Abstract
Objective
To evaluate neurotransmitter deficiencies and neurotransmitter-based treatments for frontotemporal dementia (FTD).
Methods
The authors conducted a systematic review of the literature on the mechanism and treatment of FTD and a meta-analysis of treatment studies of antidepressants for the behavioral symptoms of FTD.
Results
Patients with FTD show deficiencies in the serotonin and dopamine neurotransmitter systems, while the acetylcholine system appears relatively intact. Antidepressant treatment significantly improves behavioral symptoms in FTD, but most studies are small and uncontrolled. Serotonergic treatments appear to improve the behavioral but not cognitive symptoms of FTD.
Conclusions
Studies of neurotransmitter deficiencies in frontotemporal dementia (FTD) can be helpful in developing treatments. Treatment studies on FTD are scarce, given the prevalence and severity of this illness. Larger, well-controlled treatment studies are required to reach more definitive conclusions about treatment efficacy. Multicenter studies are likely the best way to complete treatment studies in a timely manner.
Frontotemporal dementia (FTD) is increasingly recognized as an important cause of dementia.1 The symptoms of FTD include behavioral symptoms such as disinhibition, inappropriate social behavior, and apathy. Other symptoms include language and executive dysfunction.2,3 The behavioral symptoms of FTD can be difficult to manage for caregivers and clinicians.
The paucity of pharmacologic trials for FTD is likely due to the only recent clinical definition of the illness, limitations in understanding the biology of FTD, and the difficulty of assembling well-characterized groups of patients.
Currently, the medication strategies used for FTD are based mostly on the neurotransmitter replacement/augmentation strategies used for other neurodegenerative diseases such as Parkinson disease (PD) and Alzheimer disease (AD), and on medications used to treat the behavioral symptoms of illnesses such as AD, major depressive disorder, obsessive-compulsive disorder, and schizophrenia.4 Hopefully, medications will eventually be developed that affect the underlying disease process of FTD. However, there are several reasons to investigate neurotransmitter-based strategies for FTD: there is evidence that neurotransmitter augmentation strategies can decrease the behavioral symptoms of FTD, and these medications are in current use in patients with FTD and their safety, efficacy, and long-term effects should be evaluated. Also, even when medications that affect the specific disease process of FTD are developed, medications based on augmenting neurotransmitter systems will likely continue to be useful to ameliorate symptoms. In this article we systematically review the biologic mechanisms of FTD, focusing on neurotransmitter studies, and reports of treatments for FTD. We sought to provide a basis for the rational evaluation and investigation of the pharmacologic treatment of FTD.
Methods
Mechanism review
A number of terms have been used to describe patients with FTD. Accordingly, many diagnoses were used in the searches. However, these diagnoses are not synonymous. In supplementary tables E-1 and E-2, we report the diagnoses, criteria, and imaging modality used in all studies reviewed that evaluated living subjects (i.e., did not have a diagnosis based on autopsy). See supplementary reference list and tables E-1 and E-2 listing the reviewed studies (go to the Neurology Web site at www.neurology.org). The commonly used diagnostic criteria for FTD do not explicitly address psychiatric illness despite considerable symptom overlap.2,5 This hinders comparisons between FTD and psychiatric disorders. Future revisions of the diagnostic criteria for FTD should explicitly address psychiatric illness.
Searches of MEDLINE, EMBASE, and The Cochrane Library were performed through June 2005 with the following diagnosis terms: “frontotemporal dementia or dementia lacking distinctive histopathologic features or dementia lacking distinctive histology or Pick complex or Pick's complex or lobar atrophy or Pick's disease or dementia of the frontal type or frontal lobe degeneration or frontal lobe dementia.” These terms were linked to the following terms: “neurotransmitter or monoamine or serotonin or dopamine or norepinephrine or acetylcholine or glutamate or GABA or somatostatin or positron emission tomography or PET or single photon emission computed tomography or SPECT.” The search was limited to human subjects and English language publications. The results of the search were evaluated by one of the authors (E.D.H.) and a study was reviewed if it met the following criteria: it was performed on patients with one of the diagnoses listed above, and it contained original data pertaining to neurotransmitter or neuromodulator alterations. Patients with corticobasal degeneration or progressive supranuclear palsy were not included. Imaging studies were included if they investigated neurotransmitter systems, but not if they analyzed only regional blood flow. If an appropriate article was referenced, it was also reviewed. A total of 48 studies were reviewed for this section.
Treatment review
Searches of MEDLINE, EMBASE, and The Cochrane Library were performed through June 2005 with the term “treatment” linked with the diagnosis terms listed in the Mechanism section. The search was limited to human subjects and English language publications. The results of the search were evaluated by one of the authors (E.D.H.) and the study was reviewed if it met the following criteria: it was performed on patients with one of the diagnoses listed above, and it contained original data on pharmacologic or somatic treatment. A study would be included if it looked at FTD in addition to patients with other diagnoses, but only if the data from the FTD patients were reported separately. Studies were not included that evaluated subjects with frontal lobe dysfunction due to other causes, such as traumatic brain injury or stroke. A total of 24 studies were reviewed for this section.
