is released into the air to produce respiratory disease (Egilman and Schilling, 2012).
Over 150 volatile agents are present in popcorn butter flavoring (Boylstein et al., 2006). The primary component in butter flavoring mixture that is thought to be toxic to the lungs of workers is diacetyl (2,3-butanedione; C4H6O2) (Hubbs et al., 2008; van Rooy et al., 2007). Diacetyl is a low-molecular-weight, four-carbon α-diketone largely responsible for the taste of butter (Harber et al., 2006; Hubbs et al., 2008). 2,3-Pentanedione (acetyl propionyl; C5H8O2), an α-diketone with a structure similar to diacetyl’s, is used as a diacetyl substitute. This compound produces pulmonary toxicity in animal models, although its toxicity is not as well characterized as that of diacetyl (Day et al., 2011; Hubbs et al., 2012; Morgan et al., 2012a, 2012b). Both chemicals are volatile, yielding vapors that can be inhaled. Other volatile components in flavoring mixtures, alone or in combination with diacetyl, may exert pulmonary toxicity. In particular, acetoin and acetic acid are present in vapor mixtures associated with popcorn workers’ lung (Boylstein et al., 2006; Kreiss et al., 2002; van Rooy et al., 2007, 2009).
Histological investigation in animal models demonstrated that a 6-h exposure inhalation exposure to diacetyl and 2,3-pentanedione causes some epithelial damage 0 h postexposure, which progresses in intensity by 18 to 20 h postexposure (Hubbs et al., 2002, 2008, 2010, 2012; Morris and Hubbs, 2009; Morgan et al., 2008, 2012a, 2012b; Palmer et al., 2011). In addition, inhaled 2,3-pentanedione was found to result in olfactory neuroepithelial injury in rats (Hubbs et al., 2012), and, interestingly, diacetyl may induce long-lasting neurotoxicity by binding to β-amyloid and interfering with pleated sheet structures (More et al., 2012a). Thus, the toxicity of these volatile α-diketones extends beyond the lung.
Functionally, acute diacetyl inhalation produces pulmonary irritation in mice but, surprisingly, high-concentration diacetyl exposures result in desensitization of the sensory response to subsequent diacetyl inhalations (Larsen et al., 2009). The rat has been used to model the bronchiolitis-like changes in the lung following exposures to diacetyl or 2,3-pentanedione (Morgan et al., 2012a; Palmer et al., 2011). Understanding the pulmonary function changes after acute diacetyl exposure in the rat is important because it is an established model of flavorings-related lung disease and the spectrum of flavorings-related lung disease recently expanded to include functionally important pulmonary conditions in addition to bronchiolitis obliterans and pulmonary function (Kreiss et al., 2002; Sahakian et al., 2008).
While intratracheal instillation of diacetyl is known to alter pulmonary function in rats at 7 d postexposure when aberrant epithelial repair and intraluminal airway fibrosis have developed (Palmer et al., 2011), little is known regarding changes in rat pulmonary function and airway reactivity after acute inhalation exposures to diacetyl, 2,3-pentanedione, or diacetyl-containing mixtures of flavorings. This information is critical to understanding the full spectrum of toxicities associated with these flavorings.
Many studies demonstrated increased responsiveness of airway smooth muscle in vitro to contractile agonists such as acetylcholine (ACh) and 5-hydroxytryptamine (5-HT), as well as sensory neuropeptides, including substance P and neurokinin A, following removal of the epithelium (Aarhus et al., 1984; Hay et al., 1986; Spina, 1998). Therefore, damage to the epithelium by flavorings would be expected to increase reactivity to contractile agonists in vivo and in vitro, especially after contractile agonists are applied to the mucosal surface. For example, ozone (O3) inhalation results in injury to airway epithelial cells and in hyperreactivity to MCh in vitro and in vivo (Fedan et al., 2000; Savov et al., 2004). The increase in reactivity to inhaled MCh after O3 exposure was thought to be a consequence of greater accessibility of the drug through the airway wall to the smooth muscle and to loss of epithelial-derived relaxant factor (EpDRF) (Fedan et al., 2000).
The overall hypothesis of this study is popcorn flavorings that produce damage to the airway epithelium result in airway hyperreactivity to MCh in vivo and in vitro. A major purpose of the investigation was to compare and contrast the effects of diacetyl and 2,3-pentanedione in airways in vivo and in vitro. In the first series of experiments, alterations were determined in respiratory mechanics, that is, lung resistance and dynamic compliance (RL and Cdyn, respectively), as well as reactivity to inhaled MCh, following diacetyl or 2,3-pentanedione inhalation (Poole et al., 2009; Yao et al., 2010). It was of interest to identify the concentration range over which diacetyl and 2,3-pentanedione may produce such effects. In the second series of experiments, the isolated, perfused trachea (IPT) method was employed using tracheas from exposed rats to investigate and compare the effects of diacetyl or 2,3-pentanedione inhalation on airway reactivity to MCh in vitro, again with the goal of examining concentration dependence of effects. It was postulated that reactivity to MCh applied to the mucosal surface bath of the perfused trachea would be increased following these exposures. In the third series of experiments, the IPT preparation was employed to investigate the direct effects of diacetyl and 2,3-pentanedione in rat airways in vitro. Earlier findings that diacetyl elicits contraction and relaxation of guinea pig airways were extended (Fedan et al., 2006). The potential role of the epithelium in the development of these responses was investigated. Finally, other flavorings, such as acetic acid and acetoin, are often present in abundant amounts in the vapors of butter flavorings and also were reported in a flavoring manufacturing workplace with affected workers (Boylstein et al., 2006; van Rooy et al., 2007, 2009). They are not currently regarded as being key compounds in the development of toxicity in humans. However, being inhaled along with diacetyl they could worsen or mitigate its toxic effects in the lung. Thus, studies also investigated the effects of acetic acid and acetoin, alone and in combination with inhaled diacetyl as a mixed exposure.
MATERIALS AND METHODS
Animals
These studies were conducted in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International and were approved by the institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Hilltop Lab Animals; Scottdale, PA; 300–450 g) were housed in pairs in ventilated micro-isolator units supplied with HEPA-filtered laminar flow air (Thoren Caging Systems; Hazleton, PA), with autoclaved Alpha-DriTM virgin cellulose chips (Shepherd Specialty Papers, Watertown, TN) and hardwood Beta-chips (NEPCO; Warrensburg, NY) for bedding, and provided tap water and autoclaved HaCXan Teklad Global 18% protein rodent diet (Harlan Teklad; Madison, WI) ad libitum. Rats were housed under controlled light cycle (12-h light) and temperature (22–25°C) conditions.
Exposure of Rats to Diacetyl, 2,3-Pentanedione, Acetoin and Acetic Acid Vapors
Two inhalation systems were designed to expose rats to butter flavoring constituents. In the first system, the whole-body inhalation exposure system (Hubbs et al., 2008) was modified and automated to expose rats for 6 h to diacetyl (100, 200, 300, or 360 ppm) or 2,3-pentanedione (120, 240, 320, or 360 ppm) (Figure 1). The concentrations of diacetyl and 2,3-pentanedione were selected based upon previously described airway epithelial toxicity (Hubbs et al., 2008, 2012). Filtered-air-breathing control animals were placed for 6 h in chambers that were similar to ones used for exposure to flavorings, with matched environmental conditions. A photo-ionization detector (PID, PGM-7600; RAE Systems, San Jose, CA) measured the real-time concentration levels of diacetyl and 2,3-pentanedione during the exposures, in which the measured concentrations of components were closely related to target concentrations (Table 1). Water-sealed, compressed air was dried, HEPA-filtered, and charcoal-filtered and then passed through a mass flow controller (GFC37, Aalborg; Orangeburg, NY) and into a custom humidifier, which maintained the relative humidity. Airflow was regulated at 20 L/min, which corresponded to more than 15 air changes/h. Chamber CO2, ammonia, temperature, and relative humidity for both exposed and control rats were regulated within acceptable limits for animal comfort (Institute for Laboratory Animal Research, 2011). The air then entered a stainless-steel mixer where it was heated. Liquid diacetyl or 2,3-pentanedione was injected with a syringe pump (210, KD Scientific Inc.; Holliston, MA) into the airstream, where it vaporized in the heated air (70°C). The diacetyl or 2,3-pentanedione vapor was passed into a custom stainless-steel and glass exposure chamber (McKinney and Frazer, 2008) that could house up to six animals. Proportional–integral–derivative (PID) measurements from the chamber were used in a feedback loop that adjusted the flow of the syringe pump to maintain the concentration at a constant, user-defined value (Table 1). Custom data acquisition and control software was developed that permitted the user to set parameters such as flavoring concentration and relative humidity. Data were acquired at a rate of 1 sample/s and recorded for subsequent analysis.
