Abstract
The noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist ketamine produces consistent, rapid, and sustained antidepressant effects in patients suffering from treatment-resistant depression. However, ketamine-induced cognitive impairments remain a major concern. The present study sought to extend the preclinical evaluation of ketamine-induced cognitive impairments by evaluating the dose (1.0-18.0 mg/kg) and time-course (10 min-24 hr) of effects of ketamine on sustained attention using a visual signal detection procedure in rats. Overall, ketamine (10.0-18.0 mg/kg) dose dependently decreased percent hit and correct rejection accuracy. Additionally, these same doses of ketamine increased response latency and trial omissions. In the time-course study, treatment with 18.0 mg/kg ketamine produced the greatest decrease in visual signal detection performance at 10 min, when ketamine decreased percent hit and correct rejection accuracy as well as increased response latency and trial omissions, but returned to saline baseline controls by 100 min. In conclusion, acute ketamine inhibited sustained attention in rats performing a visual signal detection task; however, these effects were short in duration, similar to the short duration (< 2 hours) of psychotomimetic effects reported in low-dose ketamine treatment in depressed patients.
Keywords: Ketamine, NMDA receptor antagonist, antidepressant, cognition, attention, signal detection task, rat
Introduction
On the basis of preclinical and clinical research, the glutamatergic system has emerged as a viable target for the treatment of depression (for review see, Paul and Skolnick, 2003). For example, subanesthetic infusions of the noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist ketamine produce rapid and sustained antidepressant effects in patients suffering from treatment-resistant depression (for review see, Hillhouse and Porter, 2015). However, ketamine-induced cognitive impairments remain a major concern. For example, frequent and repeated ketamine users display cognitive deficits across a broad range of cognitive domains, including attention, spatial memory, semantic memory, and working memory (Curran and Morgan, 2000; Morgan et al., 2014). In healthy volunteers, a subanesthetic infusion of ketamine (0.1-0.8 mg/kg), which is similar to the antidepressant dose of ketamine, was found to produce cognitive impairments during the infusion (Krystal et al., 1994; Morgan et al., 2004a,b). However, these cognitive impairments were not observed at the 3 day follow up, and thus, it is possible that chronic ketamine use is needed to produce residual cognitive impairments (Morgan et al., 2004b). Furthermore, there is limited research, both preclinical and clinical, on the duration of the cognitive deficits produced by acute ketamine.
To address these limitations, the present study evaluated the effects of ketamine on sustained attention, which is a cognitive domain that has been shown to be impaired by repeated ketamine use. To thoroughly address the effects of ketamine on sustained attention, we evaluated the dose- and time-dependent effects of ketamine using the visual signal detection procedure in rats. The visual signal detection procedure has been used to evaluate the effects of antipsychotic drugs, nicotinic agonists, muscarinic antagonists, dopamine reuptake inhibitors and other NMDA receptor antagonists on sustained attention (Hillhouse and Prus, 2013; Levin et al., 2011; Rezvani et al. 2008, 2009).
Methods
Subjects.
Fifteen adult male Sprague-Dawley rats (Harlan Laboratories Inc, Frederick, MD) weighing between 300 and 350 grams at the start of the experiment were used. Rats were housed individually with a 12-h/12-h light/dark cycle (lights on 06.00 h). Training and testing sessions were conducted during the light portion of the cycle. After one week of acclimation to the vivarium, daily access to food (Harlan Teklad Lab Diets, Teklad LM-485) was restricted in order to maintain the rats at 85% of their free feeding body weights; water was freely available in the home cages. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University and conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources, 2011).
Apparatus
Rats were trained and tested in four operant chambers enclosed within a sound attenuating cabinet equipped with a fan for ventilation (Med-Associates Inc., St. Albans. VT, USA). Each operant chamber was equipped with a signal (stimulus) light, a houselight, two retractable levers and a food pellet dispenser. The signal light was located directly above the food receptacle (center of the front panel). The levers were located on either side of the food receptacle. Signal light intensity was adjusted using a fader control (ENV-226A, Med-Associates Inc.) that allowed for four different illumination levels (i.e. background [blank] illumination and 3 signal intensities), which were calibrated using a light meter (LX1330B, HisGadget, Union City, CA). Data were collected using Med PC version 4.1 (Med-Associates Inc.).