Meta-analysis
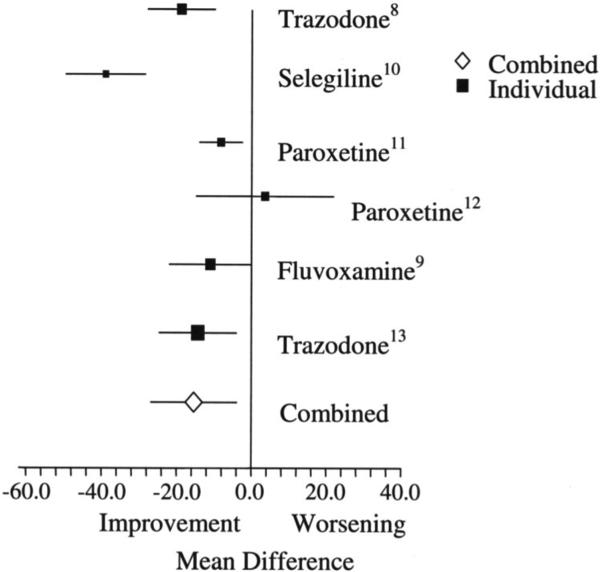
Studies were included in the meta-analysis if they tested the effects of an antidepressant medication in patients with frontotemporal dementia and if they performed the Neuropsychiatric Inventory6 (NPI) as part of their study. We limited the meta-analysis to antidepressants because this was the only class of treatments similar enough to include in a meta-analysis, yet with enough studies to be meaningful. Due to the small number of studies in this area, open-label and uncontrolled studies were included in the meta-analysis. Six studies were included (see table E-2 [on the Neurology Web site at www.neurology.org] and the figure). The change in the total NPI pre- and post-treatment was compared with a random effects model as previously described.7 Some studies used the 10-item NPI,8,9 and some used the 12-item NPI.10-13 However, when these studies were grouped separately in the meta-analysis, the difference in the average NPI difference was minimal (−15.5 vs −15.2) and so these two groups were combined for the reported meta-analysis.
Figure.
Effects of treatment on the Neuropsychiatric Inventory in patients with frontotemporal dementia. Numbers are references. Bars represent 95% CIs. Size of symbol represents sample size.
Results
Mechanism review
Serotonin
The majority of studies that have examined the serotonergic system in FTD or Pick's disease (PiD) have shown deficiencies (see table E-1). This includes autopsy,14-18 imaging,19 and CSF studies.20 Some CSF studies have failed to show a significant decrease in CSF 5-hydroxyindoleacetic acid (5-HIAA, a serotonin metabolite)18,21,22 but two of these studies showed nonsignificant trends toward decreased CSF 5-HIAA in patients with FTD.21,22
This serotonergic deficit appears to be more postsynaptic than presynaptic.14,17,18 This has been demonstrated by a recent PET study that demonstrated decreased 5-HT2A receptors in the orbitofrontal, frontal medial, and cingulate cortices of patients,19 deficiencies of non-specific serotonin binding,14 decreased 5-HT1A and 5-HT2A receptors,17 and decreased LSD 5-HT receptor binding.18 Three experiments have shown intact or increased imipramine binding, and other measures of presynaptic serotonergic activity, in FTD.14,15,18 However, one study showed neuron loss in the raphe nuclei in patients with FTD.16 This finding could be due to retrograde transsynaptic degeneration of projecting neurons.
The anatomic and clinical presentation of FTD corresponds well to serotonergic dysfunction: there are strong serotonergic projections to the frontal cortex from the raphe nuclei,23 low brain levels of serotonin have been associated with aggression and impulsiveness,24 depressive symptoms,25 and alterations in frontal cortex metabolism.26 Decreased 5-HT1A receptor activity has been associated with symptoms of depression and anxiety,27 and antagonism of 5-HT 3 and 2C blockade is associated with increased appetite and weight gain.18,28 In addition, the symptoms of FTD overlap considerably with those of psychiatric disorders treated with serotonin augmentation such as depression and obsessive-compulsive disorder.29 Interestingly, one study found that the CSF homovanillic acid (HVA, a metabolite of dopamine) to 5-HIAA ratio correlated with aggressiveness in FTD, but not AD.20
Dopamine
The dopaminergic system has interesting theoretical and practical implications for FTD. While the areas of the cortex degenerated in FTD receive serotonin projections, they also receive strong dopaminergic projections.30 There is clinical evidence of basal ganglia dopamine dysregulation (i.e., parkinsonism) in FTD and FTD variants such as frontotemporal dementia and parkinsonism (FTDP-17). This clinical observation is supported by a PET study that showed decreased presynaptic dopamine transporter in the putamen and caudate of patients with FTD.31 A SPECT study showed severely reduced presynaptic dopaminergic nerve terminals and mild reduction of postsynaptic D2 receptor binding in the striatum of a patient with FTDP-17.32 Studies have demonstrated significantly lower CSF levels of HVA and dopamine in FTD and Pick's disease (PiD),21,33,34 and decreased D2 ligand uptake in frontal cortex.35 Reports that do not support dopamine abnormalities in FTD include one that failed to demonstrate abnormal levels of CSF HVA,18 and another that failed to show abnormalities in brain dopamine concentrations.22 There is also clinical overlap between psychiatric disorders involving the dopamine system and FTD; the executive dysfunction of schizophrenia and attention-deficit/hyperactivity disorder appear to be linked to prefrontal cortex hypometabolism36,37 and can improve with dopamine augmentation.36,38
Acetylcholine
