Abstract
Ma, et al. (Reports, 10 October 2014, p. 219) report asymmetric syntheses of Sceptrin and Massadine and through a stereochemical reassignment, claim to “..uncover enantiodivergence as a new biosynthetic paradigm for natural products.” This Technical Comment challenges and clarifies this claim with relevant examples from the literature of this well-known phenomenon of enantiodivergent congener biosynthesis within the same producing organism.
Keywords: biosynthesis, enantiodivergence, congeners, natural products, pseudoenantiomeric
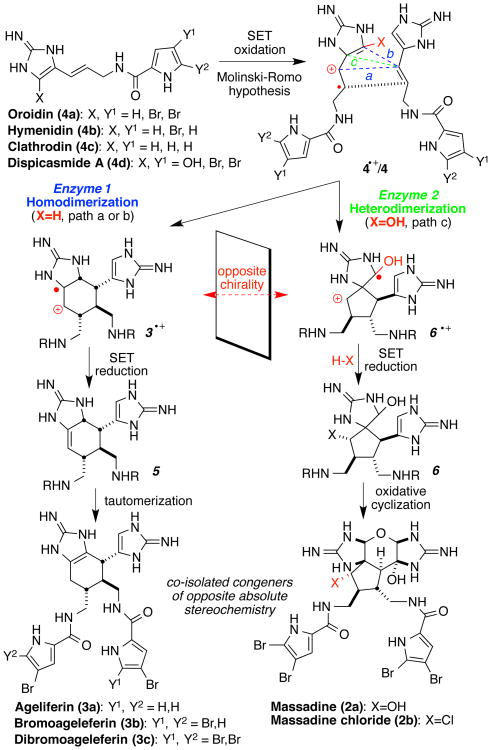
Recently, Ma et al., reported impressive syntheses of the bis-guanidine alkaloids Sceptrin and Massadine: “Asymmetric Syntheses of Sceptrin and Massadine and Evidence for Biosynthetic Enantiodivergence”. (1) Examination of the absolute configurations of the synthetic and natural materials, revealed that Sceptrin and Massadine, versus Ageliferin have mismatched chirality. The authors conclude that they: “…uncover enantiodivergence as a new biosynthetic paradigm for natural products.”, and… “Although enantiomeric biosynthesis that produces both enantiomers of a natural product has been reported (39), (2) enantiodivergent biosynthesis that produces opposite enantiomers of natural products as congeners described herein is unknown.” Unfortunately, these statements overlook a substantial body of literature unambiguously demonstrating that this phenomena is well-known. The authors' work is another affirmation that Nature has evolved enantiodivergent biogeneses leading to new secondary metabolic diversity. The biogenesis postulated (Fig. 1) accommodates the re-assigned stereochemistry providing a working hypothesis for experimental verification. (1) Although that congeneric metabolites with opposite chirality have been confirmed through this work, the details of the biosynthetic pathways to these alkaloids remains unknown.
Fig. 1. Proposed enantiodivergent biogenesis.
Re-assignment of natural product stereochemistry formed the basis upon which Ma et al. postulate the biosynthesis of the massadines and ageliferins. (1)
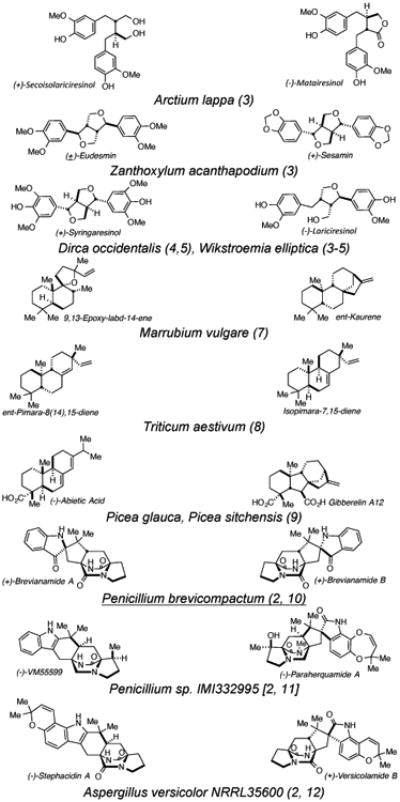
Numerous examples of enantiodivergent biogeneses producing congeners of opposite absolute stereochemistry and distinct framework connectivity within the same producing organism are well-known;[2] a non-exhaustive list is in Table 1. (3-14) Little is known about the intricate genetic, biocatalytic and mechanistic details of how enantiomeric, or pseudo-enantiomeric metabolites, are constructed. Co-isolated congeners in the same, as well as distinct oxidation states, presumably derived from the same pathway or, via parallel pathways are evident, including the biogenesis postulated by Ma. (1) Pseudoenantiomeric congeners encompasses disparate families of metabolites including polyketides, terpenes, alkaloids, and amino acid-derivatives. Pseudo-enantiomeric diastereomeric congeners, (2, 10) as well as instances of natural products sharing structural cores of opposite absolute stereochemistry, but differing in substitution patterns and oxidation states, are known (Table 1). Lignans, derived by the homodimerization of cinnamic alcohol units in plants, are particularly relevant with (+)-Syringaresinol and (-)-Lariciresinol from Dirca occidentalis and Wikstroemia elliptica being salient examples.
