Abstract
BACKGROUND
Cabazitaxel (Jevtana) has been approved for the treatment of metastatic castration-resistant prostate cancer (mCRPC). However, most patients progress and become chemoresistant, which remains a major challenge in the management of advanced PCa. In this study, we investigated whether genistein, an isoflavone abundant in soy products, could sensitize mCRPC cells to cabazitaxel treatment in experimental models.
METHODS
The in vitro and in vivo effect of genistein in enhancing the response of mCRPC cells to cabazitaxel chemotherapy was evaluated in experimental models.
RESULTS
Genistein increases the expression of pro-apoptotic protein Bax, activates apoptotic signals, and enhances the response to cabazitaxel treatment in mCRPC cells. In a PC3-luciferase xenograft model, the combined treatment with genistein and cabazitaxel significantly retarded the growth of mCRPC when compared to vehicle control, cabazitaxel, or genistein. Tissue staining confirmed the in vivo effect of genistein on the induction of Bax and activation of apoptosis.
CONCLUSION
This study provided the first preclinical evidence supporting that genistein could be beneficial in improving cabazitaxel chemotherapy in mCRPC.
Keywords: genistein, cabazitaxel, prostate cancer, experimental model, chemoresistance
INTRODUCTION
Prostate cancer (PCa) is the second leading cause of cancer death in men in the United States. An estimated 241,740 new cases of PCa will be diagnosed and 28,170 patients will die in 2012 [1]. Androgen-deprivation therapy (ADT), or hormonal therapy, remains the first choice in the treatment of both localized and metastatic PCa [2]. After an initial response to ADT, some patients eventually develop metastatic castration-resistant disease (mCRPC). In 2004, two clinical studies demonstrated a survival advantage of docetaxel (Taxotere) chemotherapy in these patients [3,4], setting a new standard of care and representing a significant milestone in the treatment of PCa [5]. Recently, the Food and Drug Administration (FDA) further approved cabazitaxel (Jevtana) as the second-line treatment of mCRPC, providing a new option for those patients progressing during or after treatment with docetaxel chemotherapy [6,7]. Nonetheless, most patients progress while receiving cabazitaxel treatment by becoming chemoresistant, which is lethal with no cure.
Epidemiologic studies have suggested a close association between soy diet and decreased PCa incidence and mortality [8–12]. For example, a cohort study in the United States involving over 12,000 men found that the consumption of soy milk was associated with a remarkable reduction (by 70%) in PCa risk after approximately 20 years of follow-up, despite extensive adjustment for potential confounding factors [11,12]. Significantly, a meta-analysis of five cohort studies and eight case-control studies [13] demonstrated an inverse relationship between PCa mortality and dietary consumption of genistein (4′,5,7-trihydroxyisoflavone), a highly bioactive isoflavone abundant in soy products [14]. In fact, genistein has repeatedly shown favorable inhibition of tumor growth and metastasis in various pre-clinical PCa models [2,13,15–23], and several clinical trials have been conducted for the therapeutic efficacy of genistein in PCa patients [16,17,24]. Recently, a Norwegian group conducted a placebo-controlled, randomized, double-blind Phase II study to determine the effect of genistein in patients with localized PCa prior to prostatectomy [17,25]. Serum prostate-specific antigen (PSA) was found to be decreased by 7.8% in the genistein group and increased by 4.4% in the placebo arm. Although the patient numbers in current trials are still relatively low, these reports support the promise of genistein in the management of PCa [11,26,27].
In this study, we evaluated the potential of genistein in improving the therapeutic efficacy of cabazitaxel in a xenograft model of mCRPC. We demonstrated that genistein could significantly increase the expression of a pro-apoptosis protein BCL-2-associated X protein (Bax), thereby enhancing the response of mCRPC to cabazitaxel treatment.
MATERIALS AND METHODS
Cell Culture
Human mCRPC cell lines C4-2 [28], ARCaPM [29,30], PC3, and luciferase-tagged PC3 (PC3-luc) were routinely maintained in T-medium (Invitrogen, Carlsbad, CA) with 5% fetal bovine serum (FBS). Normal human prostatic epithelial cell line (PrEC) was purchased from Lonza Walkersville, Inc. (Walkersville, MD), and cultured with One Prostate Epithelial Cell Medium BulletKit™. Genistein was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and dissolved in dimethyl sulfoxide (DMSO) (Sigma–Aldrich, St. Louis, MO). Cabazitaxel was obtained from Sanofi-Aventis U.S. LLC (Bridgewater, NJ).
Cell Proliferation Assay
Cell proliferation was measured using the CellTiter 96 AQueous Non-Radioactive Cell Proliferation (MTS) Assay kit (Promega, Madison, WI) according to the manufacturer’s instruction. For cell viability assay, 4 × 103 cells per well were seeded on 96-well plate overnight, and treated with genistein or other reagents at their indicated final concentrations for 72 hr. A microplate reader (Bio-Rad Laboratories, Hercules, CA) was used to determine cell viability, which was expressed as relative survival with controls recorded as 100%. Combination index (CI) was determined using CalcuSyn software (Biosoft, Inc.). A CI value of more than 1 is defined as antagonism, equal to 1 as additive, and less than 1 as synergy [31].