Given the reports of deficiencies in the cholinergic system in AD and the development of treatments related to this deficiency, several investigators have examined the cholinergic system in FTD. The majority of studies demonstrate a relatively intact (especially compared to that in AD) cholinergic system in FTD and PiD. This evidence includes a preservation or increase in cortical choline acetyltransferase,14,17,18,39-44 intact acetylcholinesterase levels,45 intact post-synaptic muscarinic receptor binding,17 preservation of neurons in the nucleus basalis of Meynert (nbM, the largest collection of pre-synaptic cholinergic neurons),41,43,46,47 and lack of response to a scopolamine challenge in one patient.39 However, some studies have shown decreased muscarinic receptor binding on autopsy41,48-50 and in living subjects with SPECT,51 and decreased neuron density in the nbM on autopsy.52
Norepinephrine
There is little evidence on norepinephrine abnormalities in FTD; one study showed decreased number and increased morphologic alterations of noradrenergic neurons in the locus coeruleus in PiD,53 while another showed relative preservation of the locus coeruleus in FTD.16 One CSF study showed 3-methoxy-4-hydroxyphenylglycol (MHPG, the major metabolite of CNS norepinephrine) levels in FTD similar to controls,18 while two others showed non-significantly lower CSF MHPG levels.21,22
One study examined the monoamine oxidase (MAO) enzyme system in FTD and showed significantly increased MAO-A activity in the hypothalamus and decreased activity in the nucleus basalis of Meynert and temporal pole in PiD. MAO-B activity was decreased in the nucleus basalis of Meynert and increased in the hypothalamus.54
Other neurotransmitter systems
There has been increased interest in the glutamate system in FTD since the FDA approval of memantine, a low to moderate affinity noncompetitive NMDA antagonist. However, there is little evidence about this neurotransmitter system in FTD. One autopsy study showed decreased α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and NMDA receptors in the frontal and temporal cortexes in patients with PiD, and a decrease in AMPA receptors in patients with frontal lobar degeneration.17 A loss of glutamatergic pyramidal cells and calbindin-D28k-immunoreactive GABAergic neurons in the frontal and temporal cortices of patients with FTD55 and FTD with motor neuron disease56 has been reported. However, other evidence on the GABA system in PiD and FTD suggests that it is intact.18
Treatment review
Overview
This section discusses results of treatment studies for FTD and presents the results of a meta-analysis of the effects of antidepressant treatment on behavioral symptoms of FTD. This literature is subject to several limitations including few studies, small sample sizes, lack of adequate controls, and publication bias. There are few randomized controlled clinical trials for FTD, especially compared to the number of treatment trials for other disorders such as AD.57
Serotonergic treatments
As reviewed in the previous section, the serotonergic neurotransmitter system has most consistently shown deficits in patients with FTD. Medications that affect this system have been most studied in FTD. There have been two double-blinded, randomized, placebo-controlled trials, and several open-label studies of serotonergic agents for the treatment of FTD (see table E-2 and figure). The first double-blinded, randomized, placebo-controlled trial reported that treatment with 40 mg of paroxetine for 6 weeks did not improve behavioral symptoms, and was associated with a worsening of cognition, compared to placebo.12 This finding disagreed with the open-label studies of selective serotonin reuptake inhibitors (SSRIs) and with anecdotal evidence from clinicians that SSRIs improve the behavioral symptoms of FTD. The worsened cognition seen in this trial may reflect the anticholinergic effects of higher dose paroxetine. Also, perhaps a larger study population (they studied 10 patients, some with incomplete data) or a longer medication period is required to detect the effects of the SSRI. However, it is concerning that the effects on behavior in this study were not only not significant, they were in the wrong direction—the paroxetine group showed a non-significant increase in their Neuropsychiatric Inventory (NPI) score.
The other medication studied in a double-blinded, randomized placebo-controlled trial is trazodone. Trazodone is a relatively weak SSRI compared to fluoxetine and sertraline.58 It is a 5-HT1A, 5-HT1C, and 5-HT2 antagonist and its active metabolite is a direct serotonin receptor agonist.58 It is also an adrenergic alpha-1 (postsynaptic), alpha-2 (presynaptic), and H1 histaminic blocker.58 A double-blind placebo-controlled crossover study of trazodone up to 300 mg/day in 26 patients with FTD showed a significant decrease in the NPI score with trazodone treatment, but no effect on the Mini-Mental State Examination (MMSE) score.13 This finding is consistent with other studies of serotonergic medications, which have generally shown an effect on behavior, but not on cognition, in patients with FTD. Of note, almost half the patients reported a treatment-emergent adverse effect when on trazodone including fatigue, dizziness, hypotension, and cold extremities. In the psychiatric community, the use of trazodone as an antidepressant has been limited by adverse effects of fatigue and somnolence.