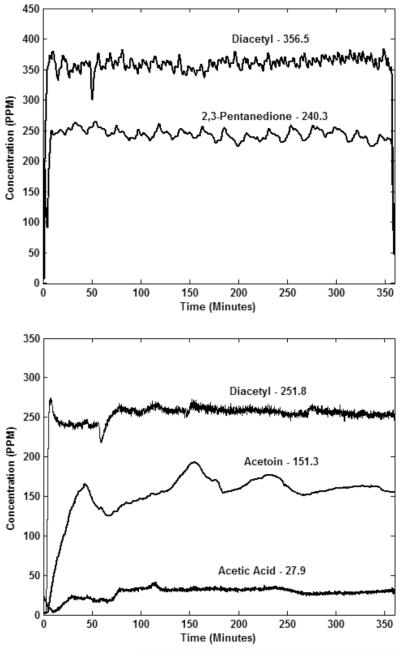
FIGURE 1.
Top, typical recordings illustrating stable diacetyl and 2,3-pentanedione levels during a 6-h inhalation exposure. Bottom, typical recordings illustrating stable diacetyl and acetoin and acetic acid levels during a 6-h inhalation exposure. Numbers above the tracings are time-weighted values.
TABLE 1.
Target Concentrations Compared to Measured Concentrations During Diacetyl and 2,3-Pentanedione Inhalation
| Target (ppm) | Measured (ppm) |
|---|---|
| Diacetyl | |
| 100 | 100±0 |
| 200 | 200±0 |
| 300 | 295±1 |
| 360 | 354±2 |
| 2,3-Pentanedione | |
| 120 | 118±0.5 |
| 240 | 242±2 |
| 320 | 315±4 |
| 360 | 356±1 |
Note. Animals were exposed to flavoring levels close to target concentrations at stable temperature and relative humidity.
The second inhalation system was similar to the first with additional alterations. This system was used to expose rats (Figure 1) to diacetyl (167 or 250 ppm), acetic acid (18 or 27 ppm), or acetoin (100 or 150 ppm) individually, or as three vapor mixtures containing low (“three low”: 167 ppm diacetyl + 100 ppm acetoin + 18 ppm acetic acid) or high (“three high”: 250 ppm diacetyl + 150 ppm acetoin + 27 ppm acetic acid) concentrations of the vapors (Table 2). To facilitate investigation of potentially enhanced toxicity to intrapulmonary airways resulting from combined exposures, the highest diacetyl concentration selected (250 ppm) was just below the lowest dose (294.6 ppm) reported previously to produce cytotoxicity in intrapulmonary airways following acute single-agent exposure (Hubbs et al., 2008). Features of this inhalation system included (1) three heated injection ports that were fed with separate syringe pumps (one for each vapor), (2) a different custom exposure chamber that could hold up to 12 animals (Goldsmith et al., 2011), (3) wet sponges that provided humidity, instead of the custom humidifier, and (4) a Fourier-transform infrared (FTIR) spectrometer gas analyzer (DX-4010, Gasmet Technologies, Inc., La Praire, Quebec), which monitored the concentration of each individual flavoring in the exposure chamber in real time. Feedback loops were used to regulate the syringe-pump flow for each of the three flavorings.
TABLE 2.
Target Concentrations Compared to Measured Concentrations During Diacetyl and Acetoin and Acetic Acid Single and Mixed Inhalation Exposures
| Vapor | Target (ppm) | Measured (ppm) |
|---|---|---|
| Single vapor | ||
| Diacetyl | 250 | 251.4±0.3 |
| Acetoin | 150 | 149.5±0.1 |
| Acetic acid | 27 | 25.9±3.4 |
| Mixed exposure: “three low” | ||
| Diacetyl | 167 | 164.9±1.7 |
| Acetoin | 100 | 99.5±1.0 |
| Acetic acid | 18 | 18.6±0.1 |
| Mixed exposure: “three high” | ||
| Diacetyl | 250 | 251.6±0.2 |
| Acetoin | 150 | 143.6±7.7 |
| Acetic acid | 27 | 28.2±0.3 |
The PID was calibrated weekly to the flavoring of interest with a custom apparatus that delivered user-defined air temperature, relative humidity, and flavoring concentrations. Measurements were taken with gravimetric filters, a scanning mobility particle sizer (SMPS, TSI, Inc., St. Paul, MN), and an aerodynamic particle sizer (APS, TSI) to determine whether any of the vapor was in an aerosol form. All results indicated that negligible aerosol was present during the exposures. Figure 1 demonstrates that vapor levels during a 6-h exposure were constant.
There was no mortality in the animals following flavoring exposures.
Effects of Flavoring Exposure on Respiratory Mechanics and Reactivity to MCh In Vivo
Eighteen hours postexposure, rats were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine given ip. A midline incision was made in the neck and a cannula was placed into the trachea. Animals received supplemental ketamine (50 mg/kg) by administration of the drug topically to the exposed muscle in the neck. Animals were placed on a warming bed in a plethysmograph for the assessment of lung RL and Cdyn (FinePointe RC; Buxco Electronics, Inc., Wilmington, NC) and were ventilated using a digital rodent ventilator (Élan Series RC; Buxco Electronics, Inc.). Ventilation settings were: maximum mouth pressure, 40 cm H2O; maximum tidal volume, 3 ml; and respiratory rate, 90 bpm. After recording baseline values of RL and Cdyn and after delivery of saline vehicle as a control, MCh aerosols were delivered from 20 μl of solutions of the following concentrations: 0.3, 1, 1.73, 3, 5.75, or 10 mg/ml. Three 5-ml (45 cm H2O mouth pressure) deep inspirations were applied just before each MCh dose was delivered. Maximum RL (RLmax) values and minimum Cdyn values (Cdynmin) were logged at 5-s intervals to quantify responses to MCh. Following completion of the experiment, rats were euthanized by exsanguination.
Effects of Diacetyl and 2,3-Pentanedione Inhalation on Reactivity to MCh in the IPT Preparations
This preparation has been described previously (Fedan et al., 2006; Fedan and Frazer, 1992). Eighteen hours after the end of air or flavoring exposure, rats were anesthetized using sodium pentobarbital (65 mg/kg, ip) and euthanized by exsanguination. A 25-mm segment of trachea was removed and cleaned. The segment was mounted on a perfusion holder at its in situ length. The holder contained indwelling, side-hole catheters in the lumen that were connected to the positive (inlet) and negative (outlet) sides of a differential pressure transducer. The holder with mounted trachea was placed into an extraluminal (EL) bath containing modified Krebs–Henseleit solution (MKHS). The trachea was perfused at a constant rate (25 ml/min) with MKHS from a separate bath, the intraluminal (IL) bath, while measuring inlet minus outlet pressure difference (△P, cm H2O) as an index of tracheal diameter. Transmural pressure was set to zero. The MHKS in the IL and EL baths was replaced at 15-min intervals with fresh solution, followed by a 30-min equilibration period in which the MHKS was not changed and the baseline was allowed to become stable. MHKS temperature was 37°C and was aerated with 95% O2/5% CO2 to give pH 7.4. After the equilibration period, MCh was added in stepwise-increasing, cumulative concentrations (10−5 to 3 × 10−2 M) to the IL bath to induce contractile responses for the generation of concentration-response curves.
Reactivity to Diacetyl and 2,3-Pentanedione in the IPT; Influence of the Epithelium
Tracheas were prepared as described earlier. After the equilibration period, diacetyl or 2,3-pentanedione (1–30 mM) was added in stepwise-increasing, cumulative concentrations to the IL or EL bath to initiate contractile responses. In order to examine the relaxant effects of the flavorings, preparations were pre-contracted with EL MCh (3 × 10−5 M; EC50) to induce contraction before administering the flavoring. The potential involvement of the epithelium in responses to the flavorings was investigated using perfused tracheas, the epithelium of which was removed by inserting a tamper pin into the lumen and gently turning the pin three to four times.