Visual Signal Detection Procedure
Rats were trained according to procedures adapted from previously published studies (e.g. Hillhouse and Prus, 2013; Rezvani et al., 2008, 2009). Lever assignments (i.e. blank and signal lever) were counter-balanced across animals. At the start of each trial, the houselight and signal light were on and the levers were retracted. The houselight and signal light remained on at all times, except during “timeout” periods. Thus, under blank conditions and in-between trials the background illumination was 0.9 lux. Each trial began with a pre-signal delay of 3, 6, or 10 s, selected in true random order and presented an equal number of times for each trial type (i.e. blank and each signal intensity). After the pre-signal delay ended, either a “blank” or “signal” occurred for 500 ms. A blank trial consisted of no change in signal light illumination during the 500 ms period. On the other hand, a signal trial consisted of an increase in signal light intensity that provided a 0.3, 0.6, and 1.5 lux increase above background illumination for the 500 ms period. Sessions consisted of 90 blank trials, and 30 trials for each signal intensity (equaling 90 signal trials). Trial type (i.e. blank and signal) was selected in true random order with no limitations on trial presentation until a trial type met the maximum number of presentations (i.e. 90 blank trials and 30 trials for each signal intensity). After the blank or signal period ended, there was a 1 s delay followed by both levers being extended from the front panel. If a rat pressed the signal-lever after a signal occurred, then this was recorded as a “hit.” If a rat pressed the blank-lever after no signal occurred, then this was recorded as a “correct rejection.” A correct response (i.e. hit or correct rejection) resulted in the delivery of a food pellet. An incorrect response on a blank or signal trial resulted in a “timeout” period, in which both the houselight and signal light were turned off resulting in complete darkness for 2 s. If a lever response failed to occur within 5s, then this was counted as a trial omission and a timeout period occurred. After a lever press or a trial omission, the levers were retracted. The training criteria were met when a rat achieved 70% or greater accuracy on both hits (the highest intensity only) and correct rejections for the overall session for 3 consecutive days. Test sessions occurred twice weekly with at least 2 days separating each test session. A training session was conducted on the day immediately preceding a test session. Test sessions consisted of 180 total trials and were approximately 30 min in duration. For the dose effect study, ketamine (1.0-18.0 mg/kg, counterbalanced) was administered with a 10 min pretreatment time. After completing a dose effect curve for a drug, there was a two week wash-out period before the time-course effect study was conducted. For more details on training and testing see Hillhouse and Prus (2013).
Data analysis
The dependent variables were: 1) percent hits; 2) percent correct rejections; 3) response latency; and 4) trial omissions. Percent hits = (number of correct responses on signal trials/the number of signal trials completed) × 100. Percent correct rejections = (number of correct responses on blank trials/the number of blank trials completed) × 100. Response latency = total time elapsed from when the levers were extended to when a lever press occurred/the number of trials completed (these data were collapsed across signal and blank trials). Response omissions = total number of trials where no response occurred (these data were collapsed across signal and blank trials). All data are reported as means +/− the standard error of the mean (SEM). A two-factor repeated measures analysis of variance (ANOVA) was conducted using signal intensity and drug dose or time as factors for percent hits. A one-way repeated measures ANOVA was used to assess the effect of drug dose or time on percent correct rejections, response latency, or trial omissions. A significant ANOVA was followed by a Holm-Sidak post hoc test, and criterion for significance was set at P<0.05. All statistical analyses were conducted using GraphPad Prism 6.0 for Windows (La Jolla, CA, USA).
Drugs
(±) Ketamine HCl (Sigma Aldrich, St. Louis, MO) was dissolved in 0.9% physiological saline, and was administered intraperitoneally at a volume of 1.0 ml/kg. Doses and pretreatment times were based on previous studies in the literature (Hillhouse and Porter, 2014; Hillhouse et al., 2014a,b; Koike et al., 2013; Páleníček et al., 2011).