Table 1.
Pseudo-enantiomeric natural product congeners co-isolated from the same producing organism.
|
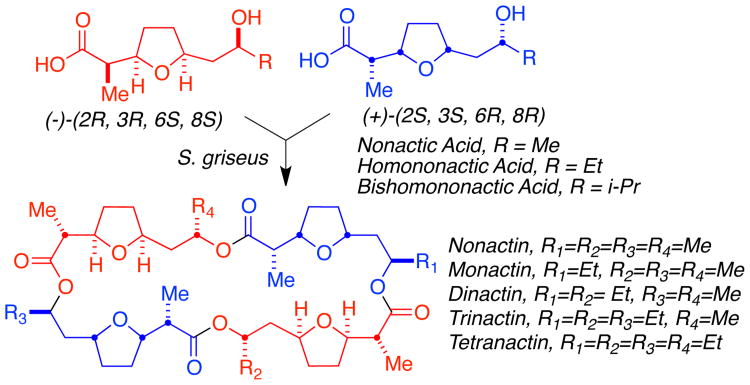
In a particularly interesting system, Shen, and others, (13) demonstrated that Nonactin and congeners are biosynthesized in Streptomyces griseus by a pair of enantiospecific pathways, which construct (+)- and (-)-Nonactic acid, as well as both enantiomers of the higher congeners Homononactic acid and Bishomononactic acid. The macrotetrolide precursors are assembled from these apparently biochemically compartmentalized, enantiomeric fragments and cyclized (Fig. 2).
Fig. 2. Nonactin Biosynthesis.
Enantiodivergent biosynthesis of monomer units and assembly of the Nonactins. (13)
Plant terpenoids, such as copalyl pyrophosphate (CPP) derivatives constitutes various families of co-isolable pseudo-enantiomeric congeners, with ent-Kaurene and 9,13-Epoxy-labd-14-ene from Marrubium vulgare and the production of (-)-Abietic acid and the ubiquitous plant hormone Gibberelin A12 from spruce trees (Picea glauca; P. sitchensis) being illustrative. It is well-known that higher plants produce both enantiomers of CPP, the core substrates from which numerous terpenoid congeners of opposite absolute stereochemistry arise. (7-10) Indeed many terpene cyclases have been documented to produce racemic or partially racemic metabolites, (2) demonstrating a further mechanism whereby Nature diversifies secondary metabolite stereochemistry. Selective catabolism or tailoring of one enantiomer of a biosynthetic racemate (ie., by an enantiospecific oxidase or reductase) is another strategy for the co-production of pseudo-enantiomeric congeners and may also be one means by which partially racemic natural products as well as pseudo-enantiomeric congeners might arise.
Fungal prenylated indole alkaloids, such as Brevianamides A/B constitute another example of pseudo-enantiomeric diasteromeric congeners from the same biosynthetic pathway with structural cores of opposite absolute configuration. (11) Related examples are Penicillium sp. congeners (-)-VM55599 and Paraherquamide A (12) and the co-isolated congeners (+)-Versicolamide B and (-)-Stephacidin A in Aspergillus versicolor. (12)
In several instances, enantiodivergent gene clusters that direct congener secondary metabolite biosynthesis appear in disparate organisms of distinct genus types, and are not restricted to speciation. Evolutionary selection pressure must be the obligatory driving force for the diversification of secondary metabolite structure and stereochemistry with enantiodivergence being among the palette of Nature's tools. As more natural products are identified from the vast reservoir of as yet undiscovered organisms on earth, it is tantalizing to ponder the adaptive significance of the evolution, conservation of genes and apparent distribution of genes between organisms that diversify secondary metabolites structurally and stereochemically.