Solutions and Reagents
The composition of MKHS was: NaCl, 113 mM; KCl, 4.8 mM; CaCl2, 2.5 mM; MgCl2, 1.2 mM; KH2PO4, 1.2 mM; NaHCO3, 25 mM; and glucose, 5.5 mM. All drugs and chemicals were obtained from Sigma-Aldrich (St. Louis, MO) and dissolved and diluted in saline. The purity of diacetyl (lot 03798LJ) and that of 2,3-pentanedione (lot 00130DJ) were 99.3% and 97%, respectively.
Statistical Analysis
The results are expressed as means ± SEM. In vivo results were analyzed for differences using analysis of variance (ANOVA) for repeated measures. For the in vitro experiments the results were analyzed for differences using ANOVA. MCh concentration-response curves were normalized with respect to each preparation’s maximum response. Geometric mean EC50 values for MCh were derived from least-squares analysis of four-parameter logit curve fits and are expressed along with 95% confidence intervals. Statistical comparisons of EC50 values were conducted using normally distributed –logEC50 values. Responses to in vitro application of diacetyl or 2,3-pentanedione were normalized by calculating the percent relaxation of the contraction induced by MCh; n is the number of separate experiments and p < .05 was considered significant.
RESULTS
Effect of Diacetyl and 2,3-Pentanedione Inhalation on Basal Pulmonary Function and Reactivity to Inhaled MCh
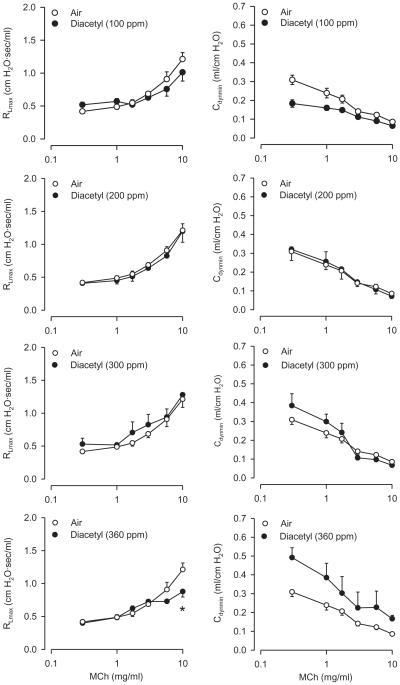
Eighteen hours following a 6-h diacetyl exposure, other than a numerical increase in basal Cdyn at 360 ppm, diacetyl did not markedly affect RL and Cdyn compared to air-exposed controls (Figure 2); this change is probably without biological significance.
FIGURE 2.
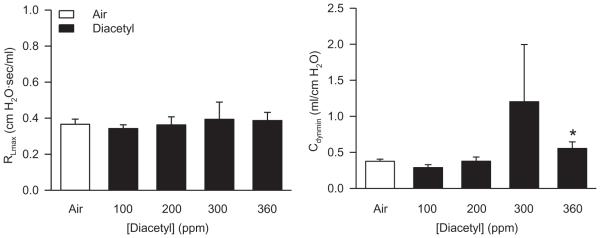
Basal values of RL (left panel) and Cdyn (right panel) after inhalation exposure of rats to air or diacetyl. Left panel: 18 h following 6-h exposure. Air, n = 24; diacetyl, n = 6–9 for each concentration. Right panel: Cdyn. Air: n = 26; diacetyl, n = 6–10 for each concentration. Asterisk indicates significantly different from air-exposed (p < .05).
Diacetyl inhalation significantly reduced the RL response to 10 mg/ml MCh at one concentration, 360 ppm. There were no marked effects of diacetyl on Cdyn responses to MCh at any concentration of diacetyl (Figure 3). Thus, diacetyl inhalation in doses that produce substantial epithelial damage (Hubbs et al., 2008) did not change reactivity to MCh.
FIGURE 3.
Effect of diacetyl inhalation 18 h following 6-h exposure of rats on reactivity to inhaled MCh. Left panels: diacetyl on effect on RL responses. Right panels: diacetyl exposure on Cdyn. Asterisk indicates significantly different from air-exposed (p < .05).
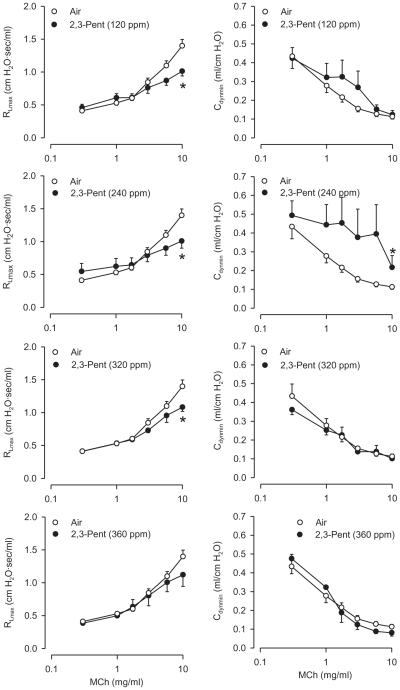
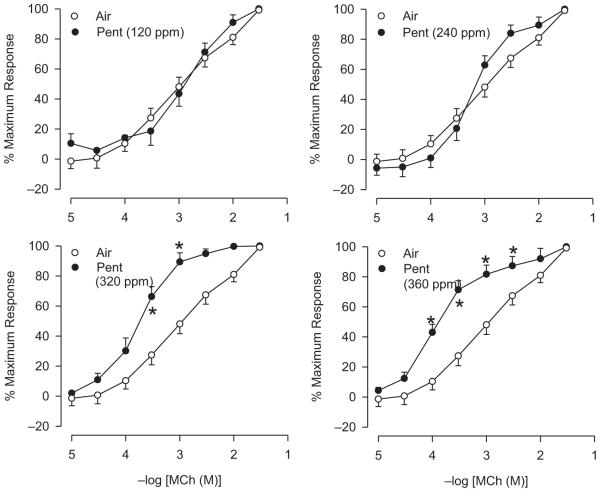
Eighteen hours following a 6-h 2,3-pentanedione inhalation, basal RL and Cdyn were largely unaffected (data not shown). 2,3-Pentanedione (120, 240, and 320 ppm) reduced RL responses at the highest doses of MCh (Figure 4); a comparable change at 360 ppm was not significant. Other than at 10 mg/ml MCh at 240 ppm 2,3-pentanedione, the flavoring exerted no marked effect on Cdyn.
FIGURE 4.
Effect of 2,3-pentanedione inhalation 18 h following 6-h exposure of rats on reactivity to inhaled. Left panel: RL responses to MCh after exposure to 120, 240, and 320 ppm 2,3-pentanedione. Right panels: effect on Cdyn. Air: n = 24; 2,3-pentanedione: n = 4–10. Asterisk indicates significantly different from air-exposed (p < .05).
Effects of Diacetyl and 2,3-Pentanedione Inhalation on Reactivity In Vitro of Rat Trachea to MCh
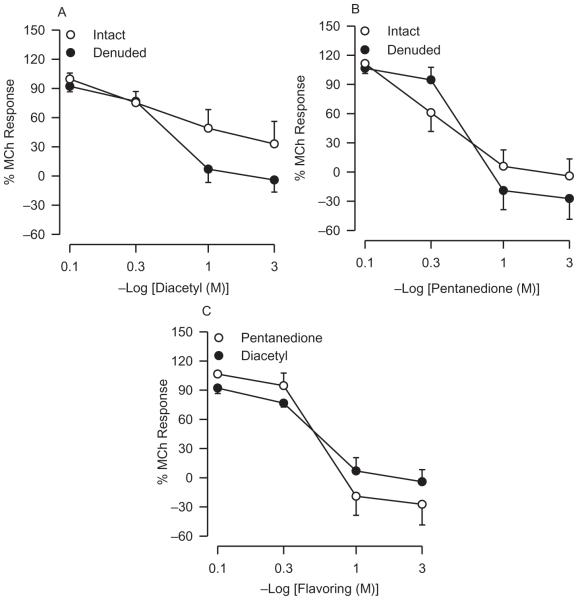
In the surprising absence of increases in vivo in reactivity to MCh after flavoring inhalation, evidence was sought for any alterations in reactivity to MCh in vitro using the IPT preparation. Before examining the effects of inhaled flavorings, reactivity to MCh was first compared in epithelium-containing and epithelium-denuded tracheas to establish what the consequences of severe epithelium damage might be. In the presence of the epithelium, the concentration-response curve obtained after adding MCh mucosally was shifted rightward of the EL curve and was characterized by a smaller maximum response (Figure 5 and Table 2). The lesser reactivity to MCh after IL addition in intact trachea is a reflection of the epithelial diffusion barrier as well as the release of epithelial-derived modulatory factors (Farmer et al., 1986, 1987; Fedan et al., 2006; Fedan and Frazer, 1992; Smith et al., 1993).