Results
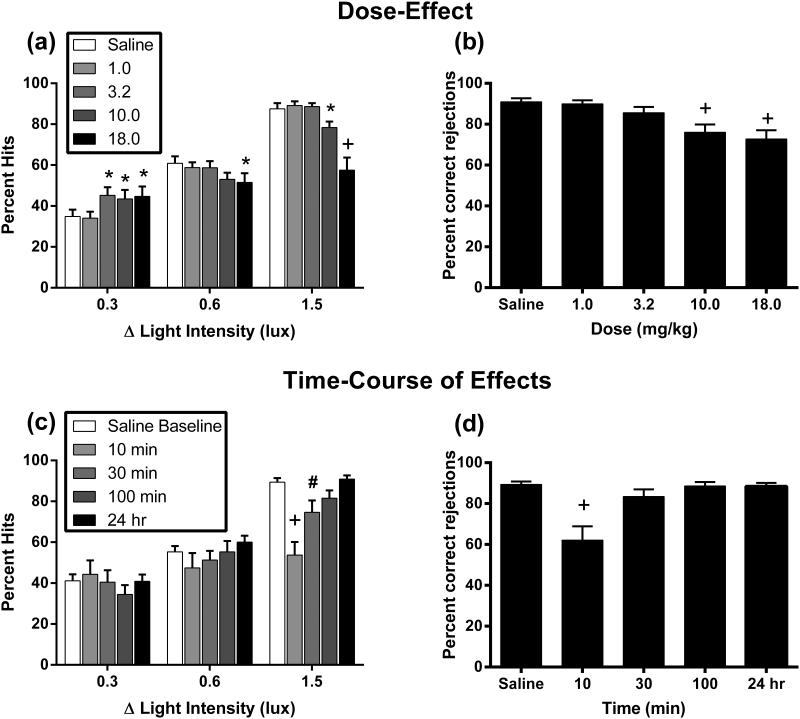
Figure 1 shows the dose effects of ketamine on percent hits (a) and percent correct rejections (b). Ketamine produced mixed effects across the three light intensities: significant main effect of dose [F(4, 56) = 4.74, P<0.01], signal intensity [F(2, 28) = 225.3, P<0.001], and interaction [F(8, 112) = 13.02, P<0.001]. Compared to saline, 3.2, 10.0, and 18.0 mg/kg ketamine significantly increased percent hits at the lowest signal intensity. The 18.0 mg/kg dose of ketamine significantly decreased percent hits at the intermediate signal intensity and the 10.0 and 18.0 mg/kg doses decreased percent hits at the highest signal intensity (Figure 1a). Ketamine produced a dose-dependent decrease in percent correct rejections [F(4, 56) = 15.93, P<0.001; Figure 1b]. Ketamine dose-dependently increased mean response latency [F(4, 56) = 48.20, P<0.001] and trial omissions [F(4, 56) = 7.87, P<0.001] (Table 1).
Figure 1.
Dose and time-course of effects of ketamine on response accuracy for signal and blank trials. Left panels show effects of ketamine on percent hits by drug dose (a) or time (c). Horizonal axis: Change in signal intensity in lux. Vertical axis: Response accuracy quantified as percent hits. Right panels show effects of ketamine on percent correct rejections by drug dose (b) or time (d). Horizontal axis: Drug dose or time. Vertical axis: Response accuracy quantified as percent correct rejections. * P<0.05, # P<0.01, + P<0.001 versus saline control as determined by Holm-Sidak post hoc test. All data show mean + SEM for 15 rats.
Table 1.
Dose and time-course of effects of ketamine on mean (±SEM) response latencies and trial omissions across all signal and blank trials
| Experiment | Dose (mg/kg) / Time | Response Latency (ms) | Omissions |
|---|---|---|---|
|
Ketamine
Dose effect |
Saline | 257 (± 14) | 0 |
| 1.0 | 269 (± 17) | 0.07 (± 0.07) | |
| 3.2 | 317 (± 15) | 0.53 (± 0.53) | |
| 10.0 | 540 (± 58)a | 8.07 (± 4.44) | |
| 18.0 | 794 (± 52)a | 34.87 (± 10.93)a | |
|
Ketamine
(18.0 mg/kg) Time Course |
Saline Baseline | 290 (± 15) | 0 |
| 10 min | 1022 (± 66)b | 69.73 (± 9.54)b | |
| 30 min | 403 (± 25) | 0.06 (± 0.06) | |
| 100 min | 374 (± 29) | 0.06 (± 0.06) | |
| 24 hr | 282 (± 14) | 0 |
P<0.001 vs. Saline
P<0.001 vs Saline Baseline
Figure 1 also shows the time-course of effects produced by 18.0 mg/kg ketamine on percent hits (c) and percent correct rejections (d). Ketamine produced a significant main effect of time [F(4, 56) = 3.50, P<0.05], signal intensity [F(2, 28) = 155.70, P<0.001], and interaction [F(8, 112) = 9.39, P<0.001]. Compared to saline baseline, 18.0 mg/kg ketamine did not significantly alter percent hits at the lowest or intermediate signal intensities at any time points; however, 18.0 mg/kg ketamine significantly decreased percent hits at the highest signal intensity at 10 and 30 min (Figure 1c). Ketamine decreased percent correct rejections only at 10 min [F(4, 56) = 14.63, P<0.001; Figure 1d]. Treatment with 18.0 mg/kg ketamine increased mean response latency [F(4, 56) = 80.29, P<0.001] and trial omissions [F(4, 56) = 53.31, P<0.001] only at 10 min (Table 1). The increase in response latencies and trial omissions in the time-course study as compared to the initial dose effect curve can be attributed to the wash-out period between these procedures. A number of the rats appeared to have lost tolerance to the rate-suppressant effects of ketamine that had developed during the dose response curve and their data account for the increases. The rate-suppressant effects of ketamine and development of tolerance to rate-suppressant effects has been shown previously in other studies (Gilmour et al., 2009; Hillhouse et al., 2014b; Negus et al., 1993; Smith et al., 2011).