Apoptosis Assay
A Cell Death Detection ELISAPlus kit (Roche Diagnostics, Indianapolis, IN) was used to determine the effect of genistein and cabazitaxel on the apoptosis in PCa cells following the manufacturer’s instruction.
Western Blot Analysis
Total cell lysates were prepared using radioimmunoprecipitation (RIPA) buffer (Santa Cruz Biotechnology). Immunoblotting analysis followed standard procedure [30]. The following antibodies were used: cleaved caspase 3, poly (ADP-ribose) polymerase (PARP), cleaved PARP (Cell Signaling); myeloid cell leukemia sequence 1 (Mcl-1), heat-shock protein 90 (HSP90), Bax (Santa Cruz Biotechnology).
Animal Study
A total of 20 athymic nude mice (BALB/c; National Cancer Institute; 6-week-old) fed with a standard diet were used. PC3-luc cells (2 × 106) per 100 µl per site were injected subcutaneously using a previously established procedure [32]. One week later, tumor-bearing mice were randomly divided into four groups (n = 5 per group) and treated with cabazitaxel (5 mg/kg body weight; 25 µl of injection volume; once per week), genistein (100 mg/kg; 50 µl of injection volume; 3 times per week), cabazitaxel (25 µl of injection volume), and genistein (50 µl of injection volume) combination, or vehicle control (DMSO; 50 µl of injection volume) via the intraperitoneal (i.p.) route, respectively. The bioluminescence intensity of region of interest (ROI) was measured as an indicator of tumor growth with a Xenogen IVIS® 100 bioluminescence imaging (BLI) system once per week following the manufacturer’s instruction (Caliper Life Sciences, Hopkinton, MA). Briefly, mice were anesthetized and received an i.p. injection of D-luciferin (125 mg/kg). Images were acquired 25 min after luciferin administration. An integration time of 1 min with a binning of 16 pixels was used for luminescent image acquisition. Signal intensity was quantified as the sum of all detected photon counts per second within the ROI after subtraction of background luminescence [33]. Tumor size was measured in two dimensions with a digital caliper twice per week, starting from week 2. The tumor volume was calculated according to the equation (l × w2)/2, where l = length and w = width. The treatment lasted for 8 weeks. Animal protocols were approved by Emory University Institutional Animal Care and Use Committee (IACUC).
Immunohistochemistry (IHC) Analysis
Expression of Bax, Mcl-1 and Ki67 in xenograft tumor tissues were analyzed by IHC staining. Briefly, tissues were deparaffinized, rehydrated, and subjected to 5-min pressure-cooking antigen retrieval, 10-min double endogenous enzyme block, and overnight primary antibody incubation, and subjected to prediluted biotinylated pan-specific universal secondary antibody (Vector laboratories) for 10 min. Signals were detected by adding 3,3′-diaminobenzidine (DAB) substrate hydrogen peroxide and counterstained by hematoxylin QS. All reagents were obtained from Vector Laboratories (Burlingame, CA). Positive expression was defined as >15% positive staining in cell population.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
TUNEL assay was performed according to the manufacturer’s instructions (TUNEL Apoptosis Detection Kit, GenScript, Piscataway, NJ). The slides were routinely dewaxed, hydrated, then enzymatically digested with 20 µg/ml protease K for 30 min at room temperature. Slides then were washed in PBS and placed in 3% H2O2 in methanol for 10 min at room temperature. After washing in PBS, 50 µl TUNEL reaction mixture was added to the tissues and incubated for 60 min at 37°C. Slides were washed in PBS and 50 µl Streptavidin-HRP solution was added to the samples, incubated for 30 min at 37°C. After washing in PBS, DAB working solution was applied to the tissues for 3 min, then slides were routinely counterstained with hematoxylin and dehydrated for coverslipping with Permount.
Statistical Analysis
Treatment effects at specific time-points were evaluated using a two-sided Student’s t-test at each measurement time-point. To assess the longitudinal effect of treatment, a mixed model was employed to test the overall difference across all groups as well as between each pair of groups during the whole study period. Kaplan–Meier method was used to generate survival curves. Log-rank test was used to test the difference in the survival times. The significance levels were set at 0.05 for all tests. The SAS statistical package V9.2 (SAS Institute, Inc., Cary, NC) was used for data management and analysis.
