Summary
Enzymes (PI3K and PTEN) controlling cellular levels of 3-phosphorylated phosphoinositides are known as important drivers or suppressors of tumorigenesis in various cancers. In this issue of Cancer Discovery, the laboratories of Sasaki and Pandolfi identify the lipid phosphatase INPP4B as a context-specific tumor suppressor that controls phosphoinositide levels and AKT2 activation in PTEN-deficient cells.
Increased signaling through phosphoinositide 3-kinases (PI3Ks) is considered an oncogenic driver in cancer. Consequently, targeting enzymes in this network has been a major goal of cancer drug development (1). Much attention has focused on class I PI3Ks (PI3Kα, P3Kβ, PI3Kδ and PI3Kγ) that convert phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) to PtdIns(3,4,5)trisphosphate (PtdIns(3,4,5)P3, hereafter abbreviated PIP3) (Fig. 1). Encouragingly, the first PI3K-targeted therapy (idelalisib, a PI3Kδ-selective agent) achieved regulatory approval recently for treatment of certain blood cancers (2). Compounds targeting other members of the class I PI3K family, or targeting downstream protein kinases including AKT and mTOR are in various phases of clinical trials (1). In addition to PI3Ks, there are numerous enzymes that modulate levels of 3-phosphorylated phosphoinositides in cells and therefore may play an important role in cancer. The PIP3 phosphatase PTEN (Fig. 1) is frequently deleted or inactivated in human cancer, but strategies to target PTEN-deficient tumors rely mainly on blocking PI3K or downstream kinases. In this issue of Cancer Discovery, the groups of Sasaki (3) and Pandolfi (4) identify the lipid phosphatase INPP4B as a tumor suppressor that controls the levels of PI3K lipid products and is potentially targetable in aggressive human thyroid cancers.
Figure 1.
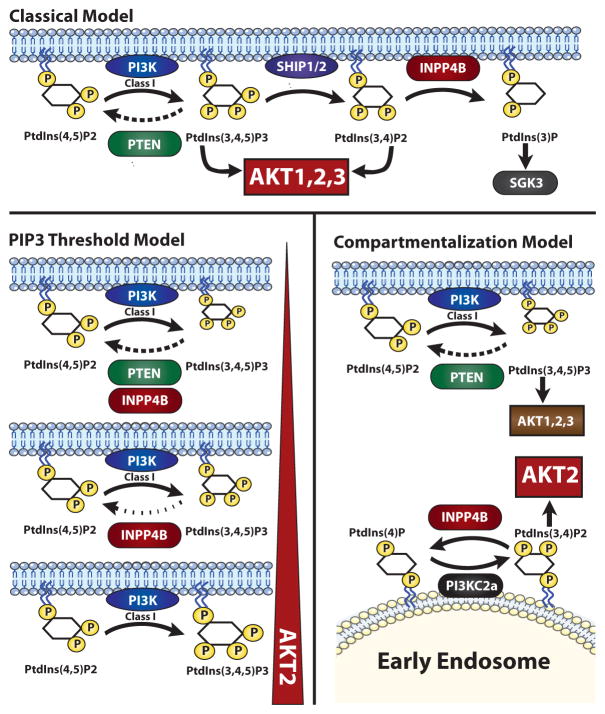
Model mechanisms of how INPP4B acts as a tumor suppressor by reducing AKT2 activation. In the classical model INPP4B reduces PtdIns(3,4)P2 at the plasma membrane. The PIP3 threshold model by Kofuji and colleagues proposes that when PTEN is absent, INPP4B restrains PtdIns(3,4,5)P3 levels by directly dephosphorylating this lipid to PtdIns(4,5)P2. The compartmentalization model by Chew and colleagues proposes that INPP4B reduces AKT2 activation specifically at the endosome by degrading PtdIns(3,4)P2 produced by PI3K-C2α.
INPP4B is an inositol phospholipid phosphatase that is best known for dephosphorylating the 4′-hydroxyl of PtdIns(3,4)P2 to produce PtdIns(3)P (Fig. 1, top). Since AKT kinases can be recruited to membranes and activated by either PIP3 or PtdIns(3,4)P2, the loss of INPP4B would be predicted to elevate AKT activity and promote transformation. Indeed, growing evidence has pointed to human INPP4B as a potential tumor suppressor gene. Gewinner and colleagues found loss of heterozygosity at the INPP4B locus in many basal-like breast cancer and ovarian cancers (5). Moreover, they found that knockdown of INPP4B enhanced AKT activation, anchorage-independent growth and cell motility. Correspondingly, Fedele and colleagues showed that expression of INPP4B in ER-negative and INPP4B-negative human breast cancer cells reduced AKT activity and anchorage-independent growth (6). Interestingly, INPP4B protein loss was frequently found in tumors that also lacked PTEN (6). Later studies provided further evidence for reduced INPP4B expression in cancer (7, 8). However, the tumor suppressor activity of INPP4B had yet to be proven or studied mechanistically in a mouse model.