FIGURE 5.
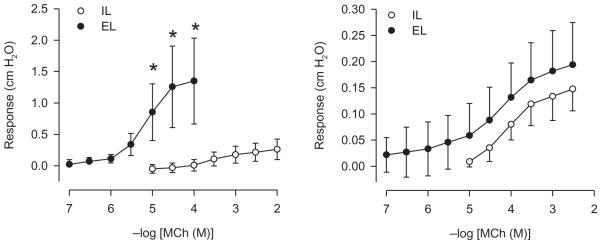
Effect of epithelium removal on reactivity to IL-applied MCh in IPT. Left panel: comparison of EL and IL MCh concentration-response curves obtained from epithelium-containing tracheas. EL, n = 6; IL, n = 6. Right panel: responses to MCh in tracheas from which the epithelium was removed. EL, n = 5; IL, n = 5. Asterisk indicates significant difference between EL and IL responses (p < .05).
In denuded tracheas, reactivities for the intraluminal and extraluminal administration of MCh were not different (Figure 5 and Table 2); that is, the IL curve was shifted leftward and upward to the location of the EL curve, as expected (Fedan and Frazer, 1992). The IL MCh concentration-response curve in the absence of the epithelium demonstrated the maximum effect on reactivity that flavorings could produce as a result of epithelial damage. It was postulated that the effects of epithelial damage produced by flavoring inhalation should resemble qualitatively and in varying degrees the increase in reactivity to IL-applied MCh after mechanical epithelial removal.
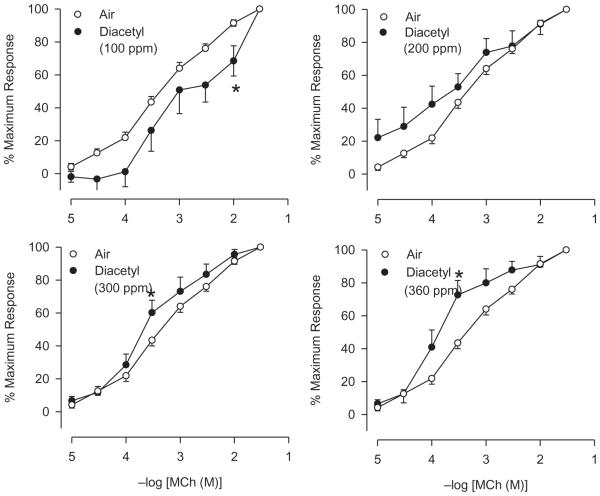
Eighteen hours after diacetyl inhalation, responses to MCh of tracheas from animals exposed to 300 and 360 ppm were potentiated (Figure 6). However, diacetyl exerted no marked effect on the EC50 values for MCh at these and the remaining diacetyl exposure levels (Table 3). Diacetyl-exposed tracheas demonstrated significant hyporeactivity compared to air controls at 100 ppm at one MCh concentration. These results indicate that epithelial damage after diacetyl exposure is accompanied by modest changes in reactivity to IL MCh in vitro.
FIGURE 6.
Effect of diacetyl inhalation on reactivity of IPT to IL MCh. MCh EC50 values and maximum responses are summarized in Table 3. Control, n = 50; 100 ppm, n = 10; 200 ppm, n = 9; 300 ppm, n = 14; 360 ppm, n = 8. Asterisk indicates significantly different from air-exposed (p < .05).
TABLE 3.
Reactivity of Rat IPT to MCh After Inhalation Exposure to Diacetyl and 2,3-Pentanedione
| Diacetyla | EC50 (95% CI) (M) | EC50 air/ EC50 exposed |
Maximum responseb |
2,3- Pentanedionea |
EC50 (95% CI) (M) | EC50 air/ EC50 exposed |
Maximum responseb |
|---|---|---|---|---|---|---|---|
| 0 (Air)c | 1.00 (0.2–0.5) × 10−3 | — | 1.02±0.18 | 0 (Air)c | 1.3 (0.7–2.2) × 10−3 | — | 0.94±0.31 |
| 100 | 2.1 (0.4–11.5) × 10−4 | 5.0 | 0.82±0.16 | 120 | 2.7 (0.7–10.3) × 10−3 | 0.48 | 0.94±0.33 |
| 200 | 1.3 (0.2–10.6) × 10−3 | 0.08 | 0.57±0.14 | 240 | 4.0 (2.0–8.0) × 10−4 | 3.25 | 0.21±0.07 |
| 300 | 3.3 (1.6–7.0) × 10−3 | 0.32 | 0.77±0.16 | 320 | 1.9 (1.1–3.2) × 10−4* | 6.84 | 0.46±0.12 |
| 360 | 0.2 (0.0–13.6) × 10−4 | 52.5 | 0.54±0.13 | 360 | 0.2 (0.3–1.8) × 10−4* | 65.0 | 0.62±0.20 |
Note. Asterisk indicates significantly different from air-exposed. CI, confidence interval.
ppm during a 6-h exposure.
cm H2O.
Control.
The two highest concentrations of 2,3-pentanedione increased significantly reactivity to IL MCh (Figure 7 and Table 3), evidenced as decreases in the EC50 values. The results indicate that the damage to the epithelium of rats following 2,3-pentanedione exposure is accompanied by an increase in reactivity to MCh in vitro.
FIGURE 7.
Effect of 2,3-pentanedione inhalation on reactivity IPT to IL MCh. MCh EC50 values and maximum responses are summarized in Table 3. Control, n = 29; 120 ppm, n = 7; 240 ppm, n = 6; 320 ppm, n = 5; 360 ppm, n = 8. Asterisk indicates EC50s were significantly different from air-exposed (p < .05).
Effects of Diacetyl and 2,3-Pentanedione in the IPT
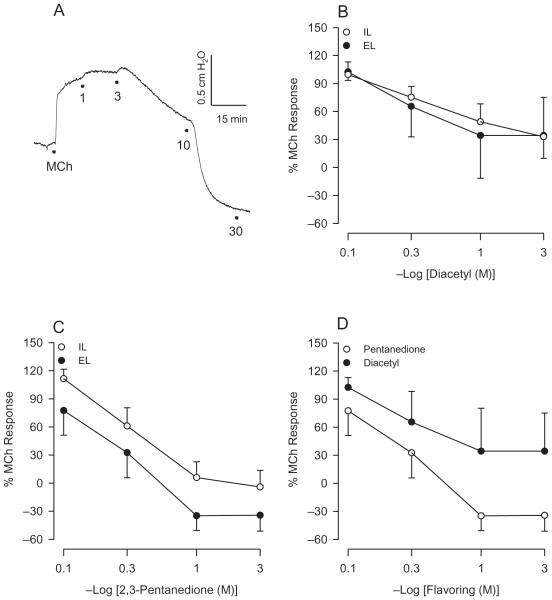
In these experiments the flavorings were administered in vitro to investigate their direct pharmacological effects on the trachea and determine whether responses to flavorings might indirectly involve the epithelium. An earlier investigation (Fedan et al., 2006) in guinea pig IPT indicated that, over its effective concentration range, the initial responses to the lowest effective concentrations of IL diacetyl consisted of contractions. At concentrations greater than 3 mM (10 mM and 30 mM), diacetyl applied to the IL bath resulted in contraction followed by net relaxation. Studies were thus extended as a consequence of these observations in the rat and findings were compared to those obtained with 2,3-pentanedione. As demonstrated in Figure 8A, IL-applied 2,3-pentanedione (1 mM) elicited small contractions. Relaxation responses were elicited subsequently during the response to 3 mM and higher 2,3-pentanedione concentrations. Similar relaxation responses were evoked when diacetyl and 2,3-pentanedione were added to the IL or EL baths in the intact trachea (Figure 8B,C). The relaxant responses to EL diacetyl and 2,3-pentanedione were not significantly different (Figure 8D), although 2,3-pentanedione appeared to more fully relax the tracheas.
FIGURE 8.