RESULTS
Genistein Sensitizes the Response to Cabazitaxel in mCRPC Cells
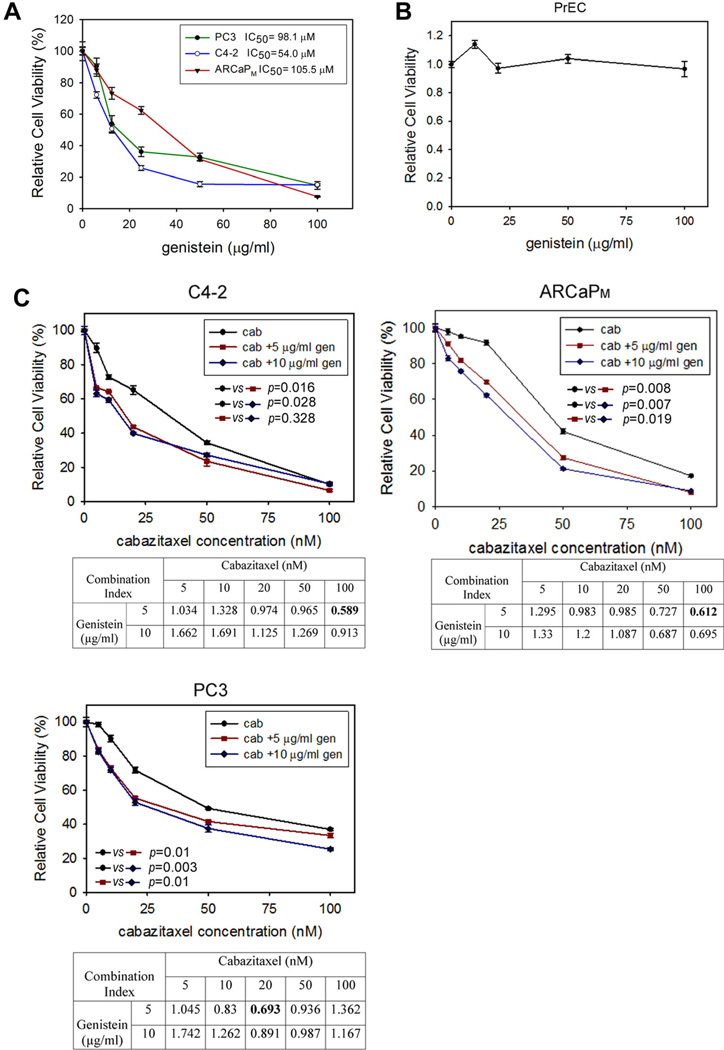
We first determined the in vitro cytotoxicity of genistein in several PCa cell lines, including PC3, C4-2, and ARCaPM. These cells have the capabilities of androgen-independent growth and proliferation, and can form distant metastatic lesions in animals, thereby closely mimicking the clinical pathophysiology of mCRPC [5]. As shown in Figure 1A, the half maximal inhibitory concentration (IC50) of genistein was 54.0 µM (14.6 µg/ml), 98.1 µM (26.5 µg/ml), and 105.5 µM (28.5 µg/ml) in C4-2, PC3, and ARCaPM cells, respectively. In comparison, genistein had negligible effect on the proliferation on a normal human prostatic epithelial cell line PrEC (Fig. 1B).
Fig. 1.
Genistein enhances the invitro cytotoxicity of cabazitaxel in human mCRPC cells. A: The effect of genistein on mCRPC cell viability. PC3, C4-2, or ARCaPM cells were seeded on 96-well plates and treated with genistein at varying concentrations. Viable cells were measured following a 72-hr incubation. Bars denote the standard error (n = 6). B: Effect of genistein on the in vitro proliferation of PrEC cells. C: The effect of cabazitaxel, in the presence of low-dose genistein or vehicle control (DMSO), on mCRPC cell viability. Viable cells were measured as described above. Combination index (CI) was determined using CalcuSyn software.
As shown in Figure 1C, cabazitaxel exhibited potent cytotoxicity in mCRPC cells, with the IC50 ranging between 40 and 55 nM. The addition of low doses (5 µg/ml or 18.5 µM; 10 µg/ml or 37.0 µM) of genistein further significantly reduced the viability of mCRPC cells in the presence of cabazitaxel. Isobologram analysis found that the interaction between cabazitaxel and genistein was highly cell- and dose-dependent. For example, the synergism (CI < 1) was the most significant when cabazitaxel was used at 20 nM in PC3 cells and at 100 nM in C4-2, and ARCaPM cells (CI in bold font). Interestingly, the lower dose of genistein (5 µg/ml) appeared to be more effective than the higher dose (10 µg/ml) in sensitizing mCRPC cells to cabazitaxel treatment.