Dopaminergic treatments
While most treatment studies for FTD have focused on the serotonergic neurotransmitter system, some have tested medications that affect the dopaminergic system. Dopamine receptor antagonists (i.e., antipsychotic medications) are used clinically to treat the behavioral symptoms of FTD, especially agitation and disinhibition. This may seem contradictory to the evidence of dopaminergic deficiencies in FTD presented in the previous section. The rationale for this use, however, is that these medications have been used successfully to treat psychotic symptoms and agitation in other types of dementia and disorders such as schizophrenia. In addition, the mechanism of action of the newer generation of “atypical” antipsychotic medications, while complex and beyond the scope of this article, is characterized by less D2 receptor occupancy relative to the occupancy of other receptors than the older, “typical” antipsychotic medications.59 The mechanism with which these medications reduce agitation is not fully understood. A case report60 with risperidone and an open-label uncontrolled study61 of olanzapine support the use of these medications in FTD. The reduction in the NPI observed with olanzapine treatment was similar in magnitude to that observed with antidepressant treatment (see table E-2). As one would expect, patients with FTD appear to have high rates of extrapyramidal symptoms.62 Accordingly, many clinicians use members of this class of medications with relatively low D2 receptor occupancy, such as quetiapine. Of note, the FDA recently determined that the use of atypical antipsychotics for the treatment of behavioral disorders in elderly patients with dementia is associated with a higher (1.6- to 1.7-fold) mortality than treatment with placebo. Most of this increase in mortality was related to heart-related events or infections, and may be related to sedation. Because of this effect, this class of medications will now have a boxed warning in their labeling describing this risk (http://www.fda.gov/cder/drug/advisory/antipsychotics.htm).
Given the dopaminergic deficiencies noted in the previous section, there has also been interest in medications that increase dopaminergic transmission. One group treated three patients with FTD with an MAO-B inhibitor (selegiline) and observed a significant decrease in their NPI scores.10 A case study of partial quantitative EEG normalization and behavioral improvement in a patient with FTD with methylphenidate use has been reported.63 A recent study demonstrated improvement in performance on a gambling task with reduced risk-taking behavior in FTD patients treated with a single dose of methylphenidate.73 Methylphenidate is a medication that, through stimulating neurotransmitter release and inhibiting re-uptake, results in increased synaptic dopamine and norepinephrine. Stimulants are used to treat depressive symptoms, especially apathy, in patients with depression and other disorders involving the frontal lobes.64 This class of medications has also demonstrated efficacy in treating the deficiencies of selective attention observed in attention-deficit/hyperactivity disorder.38 Trials of direct dopamine agonists, such as bromocriptine, have shown some efficacy in other types of dementia,65 and in patients with frontal lobe traumatic brain injury.66 While dopaminergic medications have shown interesting preliminary results in patients with FTD, follow-up controlled studies must be performed.
Other treatments
Anecdotal reports had suggested that acetylcholinesterase inhibitors do not help, and may worsen, the symptoms of FTD, which is consistent with the findings, presented in the previous section, that the cholinergic system in FTD, unlike in AD, is relatively intact.10,67 However, a recent open-label study of 20 patients with FTD showed a significant improvement in behavioral, but not cognitive, symptoms with rivastigmine treatment.68 A recent letter to the editor reported clinical improvement in seven of nine, and SPECT improvement in three of nine, patients with FTD treated with acetylcholinesterase inhibitors.69 One study reported cognitive improvement in three patients treated with the alpha-2 adrenoreceptor antagonist idazoxan, although their results were not statistically tested.74 Newer neuroprotective agents, such as memantine and riluzole, have interesting potential for the treatment of FTD. The mechanism of action of memantine (a low-affinity NMDA receptor antagonist) may be applicable to FTD as well as to AD. However, there is little evidence on the glutamatergic system in FTD (see Mechanism section). Nevertheless, neuroprotective agents such as memantine have the potential to slow disease progression in FTD.
One article reported subjective impression of response of patients with FTD to a variety of medications including antidepressants, antipsychotic medications, cholinesterase inhibitors, and anticonvulsants.70 Another retrospectively evaluated the use of amantadine in patients with executive dysfunction of various causes, including from FTD.71 The results from these articles are not included in table E-2.