Discussion
The visual signal detection procedure was used to evaluate the dose and time-course of effects of ketamine on sustained attention in rats. There were two main findings. First, ketamine (10.0-18.0 mg/kg) decreased signal detection performance at doses that have been shown to produce antidepressant-like effects in rats (Hillhouse et al., 2014a,b; Koike et al., 2013; Reus et al., 2011; Zhou et al., 2014). The effects of ketamine in the present study are consistent with the effects of the noncompetitive NMDA receptor antagonist MK-801, which also produces a decrease in percent hit and correct rejection accuracy and increases in response latency and trial omissions (Rezvani et al., 2009). The impairments produced by ketamine in the signal detection task parallel findings in other attention tasks. For example, acute and repeated ketamine administration produced cognitive impairments in the attentional set-shift and five-choice serial reaction time tasks (Nikiforuk et al., 2010, 2013; Nikiforuk and Popik, 2014; Smith et al., 2011). However, Nikiforuk and Popik (2014) found that ketamine reversed stress-induced deficits in the attentional set-shifting task, which suggests that ketamine may have procognitive effects in stressed individuals.
The second main finding was that the time-course of effects following treatment with 18.0 mg/kg ketamine was short, which coincides with the pharmacokinetics of ketamine in rat brain (Páleníček et al., 2011). In the present study, the cognitive-disrupting effects of ketamine (18.0 mg/kg) peaked at 10 min and returned to saline baseline controls by 100 min. Páleníček et al. (2011) found that ketamine concentrations peak in rat brain between 10 and 20 min following intraperitoneal administration and is completely eliminated from rat brain by 120 min. The behavioral effects from the present study also are consistent with clinical research. For example, both patients with treatment-resistant depression and healthy volunteers display ketamine-induced cognitive impairments following ketamine infusions, but these dissipate relatively quickly (Morgan et al., 2004b; Murrough et al., 2014).
In conclusion, acute ketamine produced a brief decrease in sustained attention in rats performing the visual signal detection task. While ketamine does disrupt cognitive functioning in both rodents and human, these effects are short in duration as compared to its antidepressant effects, which have been shown to last longer than a week. Further, Murrough et al. (2014) suggest that the degree of ketamine-induced cognitive effects may be predictive of antidepressant effects, such that greater cognitive impairments are predictive of a decreased antidepressant response in treatment-resistant patients.
Acknowledgements
This research was supported in part by National Institutes of Health grant T32DA07268 (TMH). The authors would like to thank Ruth Oh and Kevin Webster for their technical assistance.
Footnotes
Conflicts of interest and source of funding: There are no conflicts of interest.