Genistein Increases the Ratio of Bax/Mcl-1and Activates Apoptosis in mCRPC Cells
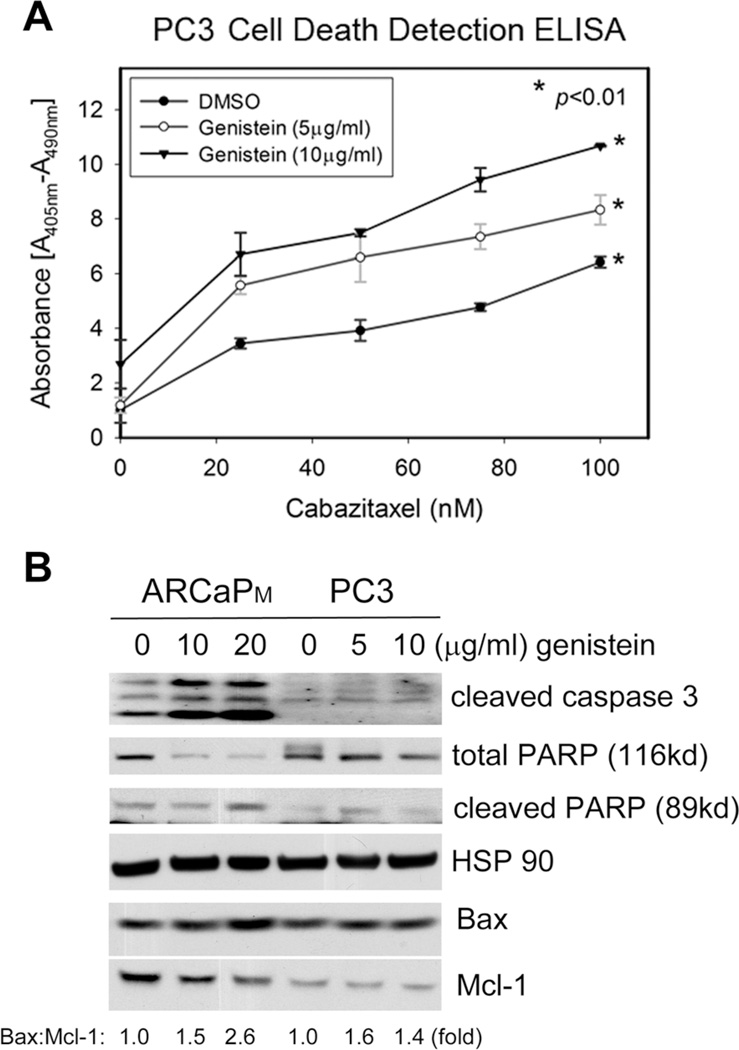
In a histone DNA enzyme-linked immunosorbent assay (ELISA)-based apoptosis analysis, genistein was found to dose-dependently increase the cytoplasmic oligonucleosomes in PC3 cells treated with varying concentrations of cabazitaxel (Fig. 2A). These data indicate that the combined treatment of genistein and cabazitaxel was more effective than cabazitaxel alone in inducing apoptosis in mCRPC cells. The effect of genistein on apoptotic signals was further examined in mCRPC cells. As shown in Figure 2B, treatment with genistein upregulated Bax and downregulated Mcl-1 in ARCaPM cells, resulting a significant increase in the ratio of Bax:Mcl-1. Consistently, the expression of cleaved caspase 3 and PARP, two general indicators of activated apoptotic process, was also increased. In PC3 cells, however, genistein treatment appeared to be less effective in increasing the ratio of Bax:Mcl-1. Further, genistein treatment at 5 µg/ml appeared to be more effective in increasing the ratio of Bax:Mcl-1 and inducing the cleavage of PARP than that at 10 µg/ml. Taken together, these data suggest that genistein may activate apoptosis and sensitize mCRPC cells to cabazitaxel chemotherapy.
Fig. 2.
Genistein treatment increases Bax and activates apoptotic signaling in human mCRPC cells. A: The effect of cabazitaxel, in the presence of low-dose genistein or vehicle control, on PC3 cell apoptosis was determined using histone DNA ELISA-based assay. Bars denote the standard error (n = 6). B: ARCaPM and PC3 were treated with genistein for 48 hr, and total cell lysates were analyzed by Western blotting. HSP90 was used as a loading control.