Meta-analysis of NPI change with antidepressant treatment in FTD
The meta-analysis presented in the figure shows a combined mean reduction of 15.4 points on the NPI with antidepressant treatment in FTD. This is likely an overestimation of the effect of these medications as these studies had small sample sizes, most were not controlled and thus subject to placebo bias, and there was likely a publication bias toward positive trials. Still, 15.4 points is a large decrease in behavioral symptoms compared to, for example, a recent review which showed that four of five randomized controlled trials showed no benefit of antidepressants in patients with AD, while one showed a 10-point NPI improvement (compared to a 2.3-point improvement on placebo),57 and a meta-analysis that showed a mean NPI decrease of 1.72 points (CI 0.87 to 2.57) with cholinesterase inhibitor use in AD.72 AD trials, however, are larger and better controlled than the FTD trials and patients with FTD tend to have higher baseline NPI scores than patients with AD.
Generally, positive serotonergic medication trials show improvement of behavioral symptoms without improvement of cognitive symptoms in patients with FTD. The ability to detect cognitive improvement, however, may be limited by the use of cognitive measures developed for use with AD (e.g., the MMSE and the ADAS-cog). These measures may not be sufficiently attuned to the symptoms of FTD to detect improvement. The choice of which antidepressant medication to use must be tailored to the individual patient. SSRIs tend to be well-tolerated. However, as described earlier, the only randomized placebo-controlled trial of an SSRI did not demonstrate an improvement in symptoms. Trazodone is the only antidepressant shown, in a blinded randomized placebo-controlled trial, to have a significant effect on the behavioral symptoms of FTD. However, trazodone may have a higher incidence of adverse effects, including fatigue and somnolence, than SSRI treatment. There is a lack of data on the long-term effects of antidepressant treatment in FTD.
Conclusions
FTD demonstrates serotonergic abnormalities in the brain, more in the post-synaptic than the pre-synaptic system. There is also evidence of dopaminergic deficiencies. The cholinergic system appears relatively intact in comparison to the deficits observed in AD. There is a dearth of treatment studies for FTD with few randomized controlled trials. However, a few points emerge from a review of this literature. The use of antidepressants as a first-line treatment to reduce the behavioral symptoms of FTD is supported by the current meta-analysis, although the studies are mostly small and uncontrolled. There is currently no evidence on the potential neuroprotective effects of neurotransmitter augmentation strategies in FTD. Further mechanism studies, including imaging studies of the dopamine and serotonin systems in FTD, are required to guide rational clinical trials. Common outcome measures should also be developed, although inclusion of the measures used in previous studies (e.g., the NPI) will facilitate comparison between studies.
Supplementary Material
Acknowledgment
The authors thank Trey Sunderland and George Chang for comments, Rita Moretti and Florence Lebert for providing supplementary data on their research projects to allow completion of the meta-analysis, and the Association for Frontotemporal Dementias (www.ftd-picks.org) for their support.
Supported by the Intramural Research Programs of the NIH, National Institute of Neurological Disorders and Stroke, and NIMH.
Footnotes
Disclosure: K.T.P. has served as a consultant for Pfizer and received fees in excess of $10,000. The authors report no conflicts of interest.
Additional material related to this article can be found on the Neurology Web site. Go to www.neurology.org and scroll down the Table of Contents for the January 10 issue to find the title link for this article.
References
- 1.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 2.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 3.Kertesz A, Munoz DG. Frontotemporal dementia. Med Clin North Am. 2002;86:501–518. doi: 10.1016/s0025-7125(02)00011-1. [DOI] [PubMed] [Google Scholar]
- 4.Litvan I. Therapy and management of frontal lobe dementia patients. Neurology. 2001;56(11 suppl 4):S41–45. doi: 10.1212/wnl.56.suppl_4.s41. [DOI] [PubMed] [Google Scholar]
- 5.The Lund and Manchester Groups Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 7.Sunderland T, Linker G, Mirza N, et al. Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 8.Lebert F, Pasquier F. Trazodone in the treatment of behavior in frontotemporal dementia. Hum Psychopharmacol Clin Exp. 1999;14:279–281. [Google Scholar]