References
- Curran HV, Morgan C. Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction. 2000;95:575–590. doi: 10.1046/j.1360-0443.2000.9545759.x. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Pioli E, Dix S, Smith J, Conway M, Jones W, et al. Diverse and often opposite behavioural effects of NMDA receptor antagonists in rats: implications for “NMDA antagonist modelling” of schizophrenia. Psychopharmacology. 2009;205:203–216. doi: 10.1007/s00213-009-1530-7. [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Prus AJ. Effects of the neurotensin NTS1 receptor agonist PD149163 on visual signal detection in rats. Eur J Pharmacol. 2013;721:201–207. doi: 10.1016/j.ejphar.2013.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH. Ketamine, but not MK-801, produces antidepressant-like effects in rats responding on a differential-reinforcement-of-low-rate operant schedule. Behavioural pharmacology. 2014;25:80–91. doi: 10.1097/FBP.0000000000000014. [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: From monoamines to glutamate. Experimental and Clinical Psychopharmacology. 2015;23:1–21. doi: 10.1037/a0038550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH, Negus SS. Comparison of antidepressant-like and abuse-related effects of phencyclidine in rats. Drug Development Research. 2014a;75:479–488. doi: 10.1002/ddr.21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH, Negus SS. Dissociable effects of the noncompetitive NMDA receptor antagonists ketamine and MK-801 on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2014b;231:2705–2716. doi: 10.1007/s00213-014-3451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. 8th Institute of Laboratory Animals Resources, Commission of Life Sciences, National Research Council; Washington DC: 2011. [Google Scholar]
- Koike H, Iijima M, Chaki S. Effects of ketamine and LY341495 on the depressive-like behavior of repeated corticosterone-injected rats. Pharmacology Biochemistry and Behavior. 2013;107:20–23. doi: 10.1016/j.pbb.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive nmda antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bushnell PJ, Rezvani AH. Attention-modulating effects of cognitive enhancers. Pharmacology Biochemistry and Behavior. 2011;99:146–154. doi: 10.1016/j.pbb.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJA, Mofeez A, Brandner B, Bromley L, Curran HV. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology. 2004a;29:208–218. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Mofeez A, Brandner B, Bromley L, Curran HV. Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose–response study. Psychopharmacology (Berl) 2004b;172:298–308. doi: 10.1007/s00213-003-1656-y. [DOI] [PubMed] [Google Scholar]
- Morgan CJA, Dodds C, Furby H, Pepper F, Fam J, Freeman T, et al. Long-term heavy ketamine use is associated with spatial memory impairment and altered hippocampal activation. Frontiers in Psychiatry. 2014;5:1–11. doi: 10.3389/fpsyt.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough J, Wan L-B, Iacoviello B, Collins K, Solon C, Glicksberg B, et al. Neurocognitive effects of ketamine in treatment-resistant major depression: association with antidepressant response. Psychopharmacology (Berl) 2014;231:481–488. doi: 10.1007/s00213-013-3255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Burke TF, Medzihradsky F, Woods JH. Effects of opioid agonists selective for mu, kappa and delta opioid receptors on schedule-controlled responding in rhesus monkeys: antagonism by quadazocine. Journal of Pharmacology and Experimental Therapeutics. 1993;267:896–903. [PubMed] [Google Scholar]
- Nikiforuk A, Popik P. Effects of quetiapine and sertindole on subchronic ketamine-induced deficits in attentional set-shifting in rats. Psychopharmacology (Berl) 2012;220:65–74. doi: 10.1007/s00213-011-2487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P. Ketamine prevents stress-induced cognitive inflexibility in rats. Psychoneuroendocrinology. 2014;40:119–122. doi: 10.1016/j.psyneuen.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Gołembiowska K, Popik P. Mazindol attenuates ketamine-induced cognitive deficit in the attentional set shifting task in rats. European Neuropsychopharmacology. 2010;20:37–48. doi: 10.1016/j.euroneuro.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Fijał K, Potasiewicz A, Popik P, Kos T. The 5-hydroxytryptamine (serotonin) receptor 6 agonist EMD 386088 ameliorates ketamine-induced deficits in attentional set shifting and novel object recognition, but not in the prepulse inhibition in rats. Journal of Psychopharmacology. 2013;27:469–476. doi: 10.1177/0269881113480991. [DOI] [PubMed] [Google Scholar]
- Páleníček T, Fujáková M, Brunovský M, Balíková M, Horáček J, Gorman I, et al. Electroencephalographic spectral and coherence analysis of ketamine in rats: Correlation with behavioral effects and pharmacokinetics. Neuropsychobiology. 2011;63:202–218. doi: 10.1159/000321803. [DOI] [PubMed] [Google Scholar]
- Paul IA, Skolnick P. Glutamate and depression. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Stringari RB, Ribeiro KF, Ferraro AK, Vitto MF, Cesconetto P, et al. Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behav Brain Res. 2011;221:166–171. doi: 10.1016/j.bbr.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Kholdebarin E, Dawson E, Levin ED. Nicotine and clozapine effects on attentional performance impaired by the NMDA antagonist dizocilpine in female rats. The International Journal of Neuropsychopharmacology. 2008;11:63–70. doi: 10.1017/S1461145706007528. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Kholdebarin E, Cauley MC, Dawson E, Levin ED. Attenuation of pharmacologically-induced attentional impairment by methylphenidate in rats. Pharmacology Biochemistry and Behavior. 2009;92:141–146. doi: 10.1016/j.pbb.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Smith J, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, et al. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology. 2011;217:255–269. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. European Psychiatry. 2014;29:419–423. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]