Genistein Retards the Growth of mCRPC and Enhances the InVivo Efficacy of Cabazitaxel
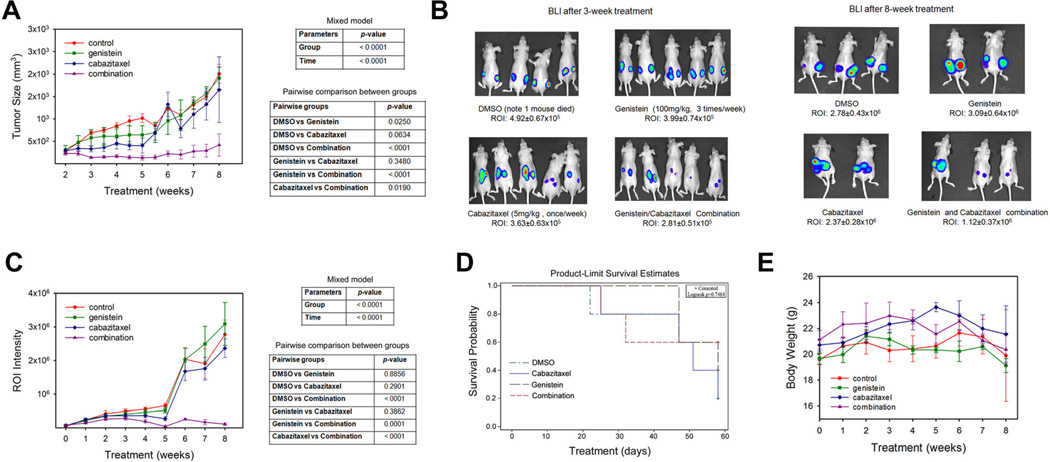
We evaluated the in vivo efficacy of genistein, alone or combined with cabazitaxel, in a subcutaneous xenograft model of PC3-luc cells. Following an 8-week treatment schedule, the average tumor sizes in each group were 2008.7 ± 214.1 mm3 (control), 1913.5 ± 248.2 mm3 (genistein), 1650.8 ± 731.4 mm3 (cabazitaxel), and 419.8 ± 249.2 mm3 (combination), respectively (Fig. 3A, left panel). Statistical analysis showed that there was significant difference in the longitudinal tumor sizes across the four treatment groups (P < 0.0001). Tumor growth over time was also significant (P < 0.0001). The pairwise comparisons in tumor sizes over time between two groups were summarized in Figure 3A, right panel. The control group has significantly higher tumor sizes over time than genistein (P = 0.025) and combination groups (P < 0.0001), and marginally significantly higher tumor sizes over time than cabazitaxel group (P = 0.0634). Combination group has significantly lower tumor sizes over time than genistein (P < 0.0001) and cabazitaxel (P = 0.019) groups.
Fig. 3.
Genistein treatment significantly enhances the in vivo efficacy of cabazitaxel in retarding PC3-luc tumor growth in a subcutaneous xenograft model. A: Tumor sizes during an 8-week treatment; (B) representative BLI of PC3-luc tumor-bearing mice at the 3-week and 8-week of treatments; (C) ROI intensity as determined by BLI; (D) Survival curve by group; (E) Animal body weights following an 8-week treatment.
The growth of PC3-luc xenograft tumors was further quantitated independently with a non-invasive BLI procedure (Fig. 3B). The average intensity of ROI in each group at the endpoint was 2.78 ± 0.43 ± 106 (control), 3.09 ± 0.64 ± 106 (genistein), 2.37 ± 0.28 ± 106 (cabazitaxel), and 1.12 ± 0.37 × 106 (combination), respectively (Fig. 3C, left panel). Similarly, there was significant difference in the longitudinal tumor sizes across the four treatment groups (P < 0.0001). Tumor growth over time was also significant (P < 0.0001). The pairwise comparisons in tumor sizes over time between two groups were summarized in Figure 3C, right panel. Combination group has significantly lower tumor sizes over time than the control (P < 0.0001), genistein (P = 0.0001), and cabazitaxel (P < 0.0001) groups.
In the survival analysis, Kaplan–Meier method did not detect any significant difference in survival between four groups (P = 0.7464) (Fig. 3D). Compared to the control group, no significant acute toxicity was observed in any of the treatment groups, as demonstrated by body weights (Fig. 3E) and ex vivo examination of major tissues (data not shown).
Taken together, these in vivo studies indicated that combined treatment with low dose of genistein and cabazitaxel could be more effective in suppressing tumor growth than either agent alone.
Genistein Increases Tissue Expression of Bax and TUNEL in PC3 Tumors
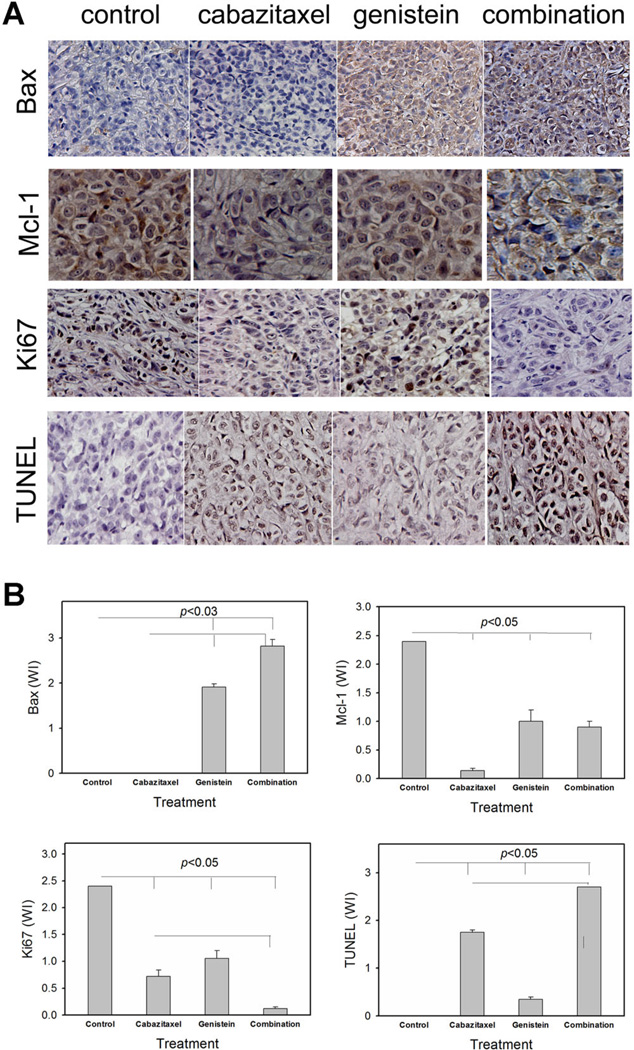
The effect of genistein treatment on the expression of Bax and Mcl-1 in PC3-luc xenograft tumors was analyzed by IHC staining (Fig. 4A) and quantitated (Fig. 4B). Compared with control group, cabazitaxel did not affect Bax at tissue levels, whereas genistein significantly increased Bax expression when administered either as a single agent or combined with cabazitaxel. On the other hand, tissue expression of Mcl-1 was reduced most significantly by the treatment with cabazitaxel. The combined treatment was effective in inhibiting Mcl-1 in PC3-luc tumors. Further, TUNEL assay confirmed an increase in apoptosis in tumor specimens from mice treated with cabazitaxel or genistein, which is further increased in the tumor tissues treated with the combined regimen. Consistently, expression of Ki67, a general proliferation marker, was significantly reduced in the combined treatment group, indicating the suppression of tumor growth.