Effects of flavorings in MCh-contracted IPT. (A) Representative responses to 1, 3, 10, and 30 mM 2,3-pentanedione added intraluminally (at dots). Note that relaxation followed contraction at 3 mM 2,3-pentanedione. The rat perfused trachea develops spontaneous tone; therefore, the relaxations to both flavorings reduced the level of △P below baseline before MCh addition. Contraction and relaxation responses were also elicited after diacetyl administration and resembled those depicted in (A). (B) The results following IL and EL diacetyl additions are summarized. Diacetyl administered to either EL or IL bath resulted in similar relaxation. IL, n = 8; EL, n = 8. (C) The results following IL and EL 2,3-pentanedione additions are summarized. (D) Comparison of relaxant responses to EL diacetyl and 2,3-pentanedione.
In the absence of the epithelium, both flavorings applied to the IL bath also resulted in relaxation responses that were nearly identical to those elicited after they were applied to the mucosal side of intact trachea (Figures 9A and 9B). A difference between the relaxant effects of the two flavorings added intraluminally to denuded tracheas was not evident (Figure 9C). Overall, these results suggest that responses to diacetyl and 2,3-pentanedione involve a direct effect on the airway smooth muscle and are independent of and are not mediated by epithelium.
FIGURE 9.
Responses of epithelium-denuded IPT to flavorings. (A) Diacetyl applied to the IL bath elicited responses that were similar to what had been observed in intact trachea. (B) Responses to IL 2,3-pentanedione in the absence of the epithelium were not different from responses to IL 2,3-pentanedione in intact trachea; see (A). (C) Comparison of responses to IL diacetyl and 2,3-pentanedione. Diacetyl, n = 5; 2,3-pentanedione, n = 6.
Effects of Mixed Inhalation Exposure to Diacetyl, Acetic Acid and Acetoin on Reactivity to MCh In Vivo
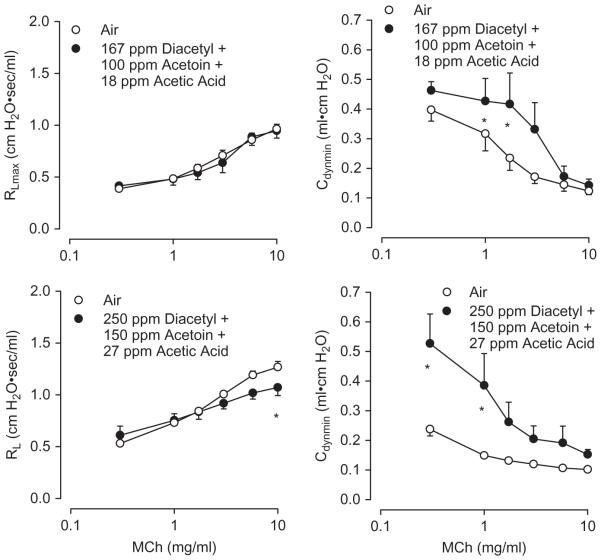
While diacetyl has been implicated as a critical etiologic agent in the development of workplace butter flavoring-induced lung toxicity, workers are exposed to a multitude of inhaled volatile chemicals in addition to diacetyl. In particular, acetic acid and acetoin are abundant in butter flavoring vapor mixtures and also were found along with diacetyl in the less complex exposure in a flavoring manufacturing workplace with affected workers (van Rooy et al., 2007, 2009). It is critical to understand whether acetic acid and acetoin, as frequent adjunct agents, might mitigate or exacerbate the effects of diacetyl inhaled concomitantly. Therefore, the effects of a 6-h mixed inhalation exposure to these three agents were investigated. First, the effects of the agents by themselves were evaluated; second, the effects of the mixtures were examined using the flavorings in “low” (“three low”: 167 ppm diacetyl + 100 ppm acetoin + 18 ppm acetic acid) and “high” (“three high”: 250 ppm diacetyl + 150 ppm acetoin + 27 ppm acetic acid) concentrations. The absolute and relative concentrations of the three flavorings are relevant to their relative concentrations in butter flavoring vapors and were measured in the workplace (Hubbs et al., 2002). Because the 250-ppm diacetyl exposure was just below the lowest acute exposure reported to damage intrapulmonary airway epithelium (294.6 ppm) and above the lowest acute exposure reported to damage tracheal epithelium (224 ppm), while the 167 ppm level was below the lowest acute diacetyl exposure reported to damage tracheal epithelium, the concentration of diacetyl chosen for this experiment was one that would permit potentiating and inhibiting effects of acetoin and acetic acid to be realized were they to occur. Figure 10 illustrates that in this series of experiments, inhalation of only diacetyl resulted in a decrease in RL; the other flavorings were without marked effect. Neither the low- nor the high-concentration flavoring mixtures altered RL. The flavorings present in high concentration in the mixture increased Cdyn.
FIGURE 10.
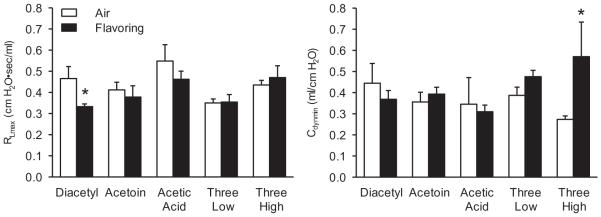
Basal values of RL (left panel) and Cdyn (right panel) after inhalation exposure of rats to air, diacetyl (250 ppm), acetoin (150 ppm), and acetic acid (27 ppm) alone and in combinations involving the three flavorings present in low (“three low”) or high (“hree high”) concentrations. The “three low” concentrations were: 167 ppm diacetyl + 100 ppm acetoin + 18 ppm acetic acid. The “three high” concentrations were: 250 ppm diacetyl + 150 ppm acetoin + 27 ppm acetic acid. Air control animals from the same shipment were run in parallel with the flavoring-exposed animals. The n values for the exposed animals and air controls, respectively, were: diacetyl (250 ppm) alone, 6 and 6; acetoin (150 ppm) alone, 7 and 7; acetic acid (27 ppm) alone, 3 and 7; “three low,” 7 and 7; and “three high,” 6 and 6. Diacetyl (250 ppm) decreased RL; “three high” increased Cdyn. Asterisk indicates significantly different from air-exposed (p < .05).
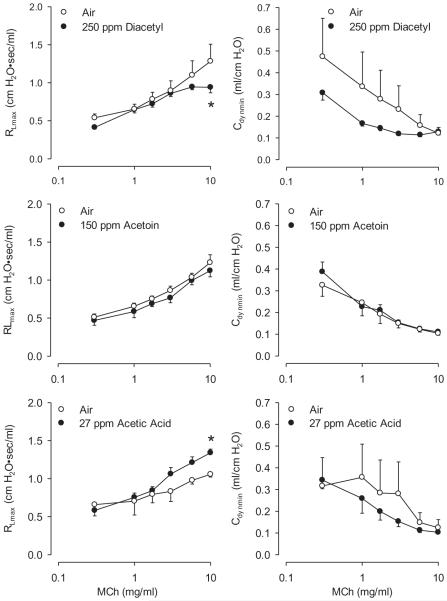
In this series of experiments, diacetyl (250 ppm) decreased the RL response to 10 mg/ml MCh (Figure 11); in the series of experiments described earlier, this effect had not been observed except at a higher concentration, that is, 360 ppm. While the reason for the reduction at 250 ppm here but not at 300 ppm earlier is not understood, these findings buttress the observation that acute diacetyl inhalation reduces, rather than increases, airway reactivity. Inhalation of acetoin (150 ppm) exerted no marked effect on reactivity to MCh (Figure 11). This is a novel observation, as no prior study of acetoin’s effects in the airways under similar experimental conditions was available.
FIGURE 11.
Effect of inhalation of diacetyl (250 ppm), acetoin (150 ppm) and acetic acid (27 ppm) 18 h following 6-h exposure of rats on reactivity to inhaled MCh. Left panels: diacetyl decreased RL responses; acetic acid increased RL responses. Asterisk indicates significantly different from air-exposed (p < .05).
Inhalation of 27 ppm acetic acid produced airway hyperreactivity to MCh (Figure 11). Acetic acid effects in the airways were reported only in the context of the use of the agent as a cough-inducing stimulus (Shimizu et al., 1997) or to examine its acute effects (Ernstgard et al., 2006; Stanek et al., 2001). The development of airway hyperreactivity to MCh after extended acetic acid inhalation appears to be a new finding.