- 9.Ikeda M, Shigenobu K, Fukuhara R, et al. Efficacy of fluvoxamine as a treatment for behavioral symptoms in frontotemporal lobar degeneration patients. Dement Geriatr Cogn Disord. 2004;17:117–121. doi: 10.1159/000076343. [DOI] [PubMed] [Google Scholar]
- 10.Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Effects of selegi-line on fronto-temporal dementia: a neuropsychological evaluation. Int J Geriatr Psychiatry. 2002;17:391–392. doi: 10.1002/gps.602. [DOI] [PubMed] [Google Scholar]
- 11.Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49:13–19. doi: 10.1159/000067021. [DOI] [PubMed] [Google Scholar]
- 12.Deakin JB, Rahman S, Nestor PJ, Hodges JR, Sahakian BJ. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology. 2003;10:10. doi: 10.1007/s00213-003-1686-5. [DOI] [PubMed] [Google Scholar]
- 13.Lebert F, Stekke W, Hasenbroekx C, Pasquier F. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17:355–359. doi: 10.1159/000077171. [DOI] [PubMed] [Google Scholar]
- 14.Sparks DL, Markesbery WR. Altered serotonergic and cholinergic synaptic markers in Pick's disease. Arch Neurol. 1991;48:796–799. doi: 10.1001/archneur.1991.00530200032014. [DOI] [PubMed] [Google Scholar]
- 15.Sparks DL, Danner FW, Davis DG, Hackney C, Landers T, Coyne CM. Neurochemical and histopathologic alterations characteristic of Pick's disease in a non-demented individual. J Neuropathol Exp Neurol. 1994;53:37–42. doi: 10.1097/00005072-199401000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Schmitt HP. Frontotemporal dementia: evidence for impairment of ascending serotoninergic but not noradrenergic innervation. Immunocytochemical and quantitative study using a graph method. Acta Neuropathol (Berl) 2001;101:256–270. doi: 10.1007/s004010000293. [DOI] [PubMed] [Google Scholar]
- 17.Procter AW, Qurne M, Francis PT. Neurochemical features of fronto-temporal dementia. Dement Geriatr Cogn Disord. 1999;10(Suppl 1):80–84. doi: 10.1159/000051219. [DOI] [PubMed] [Google Scholar]
- 18.Francis PT, Holmes C, Webster MT, Stratmann GC, Procter AW, Bowen DM. Preliminary neurochemical findings in non-Alzheimer dementia due to lobar atrophy. Dementia. 1993;4:172–177. doi: 10.1159/000107319. [DOI] [PubMed] [Google Scholar]
- 19.Franceschi M, Anchisi D, Pelati O, et al. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol. 2005;57:216–225. doi: 10.1002/ana.20365. [DOI] [PubMed] [Google Scholar]
- 20.Engelborghs S, Vloeberghs E, Maertens K, Marescau B, De Deyn PP. Evidence for an association between the CSF HVA:5-HIAA ratio and aggressiveness in frontotemporal dementia but not in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:1080. [PMC free article] [PubMed] [Google Scholar]
- 21.Sjogren M, Minthon L, Passant U, Blennow K, Wallin A. Decreased monoamine metabolites in frontotemporal dementia and Alzheimer's disease. Neurobiol Aging. 1998;19:379–384. doi: 10.1016/s0197-4580(98)00086-4. [DOI] [PubMed] [Google Scholar]
- 22.Sjogren M, Wikkelso C, Ostling S, Wallin A, Blennow K. Biological correlates of clinical subgroups of Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;14:191–197. doi: 10.1159/000066025. [DOI] [PubMed] [Google Scholar]
- 23.Wilson MA, Molliver ME. The organization of serotonergic projections to cerebral cortex in primates: retrograde transport studies. Neuroscience. 1991;44:555–570. doi: 10.1016/0306-4522(91)90077-2. [DOI] [PubMed] [Google Scholar]
- 24.Asberg M. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Ann NY Acad Sci. 1997;836:158–181. doi: 10.1111/j.1749-6632.1997.tb52359.x. [DOI] [PubMed] [Google Scholar]
- 25.Moore P, Landolt HP, Seifritz E, et al. Clinical and physiological consequences of rapid tryptophan depletion. Neuropsychopharmacology. 2000;23:601–622. doi: 10.1016/S0893-133X(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 26.Dhaenen H. Imaging the serotonergic system in depression. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 2):II76–80. [PubMed] [Google Scholar]
- 27.Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- 28.McIntyre RS, Mancini DA, Basile VS. Mechanisms of antipsychotic-induced weight gain. J Clin Psychiatry. 2001;62(Suppl 23):23–29. [PubMed] [Google Scholar]
- 29.Miller BL, Darby AL, Swartz JR, Yener GG, Mena I. Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia. 1995;6:195–199. doi: 10.1159/000106946. [DOI] [PubMed] [Google Scholar]
- 30.Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- 31.Rinne JO, Laine M, Kaasinen V, Norvasuo-Heila MK, Nagren K, Helenius H. Striatal dopamine transporter and extrapyramidal symptoms in frontotemporal dementia. Neurology. 2002;58:1489–1493. doi: 10.1212/wnl.58.10.1489. [DOI] [PubMed] [Google Scholar]