Fig. 4.
The combined treatment with genistein and cabazitaxel increases Bax and TUNEL, whereas inhibitsMcl-1and Ki67 at tissue levels in PC3-luc tumors. A: IHC staining and TUNEL assay were performed on PC3-Luc tumor tissues following an 8-week treatment with vehicle control, cabazitaxel, genistein, and combination of cabazitaxel and genistein. B: Weighted index (WI) of the relative expression of Bax, Mcl-1, Ki67, and TUNEL in PC3-Luc tumor tissues following an 8-week treatment.
DISCUSSION
Resistance to chemotherapy remains a major challenge in the management of mCRPC [7,34,35]. Recently, cabazitaxel, a semi-synthetic derivative of a natural taxoid, has been shown to improve the survival of patients with mCRPC that fail docetaxel chemotherapy. However, the benefit in overall survival is only minimal (a median of 3–4 months), at the cost of serious additional adverse effects, usually hematologic with neutropenia [35–38]. It is imperative to develop new regimens that can enhance the therapeutic efficacy of cabazitaxel and reduce its toxicity. In this report, we present the first pre-clinical evidence demonstrating that genistein could significantly augment the anti-tumor activity of low-dose cabazitaxel in a mCRPC model. These results suggested that the inclusion of genistein in the second-line treatment of mCRPC could be beneficial as an adjunct to cabazitaxel chemotherapy.
Bax is a key player in the regulation of the critical balance between apoptosis and survival in response to extracellular stimuli [39]. By increasing the expression of Bax and/or its ratio with other anti-apoptotic Bcl-1 family members, such as Mcl-1 [40], a biological agent could render cancer cells more susceptible to the cytotoxicity of chemotherapeutics. Indeed, genistein treatment resulted in an increase of Bax protein expression and its ratio against Mcl-1 in both PC3 and ARCaPM cells, which could be an underlying mechanism for genistein to potentiate the efficacy of cabazitaxel in these mCRPC cells. These observations were further supported by IHC staining of Bax and TUNEL assays on the PC3 xenograft tumors, and consistent with previous reports on the inductive effect of genistein on Bax expression in other PCa models [41,42]. On the other hand, although this study identified Bax as one of the major targets of genistein in sensitizing mCRPC cells to cabazitaxel treatment, we will not exclude other possible mechanisms by which genistein inhibits tumor growth and improve chemotherapy. Previous studies have shown that downregulation of matrix metalloproteinase-9 (MMP-9) and receptor activator of NF-κB (RANK) ligand (RANKL), and upregulation of osteoprotegerin (OPG) could also be partially responsible for the anti-metastatic activity of genistein and improved docetaxel efficacy in retarding the skeletal growth of PC3 tumors in severe combined immunodeficient (SCID) mice [18]. It is worthy to investigate whether genistein could suppress these signaling pathways and contribute to the sensitization of mCRPC to cabazitaxel treatment.
An interesting result from our animal studies was that compared with the control group, genistein was only capable of reducing PC3-luc tumor size for up to 6 weeks. The regimen using low-dose cabazitaxel exhibited a similar pattern as that of genistein, and appeared to be more effective in suppressing tumor growth than genistein at most time points, although there was no significant statistical difference between the two treatments (P > 0.34). Excitingly, however, the combined treatment with both cabazitaxel and genistein was found to markedly retard tumor growth in athymic nude mice, when compared to the control group or any other single regimens (P < 0.05). The favorable effect of combination treatment was also supported by its low acute in vivo toxicity, and relatively higher survival rate of animals (3/5, or 60%) at the end point, although the difference in survival between four groups was not statistically significant. In fact, previous studies have reported that genistein could decrease the toxicity of chemotherapy and radiation therapy [22,43]. Taken together, these evidence clearly demonstrated the potential of genistein as an effective and safe agent to improve cabazitaxel chemotherapy in the management of mCRPC.