Having characterized the effects of each flavoring, the effects of mixed exposures were investigated. Figure 12 illustrates that the low-concentration mixture exerted no marked effect on RL responses to MCh, but that Cdyn responses were increased. The high-concentration mixture resulted in hyporeactivity to MCh (RL), as observed with diacetyl inhalation alone (see earlier description), and an increase in Cdyn. Thus, the hyperreactivity observed after acetic acid exposure alone was nullified when diacetyl was included in the mixed exposure.
FIGURE 12.
Effect of “three low” (top two panels) and “three high” (bottom two panels) mixed exposures 18 h following a 6-h exposure of rats on reactivity to inhaled MCh. The “three high” exposure reduced RL responses to MCh; both the “three low” and the “three high” exposures increased Cdyn. The n values for the exposed animals and air controls, respectively, were: “three low,” 6 and 6; “three high,” 7 and 7. Asterisk indicates significantly different from air-exposed (p < .05).
DISCUSSION
In this study, the effects of acute diacetyl and 2,3-pentanedione inhalation were investigated and compared for effects on pulmonary function and airway reactivity in vivo and in vitro, the pharmacological effects of both flavorings on airways in vitro, and the consequences in vivo of inhaling a mixture of diacetyl, acetoin, and acetic acid—three common and abundant components of butter flavorings.
Despite demonstration of substantial epithelial damage in upper airways of the rat observed in previous investigations (Hubbs et al., 2008, 2012), basal RL and Cdyn were unaffected by inhalation of diacetyl or 2,3-pentanedione. This contrasts with intratracheally instilled diacetyl, which resulted in significantly increased airway RL and decreased Cdyn in rats 7 d after exposure (Palmer et al., 2011). Even more surprisingly, acute inhalation of the flavorings did not lead to airway hyperreactivity. Inhaled diacetyl reduced reactivity (RL) to MCh at one exposure concentration, and 2,3-pentanedione decreased responsiveness at three exposure concentrations. No marked effects on Cdyn responses to MCh were produced by either flavoring, with the exception that 240 ppm 2,3-pentanedione significantly increased Cdyn responses to MCh. A clear, graded dose dependency in the effects of inhaled diacetyl or 2,3-pentanedione on RL was not apparent at the inhalation doses used in this investigation. However, it can be estimated that the threshold for diacetyl, in terms of reactivity to inhaled MCh, is greater than 300 ppm and less than 360 ppm (Figure 3). On the other hand, the threshold for 2,3-pentanedione would appear to be less than 120 ppm (Figure 4). Thus, 2,3-pentanedione evoked alterations in reactivity to inhaled MCh at lower inhalation exposure doses than in the case of diacetyl. The fact that the agents decreased reactivity to MCh suggests the compounds exerted adverse effects in lower airways.
The reduced reactivity to MCh 1 d after inhaling diacetyl or 2,3-pentanedione is reminiscent of the reduced sensory irritant response to a second diacetyl exposure that was noted in mice that had inhaled 790 ppm or 1154 ppm diacetyl for 2 h the previous day (Larsen et al., 2009). Since MCh directly affects airway smooth muscle, our data suggest that smooth muscle reactivity of rat airways is reduced after a high-level acute exposure to either diacetyl or 2,3-pentanedione. Together with the previous study of the acute airway effects of diacetyl inhalation in mice (Larsen et al., 2009), these findings suggest that airway functional changes are not limited to the epithelium of α-diketone-exposed airways and that exposure also leads to reduced smooth muscle reactivity.
A reductionist approach was used to investigate whether flavoring inhalation and resultant epithelial damage would affect reactivity to MCh in vitro to a degree that mimics partly or fully the effects of epithelium removal. In tracheas that were removed from flavoring-exposed animals and perfused in vitro, treatment with diacetyl resulted in increases in reactivity that were quite modest compared to the change observed after epithelium removal (Fedan and Frazer, 1992). The inhalation dose at which diacetyl’s effect on reactivity to MCh in the IPT occurred is judged to be greater than 200 ppm and less than 300 ppm. This is in rough concordance with the doses that altered reactivity to inhaled MCh. In contrast, inhalation of 2,3-pentanedione led to larger increases in the in vitro reactivity to IL MCh at concentrations of 2,3-pentanedione greater than 240 ppm and less than 320 ppm. 2,3-Pentanedione, therefore, was more potent at decreasing reactivity to inhaled MCh than at increasing reactivity to MCh in vitro.
Diacetyl or 2,3-pentanedione inhalation by rats (>150 ppm; 6 h/d, 5 d/wk for 12 exposures over 2 wk) resulted in increases in lung resistance and decreases in compliance (diacetyl) (Morgan et al., 2012a). These findings are comparable to functional changes reported 1 wk after diacetyl aspiration in the rat (Palmer et al., 2011). These changes corresponded with the development of airway fibrosis and epithelial remodeling. Early measurements, such as those made here, as well as reactivity to MCh, were not reported in those studies. On the other hand, in the present investigation, 18 h after acute exposure, none of the agents examined affected RL and Cdyn. This is consistent with a role for airway fibrosis and/or epithelial remodeling in the development of clinical bronchiolitis obliterans and increased RL and decreased Cdyn after subchronic exposures, which are not present after acute exposure to diacetyl or 2,3-pentanedione.
It was postulated that the totality of flavorings’ effects on the airways would include more than epithelial damage and involve pharmacological effects in the airway wall. Diacetyl is believed to diffuse rapidly across the epithelium (Morris and Hubbs, 2009; Morris, 2012). An earlier investigation (Fedan et al., 2006) demonstrated that diacetyl applied to the mucosal surface of guinea pig IPT preparations elicited contractions in lower concentrations, relaxation in higher concentrations, increased reactivity to mucosally applied MCh, and reduced transepithelial potential difference and resistance. In the rat IPT preparation both diacetyl and 2,3-pentanedione elicited transient contraction and relaxation in the absence and presence of the epithelium, irrespective of the route of application, that is, IL or EL. Thus, the pharmacologic profile of diacetyl is similar in the two species (2,3-pentanedione was not investigated in the guinea pig). During inhalation the flavorings may have crossed the epithelium and induced bronchodilation that ameliorated bronchoconstriction in response to MCh. It is likely that epithelial integrity began to be compromised from the onset of exposure, worsening during the 6-h period and facilitating progressive and greater access of the flavoring to the smooth muscle. These two factors may have led to a reduction in reactivity to MCh. This notion is concordant with the lack of effect of flavorings on basal pulmonary function in our experiments.
Preparations were relaxed by flavoring concentrations that have been calculated to exist in the airway wall of exposed humans (Morris and Hubbs, 2009; Morris, 2012). It is unknown whether the relaxation reflects a nonspecific toxic action or a specific pharmacological effect, and whether or not it is reversible. The latter possibility is under investigation in our lab, but if it is determined to be irreversible, one can posit that a long-lasting effect on airway smooth muscle abrogates the ability of the smooth muscle to contract in response to inhaled MCh, regardless of the degree of epithelium integrity. Such an effect would have to be powerful enough to offset the gain in reactivity that would otherwise occur due to epithelial damage alone.
Current evidence implicates diacetyl as being a critical chemical in toxicity following butter flavoring inhalation, and the pulmonary toxicity of diacetyl has by now been clearly demonstrated (Hubbs et al., 2002, 2008, 2010; Morris and Hubbs, 2009; Morgan et al., 2008, 2012b; Palmer et al., 2011). One or more of the other flavorings in the mixture, however, may contribute to the overall toxicity of butter flavoring. An examination of the pulmonary toxicity of all the chemicals in flavoring mixtures, alone and in combination with diacetyl, has not thus far been accomplished. Many flavorings are less abundant than diacetyl (Boylstein et al., 2006), but they may be more potent. However, because they frequently accompany diacetyl exposures in the workplace and are plentiful, acetoin and acetic acid were given initial attention using workplace-relevant inhalation exposures. It was first observed that 150 ppm acetoin was without significant effect on pulmonary function and airway reactivity to MCh (RL). On the other hand, acetic acid, although without marked effect on RL and Cdyn, increased reactivity to inhaled MCh (RL). It was a surprising finding that the “diacetyl effect” dominated the reactivity change of the high-dose mixed exposure. The explanation for this flavoring interaction is not known at present.