The Sasaki lab generated Inpp4b−/− mice in which the phosphatase catalytic domain of INPP4B is deleted, and this strain was used in studies from both labs (3, 4). The two groups report very similar phenotypes. Inpp4b−/− mice are viable and have a normal life span, which does not phenocopy the malignancies and early mortality in mice heterozygous for PTEN (Pten+/−). The tumor suppressor function of INPP4B was revealed upon crossing with Pten+/− mice. Loss of INPP4B in the Pten+/− background resulted in mice that develop aggressive thyroid tumors with complete penetrance. These tumors have features resembling human follicular variant papillary thyroid carcinoma (FV-PTC), and are associated with lung metastasis. Consequently, Pten+/− Inpp4b−/− mice have significantly reduced survival compared to Pten+/− mice. To determine if loss of INPP4B is seen in human follicular thyroid carcinoma (FTC), both groups examined primary human FTC samples. Chew and colleagues noted that FTC patient cells have low mRNA expression of INPP4B compared to normal tissues. Likewise, Kofuji and colleagues noted that primary FTC samples have lower INPP4B protein expression than non-cancerous thyroid controls. Thus, reduced INPP4B is a common feature in human FTC.
Another similarity in the two studies was the finding that the AKT2 isoform has a dominant role in tumorigenesis in the Pten+/− Inpp4b−/− model. Kofuji and colleagues found that Akt2−/−Inpp4B−/−Pten+/− mice show no evidence of FV-PTC whereas AKT1−/−Inpp4B−/−Pten+/− mice were similar to Inpp4B−/−Pten+/− mice. Chew and colleagues provide biochemical evidence in cell lines for a link between INPP4B loss and selective AKT2 activation. Where these papers differ is in the proposed mechanism of how INPP4B loss increases AKT2 activation.
Kofuji and colleagues offer a threshold model whereby INPP4B acts as a “back-up” phosphatase by directly dephosphorylating PIP3 in the absence of PTEN (Fig. 1, lower left). Some lipid kinases and phosphatases can act on more than one position of the inositol head group, and indeed this study uncovers a previously unrecognized ability of INPP4B to remove the 5-phosphate from PIP3. Not surprisingly, INPP4B activity is detectable at a higher PIP3 concentration than for PTEN, consistent with a back-up function when PTEN cannot keep PIP3 levels under control. A key experiment was the use of a recently developed mass spectrometry method to measure PIP3 levels in thyroid tissue. This analysis showed that PIP3 levels increase dramatically in the Pten heterozygous background when Inpp4b is also deleted. They noted no difference in PtdIns(3,4)P2 levels between Pten+/− and Pten+/− Inpp4b−/− cells, suggesting that PtdIns(3,4)P2 is not the mediator of the increased incidence of FV-PTC. Thus in their model, the tumor suppressive role of INPP4B is to control PIP3 when PTEN is ineffective or when class I PI3K activity is very high.
Chew and colleagues propose another model whereby INPP4B controls AKT2 activation in a key subcellular compartment (Fig. 1, lower right). They show that knockdown of INPP4B in thyroid cancer cell lines selectively activates AKT2 in the early endosome. Contrary to Kofuji and colleagues, they found that loss of INPP4B in these cells is associated with increased abundance of PtdIns(3,4)P2 levels and not PIP3. Interestingly, they identify a likely role for PI3K-C2α, a class II PI3K that can generate PtdIns(3,4)P2 from PtdIns(4)P but cannot directly produce PIP3. According to this model, INPP4B exerts its tumor suppressive affects by counteracting PI3K-C2α-mediated AKT2 activation in early endosomes of thyroid cancer cells. This model is supported by their observation that PI3K-C2α knockdown decreases AKT2 activation, and by co-localization of INPP4B and PI3K-C2α at the endosome membrane. The authors emphasize that INPP4B exerts a qualitative rather than quantitative effect on PI3K signaling; in other words, the INPP4B phosphatase controls a specific pool of lipids and AKT isoforms, in contrast to PTEN that controls overall levels of signal output.