- 32.Sperfeld AD, Collatz MB, Baier H, et al. FTDP-17: an early-onset phenotype with parkinsonism and epileptic seizures caused by a novel mutation. Ann Neurol. 1999;46:708–715. doi: 10.1002/1531-8249(199911)46:5<708::aid-ana5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 33.Nagaoka S, Arai H, Iwamoto N, et al. A juvenile case of frontotemporal dementia: neurochemical and neuropathological investigations. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:1251–1261. doi: 10.1016/0278-5846(95)00264-2. [DOI] [PubMed] [Google Scholar]
- 34.Kanazawa I, Kwak S, Sasaki H, et al. Studies on neurotransmitter markers of the basal ganglia in Pick's disease, with special reference to dopamine reduction. J Neurol Sci. 1988;83:63–74. doi: 10.1016/0022-510x(88)90020-2. [DOI] [PubMed] [Google Scholar]
- 35.Frisoni GB, Pizzolato G, Bianchetti A, et al. Single photon emission computed tomography with [99Tc]-HM-PAO and [123I]-IBZM in Alzheimer's disease and dementia of frontal type: preliminary results. Acta Neurol Scand. 1994;89:199–203. doi: 10.1111/j.1600-0404.1994.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 36.Berman KF, Weinberger DR. Neuroimaging studies of schizophrenia. Oxford University Press; New York: 1999. [Google Scholar]
- 37.Zametkin AJ, Nordahl TE, Gross M, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med. 1990;323:1361–1366. doi: 10.1056/NEJM199011153232001. [DOI] [PubMed] [Google Scholar]
- 38.Rappley MD. Clinical practice. Attention deficit-hyperactivity disorder. N Engl J Med. 2005;352:165–173. doi: 10.1056/NEJMcp032387. [DOI] [PubMed] [Google Scholar]
- 39.Friedland RP, Koss E, Lerner A, et al. Functional imaging, the frontal lobes, and dementia. Dementia. 1993;4:192–203. doi: 10.1159/000107323. [DOI] [PubMed] [Google Scholar]
- 40.Wood PL, Nair NP, Etienne P, et al. Lack of cholinergic deficit in the neocortex in Pick's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:725–727. doi: 10.1016/0278-5846(83)90053-2. [DOI] [PubMed] [Google Scholar]
- 41.Hansen LA, Deteresa R, Tobias H, Alford M, Terry RD. Neocortical morphometry and cholinergic neurochemistry in Pick's disease. Am J Pathol. 1988;131:507–518. [PMC free article] [PubMed] [Google Scholar]
- 42.Wood PL, Etienne P, Lal S, et al. A post-mortem comparison of the cortical cholinergic system in Alzheimer's disease and Pick's disease. J Neurol Sci. 1983;62:211–217. doi: 10.1016/0022-510x(83)90200-9. [DOI] [PubMed] [Google Scholar]
- 43.Clark AW, Manz HJ, White CL, 3rd, Lehmann J, Miller D, Coyle JT. Cortical degeneration with swollen chromatolytic neurons: its relationship to Pick's disease. J Neuropathol Exp Neurol. 1986;45:268–284. doi: 10.1097/00005072-198605000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Bowen DM, Benton JS, Spillane JA, Smith CC, Allen SJ. Choline acetyltransferase activity and histopathology of frontal neocortex from biopsies of demented patients. J Neurol Sci. 1982;57:191–202. doi: 10.1016/0022-510x(82)90026-0. [DOI] [PubMed] [Google Scholar]
- 45.Meier-Ruge W, Iwangoff P, Reichlmeier K. Neurochemical enzyme changes in Alzheimer's and Pick's disease. Arch Gerontol Geriatr. 1984;3:161–165. doi: 10.1016/0167-4943(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 46.Tagliavini F, Pilleri G. Basal nucleus of Meynert. A neuropathological study in Alzheimer's disease, simple senile dementia, Pick's disease and Huntington's chorea. J Neurol Sci. 1983;62:243–260. doi: 10.1016/0022-510x(83)90203-4. [DOI] [PubMed] [Google Scholar]
- 47.Pilleri G. The Kluver-Bucy Syndrome in man. A clinico-anatomical contribution to the function of the medial temporal lobe structures. Psychiatr Neurol (Basel) 1966;152:65–103. [PubMed] [Google Scholar]
- 48.Odawara T, Shiozaki K, Iseki E, Hino H, Kosaka K. Alterations of muscarinic acetylcholine receptors in atypical Pick's disease without Pick bodies. J Neurol Neurosurg Psychiatry. 2003;74:965–967. doi: 10.1136/jnnp.74.7.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yates CM, Simpson J, Maloney AF, Gordon A. Neurochemical observations in a case of Pick's disease. J Neurol Sci. 1980;48:257–263. doi: 10.1016/0022-510x(80)90205-1. [DOI] [PubMed] [Google Scholar]
- 50.White P, Hiley CR, Goodhardt MJ, et al. Neocortical cholinergic neurons in elderly people. Lancet. 1977;1:668–671. doi: 10.1016/s0140-6736(77)92114-6. [DOI] [PubMed] [Google Scholar]
- 51.Weinberger DR, Gibson R, Coppola R, et al. The distribution of cerebral muscarinic acetylcholine receptors in vivo in patients with dementia. A controlled study with 123IQNB and single photon emission computed tomography. Arch Neurol. 1991;48:169–176. doi: 10.1001/archneur.1991.00530140061018. [DOI] [PubMed] [Google Scholar]
- 52.Uhl GR, Hilt DC, Hedreen JC, Whitehouse PJ, Price DL. Pick's disease (lobar sclerosis): depletion of neurons in the nucleus basalis of Meynert. Neurology. 1983;33:1470–1473. doi: 10.1212/wnl.33.11.1470. [DOI] [PubMed] [Google Scholar]