ACKNOWLEDGMENTS
This work was supported by National Cancer Institute grant 1R21CA164612-01A1, American Cancer Society Research Scholar Grant RSG-10-140-01 (D.W.), Georgia Cancer Coalition Distinguished Scholar Grant (O.K), and National Cancer Institute grant 1R43CA141870 (Y.A.W).
Footnotes
The authors declare no conflict of interest: The results in this manuscript have not been published elsewhere and have not been submitted simultaneously for publication elsewhere.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168(1):9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Pienta KJ, Smith DC. Advances in prostate cancer chemotherapy: A new era begins. CA Cancer J Clin. 2005;55(5):300–318. doi: 10.3322/canjclin.55.5.300. quiz 305–323. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 7.Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011;8(1):12–23. doi: 10.1038/nrclinonc.2010.136. [DOI] [PubMed] [Google Scholar]
- 8.Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989;49(7):1857–1860. [PubMed] [Google Scholar]
- 9.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63(6):963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Low EC, Perabo FG, Siener R, Muller SC. Review. Facts and fiction of phytotherapy for prostate cancer: A critical assessment of preclinical and clinical data. In Vivo. 2007;21(2):189–204. [PubMed] [Google Scholar]
- 11.de Souza PL, Russell PJ, Kearsley JH, Howes LG. Clinical pharmacology of isoflavones and its relevance for potential prevention of prostate cancer. Nutr Rev. 2010;68(9):542–555. doi: 10.1111/j.1753-4887.2010.00314.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen BK, Knutsen SF, Fraser GE. Does high soy milk intake reduce prostate cancer incidence? The Adventist Health Study (United States) Cancer Causes Control. 1998;9(6):553–557. doi: 10.1023/a:1008819500080. [DOI] [PubMed] [Google Scholar]
- 13.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: A meta-analysis of observational studies. Nutr Cancer. 2009;61(5):598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 14.Carter MW, Smart WW, Jr, Matrone G. Estimation of estrogenic activity of genistein obtained from soybean meal. Proc Soc Exp Biol Med. 1953;84(2):506–508. doi: 10.3181/00379727-84-20693. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002;8(7):2369–2377. [PubMed] [Google Scholar]
- 16.Hussain M, Banerjee M, Sarkar FH, Djuric Z, Pollak MN, Doerge D, Fontana J, Chinni S, Davis J, Forman J, Wood DP, Kucuk O. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47(2):111–117. doi: 10.1207/s15327914nc4702_1. [DOI] [PubMed] [Google Scholar]
- 17.Lazarevic B, Boezelijn G, Diep LM, Kvernrod K, Ogren O, Ramberg H, Moen A, Wessel N, Berg RE, Egge-Jacobsen W, Hammarstrom C, Svindland A, Kucuk O, Saatcioglu F, Tasken KA, Karlsen SJ. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: A randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutr Cancer. 2011;63(6):889–898. doi: 10.1080/01635581.2011.582221. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Kucuk O, Hussain M, Abrams J, Cher ML, Sarkar FH. Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 2006;66(9):4816–4825. doi: 10.1158/0008-5472.CAN-05-3752. [DOI] [PubMed] [Google Scholar]
- 19.Xu L, Ding Y, Catalona WJ, Yang XJ, Anderson WF, Jovanovic B, Wellman K, Killmer J, Huang X, Scheidt KA, Montgomery RB, Bergan RC. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. J Natl Cancer Inst. 2009;101(16):1141–1155. doi: 10.1093/jnci/djp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakshman M, Xu L, Ananthanarayanan V, Cooper J, Takimoto CH, Helenowski I, Pelling JC, Bergan RC. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008;68(6):2024–2032. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- 21.El Touny LH, Banerjee PP. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009;69(8):3695–3703. doi: 10.1158/0008-5472.CAN-08-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad IU, Forman JD, Sarkar FH, Hillman GG, Heath E, Vaishampayan U, Cher ML, Andic F, Rossi PJ, Kucuk O. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr Cancer. 2010;62(7):996–1000. doi: 10.1080/01635581.2010.509839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis JN, Kucuk O, Sarkar FH. Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr Cancer. 1999;35(2):167–174. doi: 10.1207/S15327914NC352_11. [DOI] [PubMed] [Google Scholar]