It is not yet clear why the two studies reached different conclusions about the mechanism of AKT2 control by INPP4B in thyroid cells. Of note, lipid measurements were carried out differently in the two studies. Kofuji applied mass spectrometry and HPLC analysis to thyroid tissue of mice with different genotypes, whereas Chew used human thyroid cancer cell lines with different knockdown constructs and assessed lipid levels by immunofluorescent staining with antibodies. Future studies may further resolve the mechanism of INPP4B tumorigenesis and provide a context for these discrepant results. Notably, Chew and colleagues observed increased anchorage-independent growth of thyroid cancer cell lines with INPP4B knockdown, consistent with a previous study (5), which could explain the observed lung metastasis. Based on evidence that endosomal trafficking regulates cell migration (9), they propose that loss of the endosomal function of INPP4B promotes an invasive phenotype. Synthesizing these ideas, one can propose that INPP4B has two roles in tumorigenesis that are not mutually exclusive. First, loss of INPP4B elevates PIP3 levels in PTEN-deficient lesions to promote tumor progression. Second, the absence of INPP4B leads to elevated PtdIns(3,4)P2 and AKT2 activity in endosomes, promoting metastasis.
Although these studies provide definitive evidence that INPP4B is a tumor suppressor, this role clearly emerges only in certain contexts since loss of Inpp4b alone does not greatly affect AKT phosphorylation in tissues and is not tumorigenic in mice. Thus INPP4B loss is one in several cooperative steps towards tumor formation. This step is particularly important in the thyroid as tumors in this organ are not observed in Pten+/− mice with functioning INPP4B. Of note, database analyses by Kofuji and colleagues reveal that loss of INPP4B in human cancer frequently co-occurs with loss of PTEN, supporting the threshold model and a selective role for INPP4B in the context of PTEN loss.
Is it possible to exploit loss of INPP4B in designing targeted therapies? Chew and colleagues provide the important insight that INPP4B is not frequently deleted or mutated in human cancers, but more commonly is downregulated by gene methylation. They show that inhibiting DNA methylation using the clinically approved drug 5′-Aza-2′-deoxycytidine can increase INPP4B expression and decrease AKT activation. Thus, applying epigenetic modulators to upregulate INPP4B expression may be a viable strategy for treatment of FTC. Another approach would be to employ selective inhibitors of AKT2. Current agents in development target both AKT1 and AKT2, but efforts to develop an AKT2-selective inhibitor might provide useful tools to treat cancers associated with INPP4B loss.
Lastly, the studies discussed here were not able to evaluate the role Inpp4b loss in breast and ovarian cancer, since Pten+/− Inpp4b−/− mice succumbed to thyroid tumors at an early age. It will be particularly interesting to resolve the role of INPP4B in breast cancer, given that Toker and colleagues recently reported that INPP4B has oncogenic potential in breast cancer by promoting SGK3 activation (Fig. 1, top) (10). Future studies applying tissue-specific deletion of Inpp4b should shed light on the role of this phosphatase in controlling tumor progression and metastasis in other contexts.
Footnotes
The authors disclose no conflicts of interest.
References
- 1.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discovery. 2014;13:140–56. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Q, Modi P, Newcomb T, Queva C, Gandhi V. Idelalisib: First-in-Class PI3K Delta Inhibitor for the Treatment of Chronic Lymphocytic Leukemia, Small Lymphocytic Leukemia, and Follicular Lymphoma. Clin Cancer Res. 2015;21:1537–42. doi: 10.1158/1078-0432.CCR-14-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kofuji S, Kimura H, Nakanishi H, Nanjo H, Takasuga S, Liu H, et al. INPP4B is a PtdIns(3,4,5)P3 phosphatase that can act as a tumor suppressor. Cancer Discovery. 2015 doi: 10.1158/2159-8290.CD-14-1329. [DOI] [PubMed] [Google Scholar]
- 4.Chew CL, Lunardi A, Gulluni F, Ruan DT, Chen M, Salmena LPD, et al. In vivo role of INPP4B in tumor and metastasis suppression through regulation of PI3K/AKT signaling at endosomes. Cancer Discovery. 2015 doi: 10.1158/2159-8290.CD-14-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–25. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedele CG, Ooms LM, Ho M, Vieusseux J, O’Toole SA, Millar EK, et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc Natl Acad Sci USA. 2010;107:22231–6. doi: 10.1073/pnas.1015245107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rynkiewicz NK, Fedele CG, Chiam K, Gupta R, Kench JG, Ooms LM, et al. INPP4B is highly expressed in prostate intermediate cells and its loss of expression in prostate carcinoma predicts for recurrence and poor long term survival. Prostate. 2015;75:92–102. doi: 10.1002/pros.22895. [DOI] [PubMed] [Google Scholar]
- 8.Stjernstrom A, Karlsson C, Fernandez OJ, Soderkvist P, Karlsson MG, Thunell LK. Alterations of INPP4B, PIK3CA and pAkt of the PI3K pathway are associated with squamous cell carcinoma of the lung. Cancer Med. 2014;3:337–48. doi: 10.1002/cam4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, et al. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–47. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Gasser JA, Inuzuka H, Lau AW, Wei W, Beroukhim R, Toker A. SGK3 mediates INPP4B-dependent PI3K signaling in breast cancer. Mol Cell. 2014;56:595–607. doi: 10.1016/j.molcel.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]