- 53.Luque JM, Chan-Palay V. Alterations in tyrosine hydroxylase immuno-reactive neurons of the locus ceruleus in Pick's disease. Dementia. 1991;2:291–296. [Google Scholar]
- 54.Sparks DL, Woeltz VM, Markesbery WR. Alterations in brain monoamine oxidase activity in aging, Alzheimer's disease, and Pick's disease. Arch Neurol. 1991;48:718–721. doi: 10.1001/archneur.1991.00530190064017. [DOI] [PubMed] [Google Scholar]
- 55.Ferrer I. Neurons and their dendrites in frontotemporal dementia. Dement Geriatr Cogn Disord. 1999;10(Suppl 1):55–60. doi: 10.1159/000051214. [DOI] [PubMed] [Google Scholar]
- 56.Ferrer I, Tunon T, Serrano MT, et al. Calbindin D-28k and parvalbumin immunoreactivity in the frontal cortex in patients with frontal lobe dementia of non-Alzheimer type associated with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1993;56:257–261. doi: 10.1136/jnnp.56.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293:596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- 58.Schatzberg AF, Nemeroff CB. The American Psychiatric Publishing textbook of psychopharmacology. 3rd ed. American Psychiatric Publishing; Washington, DC: 2004. [Google Scholar]
- 59.Tauscher J, Hussain T, Agid O, et al. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: differentiation from other atypical antipsychotics. Am J Psychiatry. 2004;161:1620–1625. doi: 10.1176/appi.ajp.161.9.1620. [DOI] [PubMed] [Google Scholar]
- 60.Curtis RC, Resch DS. Case of Pick's central lobar atrophy with apparent stabilization of cognitive decline after treatment with risperidone. J Clin Psychopharmacol. 2000;20:384–385. doi: 10.1097/00004714-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Moretti R, Torre P, Antonello RM, Cazzato G, Griggio S, Bava A. Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer's disease and other dementias: a 24-month follow-up of 68 patients. Am J Alzheimers Dis Other Dement. 2003;18:205–214. doi: 10.1177/153331750301800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pijnenburg YA, Sampson EL, Harvey RJ, Fox NC, Rossor MN. Vulnerability to neuroleptic side effects in frontotemporal lobar degeneration. Int J Geriatr Psychiatry. 2003;18:67–72. doi: 10.1002/gps.774. [DOI] [PubMed] [Google Scholar]
- 63.Goforth HW, Konopka L, Primeau M, et al. Quantitative electroencephalography in frontotemporal dementia with methylphenidate response: a case study. Clin EEG Neurosci. 2004;35:108–111. doi: 10.1177/155005940403500212. [DOI] [PubMed] [Google Scholar]
- 64.Marin RS, Fogel BS, Hawkins J, Duffy J, Krupp B. Apathy: a treatable syndrome. J Neuropsychiatry Clin Neurosci. 1995;7:23–30. doi: 10.1176/jnp.7.1.23. [DOI] [PubMed] [Google Scholar]
- 65.Imamura T, Takanashi M, Hattori N, et al. Bromocriptine treatment for perseveration in demented patients. Alzheimer Dis Assoc Disord. 1998;12:109–113. doi: 10.1097/00002093-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 66.McDowell S, Whyte J, D'Esposito M. Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients. Brain. 1998;121(Pt 6):1155–1164. doi: 10.1093/brain/121.6.1155. [DOI] [PubMed] [Google Scholar]
- 67.Merrilees JJ, Miller BL. Long-term care of patients with frontotemporal dementia. J Am Med Dir Assoc. 2003;4(6 Suppl):S162–164. doi: 10.1097/01.JAM.0000095366.91533.22. [DOI] [PubMed] [Google Scholar]
- 68.Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, Bava A. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21:931–937. doi: 10.2165/00002512-200421140-00003. [DOI] [PubMed] [Google Scholar]
- 69.Lampl Y, Sadeh M, Lorberboym M. Efficacy of acetylcholinesterase inhibitors in frontotemporal dementia. Ann Pharmacother. 2004;38:1967–1968. doi: 10.1345/aph.1D445. [DOI] [PubMed] [Google Scholar]
- 70.Chow TW, Mendez MF. Goals in symptomatic pharmacologic management of frontotemporal lobar degeneration. Am J Alzheimers Dis Other Dement. 2002;17:267–272. doi: 10.1177/153331750201700504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drayton SJ, Davies K, Steinberg M, Leroi I, Rosenblatt A, Lyketsos CG. Amantadine for executive dysfunction syndrome in patients with dementia. Psychosomatics. 2004;45:205–209. doi: 10.1176/appi.psy.45.3.205. [DOI] [PubMed] [Google Scholar]
- 72.Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA. 2003;289:210–216. doi: 10.1001/jama.289.2.210. [DOI] [PubMed] [Google Scholar]
- 73.Rahman S, Robbins TW, Hodges JR, et al. Methylphenidate (“Ritalin”) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology. doi: 10.1038/sj.npp.1300886. Epub ahead of print September 7, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coull JT, Sahakian BJ, Hodges JR. The alpha(2) antagonist idazoxan remediates certain attentional and executive dysfunction in patients with dementia of frontal type. Psychopharmacology (Berl) 1996;123:239–249. doi: 10.1007/BF02246578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.