- 24.Russo M, Spagnuolo C, Tedesco I, Russo GL. Phytochemicals in cancer prevention and therapy: Truth or dare? Toxins. 2010;2(4):517–551. doi: 10.3390/toxins2040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazarevic B, Hammarstrom C, Yang J, Ramberg H, Diep LM, Karlsen SJ, Kucuk O, Saatcioglu F, Tasken KA, Svindland A. The effects of short-term genistein intervention on prostate biomarker expression in patients with localised prostate cancer before radical prostatectomy. Br J Nutr. 2012;108(12):2138–2147. doi: 10.1017/S0007114512000384. [DOI] [PubMed] [Google Scholar]
- 26.Messina M, Kucuk O, Lampe JW. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J AOAC Int. 2006;89(4):1121–1134. [PubMed] [Google Scholar]
- 27.Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010;29(3):465–482. doi: 10.1007/s10555-010-9238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC, Chung LW. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54(10):2577–2581. [PubMed] [Google Scholar]
- 29.Xu J, Wang R, Xie ZH, Odero-Marah V, Pathak S, Multani A, Chung LW, Zhau HE. Prostate cancer metastasis: Role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate. 2006;66(15):1664–1673. doi: 10.1002/pros.20488. [DOI] [PubMed] [Google Scholar]
- 30.Wu D, Zhau HE, Huang WC, Iqbal S, Habib FK, Sartor O, Cvitanovic L, Marshall FF, Xu Z, Chung LW. cAMP-responsive element-binding protein regulates vascular endothelial growth factor expression: Implication in human prostate cancer bone metastasis. Oncogene. 2007;26(35):5070–5077. doi: 10.1038/sj.onc.1210316. [DOI] [PubMed] [Google Scholar]
- 31.Shin DM, Zhang H, Saba NF, Chen AY, Nannapaneni S, Amin AR, Muller S, Lewis M, Sica G, Kono S, Brandes JC, Grist WJ, Moreno-Williams R, Beitler JJ, Thomas SM, Chen Z, Shin HJ, Grandis JR, Khuri FR, Chen ZG. Chemoprevention of head and neck cancer by simultaneous blocking of epidermal growth factor receptor and cyclooxygenase-2 signaling pathways: Preclinical and clinical studies. Clin Cancer Res. 2013;19(5):1244–1256. doi: 10.1158/1078-0432.CCR-12-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991;51(14):3753–3761. [PubMed] [Google Scholar]
- 33.Hsieh CL, Xie Z, Yu J, Martin WD, Datta MW, Wu GJ, Chung LW. Non-invasive bioluminescent detection of prostate cancer growth and metastasis in a bigenic transgenic mouse model. Prostate. 2007;67(7):685–691. doi: 10.1002/pros.20510. [DOI] [PubMed] [Google Scholar]
- 34.Seruga B, Tannock IF. Chemotherapy-based treatment for castration-resistant prostate cancer. J Clin Oncol. 2011;29(27):3686–3694. doi: 10.1200/JCO.2010.34.3996. [DOI] [PubMed] [Google Scholar]
- 35.Keizman D, Maimon N, Gottfried M. Metastatic hormone refractory prostate cancer: Recent advances in standard treatment paradigm, and future directions. Am J Clin Oncol. 2012 doi: 10.1097/COC.0b013e318248dc1e. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 36.Kao SC, Hovey E, Marx G. Second-line therapy for castrate-resistant prostate cancer: A literature review. Asia Pac J Clin Oncol. 2011;7(3):212–223. doi: 10.1111/j.1743-7563.2011.01421.x. [DOI] [PubMed] [Google Scholar]
- 37.Lheureux S, Joly F. Cabazitaxel after docetaxel: A new option in metastatic castration-resistant prostate cancer. Bull Cancer. 2012;99(9):875–880. doi: 10.1684/bdc.2012.1617. [DOI] [PubMed] [Google Scholar]
- 38.Goetz D. New options for the management of castration-resistant prostate cancer: A case perspective. J Natl Compr Canc Netw. 2011;9(Suppl. 3):S13–S23. doi: 10.6004/jnccn.2011.0128. quiz S24. [DOI] [PubMed] [Google Scholar]
- 39.Walensky LD, Gavathiotis E. BAX unleashed: The biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends Biochem Sci. 2011;36(12):642–652. doi: 10.1016/j.tibs.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci USA. 1993;90(8):3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raffoul JJ, Banerjee S, Che M, Knoll ZE, Doerge DR, Abrams J, Kucuk O, Sarkar FH, Hillman GG. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int J Cancer. 2007;120(11):2491–2498. doi: 10.1002/ijc.22548. [DOI] [PubMed] [Google Scholar]
- 42.Zhao R, Xiang N, Domann FE, Zhong W. Effects of selenite and genistein on G2/M cell cycle arrest and apoptosis in human prostate cancer cells. Nutr Cancer. 2009;61(3):397–407. doi: 10.1080/01635580802582751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tacyildiz N, Ozyoruk D, Yavuz G, Unal E, Dincaslan H, Dogu F, Sahin K, Kucuk O. Soy isoflavones ameliorate the adverse effects of chemotherapy in children. Nutr Cancer. 2010;62(7):1001–1005. doi: 10.1080/01635581.2010.509841. [DOI] [PubMed] [Google Scholar]