The flavoring effects described in this study reflect cytotoxicity in the airway wall, probably to both epithelium and smooth muscle, at the least. Diacetyl and similar α-diketones are highly selective for the modification of arginyl residues in proteins (Borders and Riordan, 1975; Epperly and Dekker, 1989; Mueller et al., 1995) and enzymes responsible for protecting cells from oxidative damage (Borders et al., 1985). Injury to the epithelium may involve covalent bonding of α-diketones with arginine-containing proteins, altering protein ternary structure and function (Mathews et al., 2010). Apoptosis may result from DNA modification resulting from diacetyl covalently binding to guanyl nucleotides (More et al., 2012b). α-Diketones may disrupt normal electron transfer processes and generate reactive oxygen species (Kovacic and Cooksy, 2010), which are well known to result in cell injury. Caspase 3 in main-stem bronchus epithelium (Gardiner et al., 2009) and in olfactory nerve bundles (Hubbs et al., 2012) is activated by α-diketones.
Dicarbonyl/xylulose reductase (DCXR), which metabolizes diacetyl and 2,3-pentanedione in rat nasal and tracheal tissues (Gardiner et al., 2009; Hubbs et al., 2012), may also augment cytotoxicity and death in cells that have DCXR activity (Hubbs et al., 2012; Matsunaga et al., 2008). Thus, a multitude of changes in the airways may coalesce and contribute to the development of airway hyporeactivity to MCh after α-diketone inhalation.
CONCLUSIONS
Collectively, our findings indicate that a 6-h inhalation of diacetyl or 2,3-pentanedione leads to airway hyporeactivity in vivo and airway hyperreactivity in vitro. The results suggest that 2,3-pentanedione elicits greater effects than diacetyl in the airways in vivo and in vitro. The α-diketones possess complex pharmacological characteristics, being able to produce mild contraction and profound relaxation at concentrations that have been calculated to exist in the airway wall of humans during inhalation exposures. In mixed inhalation exposures neither acetoin nor acetic acid appreciably influenced the effects of diacetyl itself.
Footnotes
Mention of brand names does not constitute product endorsement. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
REFERENCES
- Aarhus LL, Rimele TJ, Vanhoutte PM. Removal of epithelium causes bronchial supersensitivity to acetylcholine and 5-hydroxytryptamine. Fed. Proc. 1984;43:995. [Google Scholar]
- Borders CL, Jr., Riordan JF. An essential arginyl residue at the nucleotide binding site of creatine kinase. Biochemistry. 1975;14:4699–4704. doi: 10.1021/bi00692a021. [DOI] [PubMed] [Google Scholar]
- Borders CL, Jr., Saunders JE, Blech DM, Fridovich I. Essentiality of the active-site arginine residue for the normal catalytic activity of Cu,Zn superoxide dismutase. Biochem. J. 1985;230:771–776. doi: 10.1042/bj2300771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylstein R, Piacitelli C, Grote A, Kanwal R, Kullman G, Kreiss K. Diacetyl emissions and airborne dust from butter flavorings used in microwave popcorn production. J. Occup. Environ. Hyg. 2006;3:530–535. doi: 10.1080/15459620600909708. [DOI] [PubMed] [Google Scholar]
- Cavalcanti Zdo R, Albuquerque Filho AP, Pereira CA, Coletta EN. Bronchiolitis associated with exposure to artificial butter flavoring in workers at a cookie factory in Brazil. J. Bras. Pneumol. 2012;38:395–399. doi: 10.1590/s1806-37132012000300016. [DOI] [PubMed] [Google Scholar]
- Day G, LeBouf R, Grote A, Pendergrass S, Cummings K, Kreiss K, Kullman G. Identification and measurement of diacetyl substitutes in dry bakery mix production. J. Occup. Environ. Hyg. 2011;8:93–103. doi: 10.1080/15459624.2011.547148. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health . NIOSH health hazard evaluation report. ConAgra Snack Foods; Marion, OH: 2004. HETA number 2-3-0112-2949. [Google Scholar]
- Egilman DS, Schilling JH. Bronchiolitis obliterans and consumer exposure to butter-flavored microwave popcorn: A case series. Int. J. Occup. Environ. Health. 2012;18:29–42. doi: 10.1179/1077352512Z.0000000005. [DOI] [PubMed] [Google Scholar]
- Epperly BR, Dekker EE. Inactivation of Escherichia coli L-threonine dehydrogenase by 2,3-butanedione. Evidence for a catalytically essential arginine residue. J. Biol. Chem. 1989;264:18296–18301. [PubMed] [Google Scholar]
- Ernstgard L, Iregren A, Sjogren B, Johanson G. Acute effects of exposure to vapours of acetic acid in humans. Toxicol. Lett. 2006;165:22–30. doi: 10.1016/j.toxlet.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Farmer SG, Fedan JS, Hay DW, Raeburn D. The effects of epithelium removal on the sensitivity of guinea-pig isolated trachealis to bronchodilator drugs. Br. J. Pharmacol. 1986;89:407–414. doi: 10.1111/j.1476-5381.1986.tb10274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SG, Hay DW, Raeburn D, Fedan JS. Relaxation of guinea-pig tracheal smooth muscle to arachidonate is converted to contraction following epithelium removal. Br. J. Pharmacol. 1987;92:231–236. doi: 10.1111/j.1476-5381.1987.tb11316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedan JS, Dowdy JA, Fedan KB, Hubbs AF. Popcorn worker’s lung: In vitro exposure to diacetyl, an ingredient in microwave popcorn butter flavoring, increases reactivity to methacholine. Toxicol. Appl. Pharmacol. 2006;215:17–22. doi: 10.1016/j.taap.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Fedan JS, Frazer DG. Influence of epithelium on the reactivity of guinea pig isolated, perfused trachea to bronchoactive drugs. J. Pharmacol. Exp. Ther. 1992;262:741–750. [PubMed] [Google Scholar]
- Fedan JS, Millecchia LL, Johnston RA, Rengasamy A, Hubbs A, Dey RD, Yuan LX, Watson D, Goldsmith WT, Reynolds JS, Orsini L, Dortch-Carnes J, Cutler D, Frazer DG. Effect of ozone treatment on airway reactivity and epithelium-derived relaxing factor in guinea pigs. J. Pharmacol. Exp. Ther. 2000;293:724–734. [PubMed] [Google Scholar]
- Gardiner DW, Goldsmith W, Morris JB, Battelli LA, Friend S, Castranova V, Hubbs A. Adhesion molecule expression in diacetyl exposed rat lungs. Toxicol. Sci. 2009;108(S-1):432–433. [Google Scholar]
- Goldsmith WT, McKinney W, Jackson M, Law B, Bledsoe T, Siegel P, Cumpston J, Frazer D. A computer-controlled whole-body inhalation exposure system for the oil dispersant COREXIT EC9500A. J. Toxicol. Environ. Health A. 2011;74:1368–1380. doi: 10.1080/15287394.2011.606793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber P, Saechao K, Boomus C. Diacetyl-induced lung disease. Toxicol. Rev. 2006;25:261–272. doi: 10.2165/00139709-200625040-00006. [DOI] [PubMed] [Google Scholar]
- Hay DW, Farmer SG, Raeburn D, Robinson VA, Fleming WW, Fedan JS. Airway epithelium modulates the reactivity of guinea-pig respiratory smooth muscle. Eur. J. Pharmacol. 1986;129:11–18. doi: 10.1016/0014-2999(86)90330-4. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Battelli LA, Goldsmith WT, Porter DW, Frazer D, Friend S, Schwegler-Berry D, Mercer RR, Reynolds JS, Grote A, Castranova V, Kullman G, Fedan JS, Dowdy J, Jones WG. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol. Appl. Pharmacol. 2002;185:128–135. doi: 10.1006/taap.2002.9525. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Cumpston AM, Goldsmith WT, Battelli LA, Kashon ML, Jackson MC, Frazer DG, Fedan JS, Goravanahally MP, Castranova V, Kreiss K, Willard PA, Friend S, Schwegler-Berry D, Fluharty KL, Sriram K. Respiratory and olfactory cytotoxicity of inhaled 2,3-pentanedione in Sprague-Dawley rats. Am. J. Pathol. 2012;181:829–844. doi: 10.1016/j.ajpath.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbs AF, Goldsmith WT, Kashon ML, Frazer D, Mercer RR, Battelli LA, Kullman GJ, Schwegler-Berry D, Friend S, Castranova V. Respiratory toxicologic pathology of inhaled diacetyl in Sprague-Dawley rats. Toxicol. Pathol. 2008;36:330–344. doi: 10.1177/0192623307312694. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Moseley AE, Goldsmith WT, Jackson MC, Kashon ML, Battelli LA, Schwegler-Berry D, Goravanajally MP, Frazer D, Fedan JS, Kreiss K, Castranova V. Airway epithelial toxicity of the flavoring agent, 2,3-pentanedione. Toxicol. Sci. 2010;114(suppl.):319. [Google Scholar]