Although little is understood regarding genetic and biochemical mechanisms that control enantioselectivity in pathways generating enantiodivergent congeners, preliminary information is emerging. (7,-9, 13-15) For example, genome sequencing, gene cluster mining and annotation of enzymes specifying the biosynthesis of (−)-Stephacidin A and (+)-Versicolamide B reveals high amino acid sequence identity (79 - 88%) between every structural pathway protein. (15) Here, enantiodivergence is clearly a result of orthologous divergent, rather than paralogous convergent evolutionary processes. That Nature clearly interrogates stereochemical diversity in the context of structural diversity, is firmly established and many more examples are anticipated. The interesting pyrrole-imidazole alkaloids (1) provides a provocative working hypothesis from which to investigate the putative existence of yet another striking example of enantiodivergent congener biosynthesis. The claim to having discovered enantiodivergent congener biosynthesis must be tempered in the context of a substantial body of published evidence dating back four decades.
Acknowledgments
We are grateful to the National Institutes of Health for financial support (CA70375 to RMW & DHS). We thank Dr. Jennifer Finefield for assistance in preparing the manuscript and thank Ben Shen, Joerg Bohlmann, Rod Croteau and Reuben Peters for helpful discussions. We confirm no competing financial interests
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Ma Z, Wang X, Wang X, Rodriguez RA, Moore CE, Gao S, Tan X, Ma Y, Rheingold AL, Baran PS, Chen C. Science. 2014;346:219–224. doi: 10.1126/science.1255677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finefield J, Sherman DH, Kreitman M, Williams RM. Angew Chem Int Ed. 2012;51:4802. doi: 10.1002/anie.201107204. This corresponds to ref. #39 in Ma Ref. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki S, Umezawa T, Shimada M. Biosci Biotechnol Biochem. 2002;66:1262–1269. doi: 10.1271/bbb.66.1262. [DOI] [PubMed] [Google Scholar]
- 4.Umezawa T, Okunishi T, Shimada M. Wood Res. 1997;84:62–75. [Google Scholar]
- 5.Badawi MM, Handa SS, Kinghorn AD, Cordell GA, Farnsworth NA. J Pharm Sci. 1983;72:1285–1287. doi: 10.1002/jps.2600721112. [DOI] [PubMed] [Google Scholar]
- 6.Okunishi T, Umezawa T, Shimada M. J Wood Sci. 2000;46:234–242. [Google Scholar]
- 7.Zerbe P, Chiang A, Dullat H, O'Neil-Johnson M, Starks C, Maberger B, Bohlmann J. The Plant Journal. 2014;79:914–927. doi: 10.1111/tpj.12589. [DOI] [PubMed] [Google Scholar]
- 8.Zhou K, Xu M, Tiernan M, Zie Q, Toyomasu T, Sugawara C, Oku M, Usui M, Mitsuhashi W, Chono M, Chandler PM, Peters RJ. Phytochem. 2012;84:47–55. doi: 10.1016/j.phytochem.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keeling CI, Dullat HK, Yuen M, Ralph SG, Jancsik S, Bohlman J. Plant Physiol. 2010;152:1197–1208. doi: 10.1104/pp.109.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birch AJ, Russell RA. Tetrahedron. 1972;28:2999–3008. the absolute stereochemistry of (+)-Brevianamide B was later reassigned to that shown (see refs. cited in ref. 2) [Google Scholar]
- 11.Blanchflower SE, Banks RM, Everett JR, Reading C. J Antibiot. 1993;46:1355. doi: 10.7164/antibiotics.46.1355. [DOI] [PubMed] [Google Scholar]
- 12.Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. Angew Chem Int Ed. 2008;47:3573–3577. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon HJ, Smith WC, Scharon AJ, Hwang SH, Kurth MJ, Shen B. Science. 2002;297:1327–1330. doi: 10.1126/science.1073175. [DOI] [PubMed] [Google Scholar]
- 14.Smith WC, Xiang L, Shen B. Antimicrob Agents Chemother. 2000;44:1809–1817. doi: 10.1128/aac.44.7.1809-1817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Srinivasan K, Tran H, Yu F, Finefield JM, Sunderhaus JD, McAfoos TJ, Tsukamoto S, Williams RM, Sherman DH. Med Chem Comm. 2012;3:987–996. doi: 10.1039/C2MD20029E. [DOI] [PMC free article] [PubMed] [Google Scholar]