- Institute for Laboratory Animal Research . Guide for the care and use of laboratory animals. 8th National Academies Press; Washington, DC: 2011. [Google Scholar]
- King TE., Jr. Bronchiolitis obliterans. Lung. 1989;167:69–93. doi: 10.1007/BF02714935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic P, Cooksy AL. Electron transfer as a potential cause of diacetyl toxicity in popcorn lung disease. Rev. Environ. Contam. Toxicol. 2010;204:133–148. doi: 10.1007/978-1-4419-1440-8_2. [DOI] [PubMed] [Google Scholar]
- Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N. Engl. J. Med. 2002;347:330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- Larsen ST, Alarie Y, Hammer M, Nielsen GD. Acute airway effects of diacetyl in mice. Inhal. Toxicol. 2009;21:1123–1128. doi: 10.3109/08958370902795311. [DOI] [PubMed] [Google Scholar]
- Lockey JE, Hilbert TJ, Levin LP, Ryan PH, White KL, Borton EK, Rice CH, McKay RT, LeMasters GK. Airway obstruction related to diacetyl exposure at microwave popcorn production facilities. Eur. Respir. J. 2009;34:63–71. doi: 10.1183/09031936.00050808. [DOI] [PubMed] [Google Scholar]
- Martyny JW, Van Dyke MV, Arbuckle S, Towle M, Rose CS. Diacetyl exposures in the flavor manufacturing industry. J. Occup. Environ. Hyg. 2008;5:679–688. doi: 10.1080/15459620802368463. [DOI] [PubMed] [Google Scholar]
- Mathews JM, Watson SL, Snyder RW, Burgess JP, Morgan DL. Reaction of the butter flavorant diacetyl (2,3-butanedione) with N-alpha-acetylarginine: A model for epitope formation with pulmonary proteins in the etiology of obliterative bronchiolitis. J. Agric. Food Chem. 2010;58:12761–12768. doi: 10.1021/jf103251w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga T, Kamiya T, Sumi D, Kumagai Y, Kalyanaraman B, Hara A. L-Xylulose reductase is involved in 9,10-phenanthrenequinone-induced apoptosis in human T lymphoma cells. Free Radical Biol. Med. 2008;44:1191–1202. doi: 10.1016/j.freeradbiomed.2007.12.024. [DOI] [PubMed] [Google Scholar]
- McKinney W, Frazer D. Computer-controlled ozone inhalation exposure system. Inhal. Toxicol. 2008;20:43–48. doi: 10.1080/08958370701758544. [DOI] [PubMed] [Google Scholar]
- More SS, Vartak AP, Vince R. The butter flavorant, diacetyl, exacerbates beta-amyloid cytotoxicity. Chem. Res. Toxicol. 2012a;25:2083–2091. doi: 10.1021/tx3001016. [DOI] [PubMed] [Google Scholar]
- More SS, Raza A, Vince R. The butter flavorant, diacetyl, forms a covalent adduct with 2-deoxyguanosine, uncoils DNA, and leads to cell death. J. Agric. Food Chem. 2012b;60:3311–3317. doi: 10.1021/jf300180e. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Flake GP, Kirby PJ, Palmer SM. Respiratory toxicity of diacetyl in C57BL/6 mice. Toxicol. Sci. 2008;103:169–180. doi: 10.1093/toxsci/kfn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Jokinen MP, Johnson CL, Gwinn WM, Price HC, Flake GP. Bronchial fibrosis in rats exposed to 2,3-butanedione and 2 3-pentanedione vapors. Toxicol. Sci. 2012a;126:186. doi: 10.1177/0192623311431946. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Jokinen MP, Price HC, Gwinn WM, Palmer SM, Flake GP. Bronchial and bronchiolar fibrosis in rats exposed to 2,3-pentanedione vapors: Implications for bronchiolitis obliterans in humans. Toxicol. Pathol. 2012b;40:448–465. doi: 10.1177/0192623311431946. [DOI] [PubMed] [Google Scholar]
- Morris JB. Biologically-based modeling insights in inhaled vapor absorption and dosimetry. Pharmacol. Ther. 2012;136:401–413. doi: 10.1016/j.pharmthera.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Morris JB, Hubbs AF. Inhalation dosimetry of diacetyl and butyric acid, two components of butter flavoring vapors. Toxicol. Sci. 2009;108:173–183. doi: 10.1093/toxsci/kfn222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MJ, Samuelsson B, Haeggstrom JZ. Chemical modification of leukotriene A4 hydrolase. Indications for essential tyrosyl and arginyl residues at the active site. Biochemistry. 1995;34:3536–3543. doi: 10.1021/bi00011a007. [DOI] [PubMed] [Google Scholar]
- Palmer SM, Flake GP, Kelly FL, Zhang HL, Nugent JL, Kirby PJ, Foley JF, Gwinn WM, Morgan DL. Severe airway epithelial injury, aberrant repair and bronchiolitis obliterans develops after diacetyl instillation in rats. PLoS One. 2011;6:e17644. doi: 10.1371/journal.pone.0017644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L1085–L1095. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian N, Kullman G, Lynch D, Kreiss K. Asthma arising in flavoring-exposed food production workers. Int. J. Occup. Med. Environ. Health. 2008;21:173–177. doi: 10.2478/v10001-008-0019-7. [DOI] [PubMed] [Google Scholar]
- Savov JD, Whitehead GS, Wang J, Liao G, Usuka J, Peltz G, Foster WM, Schwartz DA. Ozone-induced acute pulmonary injury in inbred mouse strains. Am J Respir Cell Mol Biol. 2004;31:69–77. doi: 10.1165/rcmb.2003-0001OC. [DOI] [PubMed] [Google Scholar]
- Schachter EN. Popcorn worker’s lung. N. Engl. J. Med. 2002;347:360–361. doi: 10.1056/NEJMe020064. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Mochizuki H, Morikawa A. Effect of influenza A virus infection on acid-induced cough response in children with asthma. Eur. Respir. J. 1997;10:71–74. doi: 10.1183/09031936.97.10010071. [DOI] [PubMed] [Google Scholar]
- Smith JA, Frazer DG, Fedan JS. Alteration in the modulatory role of respiratory epithelium after exposure of guinea pigs to respirable cotton dust. J. Pharmacol. Exp. Ther. 1993;264:683–688. [PubMed] [Google Scholar]
- Spina D. Epithelium smooth muscle regulation and interactions. Am. J. Respir. Crit. Care Med. 1998;158:S141–S145. doi: 10.1164/ajrccm.158.supplement_2.13tac100a. [DOI] [PubMed] [Google Scholar]
- Stanek J, Symanowicz PT, Olsen JE, Gianutsos G, Morris JB. Sensory-nerve-mediated nasal vasodilatory response to inspired acetaldehyde and acetic acid vapors. Inhal. Toxicol. 2001;13:807–822. doi: 10.1080/08958370120057. [DOI] [PubMed] [Google Scholar]
- van Rooy FG, Rooyackers JM, Prokop M, Houba R, Smit LA, Heederik DJ. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am. J. Respir. Crit. Care Med. 2007;176:498–504. doi: 10.1164/rccm.200611-1620OC. [DOI] [PubMed] [Google Scholar]
- van Rooy FG, Smit LA, Houba R, Zaat VA, Rooyackers JM, Heederik DJ. A cross-sectional study of lung function and respiratory symptoms among chemical workers producing diacetyl for food flavourings. Occup. Environ. Med. 2009;66:105–110. doi: 10.1136/oem.2008.039560. [DOI] [PubMed] [Google Scholar]
- Yao X, Fredriksson K, Yu ZX, Xu X, Raghavachari N, Keeran KJ, Zywicke GJ, Kwak M, Amar MJ, Remaley AT, Levine SJ. Apolipoprotein E negatively regulates house dust mite-induced asthma via a low-density lipoprotein receptor-mediated pathway. Am. J. Respir. Crit. Care Med. 2010;182:1228–1238. doi: 10.1164/rccm.201002-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotolow R, Brautbar N, Wu MP. Occupational bronchiolitis obliterans in food flavoring; Presented during the 25th Anniversary of the Collegium Ramazzini; Carpi, Italy. Oct 25–28, 2007. [Google Scholar]