Abstract
There is strong evidence indicating that disease in adult humans stems from a combination of genetic and environmental factors. A problem in identifying environmental factors is that subacute exposures during early life are often unnoticed, or exposures are variable among a diverse population. This leads to a confusing pattern in adulthood. An additional problem in following exposure effects in humans is the length of time needed to study outcomes spanning a human generation. We have recently developed a zebrafish model for studying the effects of sublethal juvenile exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin). Although the initial exposure produces no effect at the time, we find skeletal and reproductive defects in adulthood and into subsequent generations. The short generation time of zebrafish along with the ability to maintain large cohorts of exposed individuals and their offspring allows us to overcome variation in exposure and genetic background. Here we describe progress in studying TCDD as an endocrine and developmental disruptor, and our results showing adult consequences of early exposure.
Keywords: dioxin, zebrafish, adult disease, TCDD, AHR, scoliosis, infertility, reproduction
Chemicals, Development, and Adult Disease
Humans and wildlife are now exposed to numerous man-made chemicals in the environment. While most of these are benign, some have proven to produce toxicity. Examples include herbicides such as atrazine, the fungicide vinclozolin, halogenated aromatic hydrocarbons including dioxin and PCBs, bisphenol A, and pthalates, used in plastics, and contraceptive hormones, originating as pharmaceuticals. The effects of these compounds can often be traced to endocrine systems, and they are therefore classified under the very broad term of endocrine disruptors.1 Endocrine disruption is not confined to reproductive disease because hormones affect virtually every system in the body. Exposure causes diabetes, obesity, bone disorders, as well as effects on the immune system, behavior, and male and female reproduction.2-8
Chemical Disruption of Development
We now know that exposure to low doses of chemicals, including bisphenol A, diethylstilbestrol, PCBs, and TCDD, during early development produce toxicity later in adulthood.9-15 Exposure to chemicals during early development may predispose individuals to disease in adulthood.16,17
Developing organisms are especially vulnerable to xenobiotic compounds for a variety of reasons. First, it is thought that the dividing cells are dependent on countless chemical signals in order to properly migrate and differentiate to form the complete organism. This provides targets for toxicity in the form of receptors that foreign chemicals can either block or activate. In addition, the cells in developing organisms form distinct cell lineages with specific properties, yielding the arrays of unique progenitor cells needed to complete the body. Alterations in these lineages may have profound effects, in some cases not manifested until maturity. Thus, in addition to being especially vulnerable to signal disruption by chemicals, developing cells can carry the effects of an early exposure event far into the future, producing disease during adulthood. The blood-brain barrier forms during development, protecting the mature CNS from chemicals in adulthood. In addition, for most vertebrate species, the immune system, DNA repair mechanisms, detoxifying enzymes, and liver metabolism are underdeveloped early in development, and are only fully functional in adults.18 Not all chemicals that alter development do so by affecting the endocrine pathways, so it can also be useful to think in terms of developmental disruption.
TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD, dioxin) is released into the environment mostly as a by-product of human activities, including industrial emissions and waste incineration. TCDD is lipophilic and bioaccumulative, leaving almost all humans with some body burden of TCDD.19 TCDD is a member of a group of chemicals called dioxin-like compounds (DLCs). The DLCs include polychlorinated biphenyls (PCBs), dibenzofurans (PCDFs), and dioxins (PCDDs). TCDD is a potent, prototypical DLC. In addition, TCDD is a very poor substrate for metabolism, and can remain in the body for weeks, months, and even years.19-21 The receptor for TCDD is the aryl hydrocarbon receptor (AHR), a ligand-activated transcription factor.22
In humans, acute TCDD exposure has occurred through contamination of the heavily used defoliant Agent Orange during the Vietnam War, and industrial accidents. Individuals exposed through these routes have been followed over time, revealing cancer, heart disease, skeletal malformations, and reproductive abnormalities in humans.21,23-28 Due to increased regulations, the concentrations of TCDD and many other DLCs is decreasing in the environment. In addition, the source of many of these compounds is changing from large industrial point sources, to more widespread waste incineration.
Environmental exposure to pollutants including TCDD may be playing an important role in rising infertility.29-31 In humans, TCDD exposure is associated with increased spontaneous miscarriage and decreased spermatogenesis.32-34 A study examining human semen quality after an accidental industrial release of TCDD showed that males exposed during infancy had decreased sperm concentration and motility.26 Studies using rodent models have shown that TCDD exposure in utero or as neonates produces effects that cannot be caused by adult exposure.35-37
Linking Exposure to Disease
Epidemiological studies are powerful in building associations between exposure and disease. Part of this success has been due to the very obvious nature of some of the exposures, and incisive decisions to follow individuals after noteworthy exposures in Italy and Vietnam. Even under these circumstances this approach faces numerous challenges, including differences in exposure, heterogeneity in other exposures, diet, lifestyle, and genetics. Because of the large number of confounding genetic and exposure variables for each individual, it is hard to make a clear connection between specific chemical exposures and specific health outcomes. In addition, major accidents are not the norm for exposures: often only the adult disease consequence is noted, not the initial stressor. Because the life spans of the investigators match those of the subjects: it is hard for a single investigator or team to accomplish this work.
A solution to overcoming these challenges is to use model systems that make it easier to link early life stage chemical exposure to disease in adulthood and later generations. The zebrafish (Danio rerio) is a well-established model for investigating human development and disease.38 They have a short 3–4 mo generation time, it is easy to obtain large simultaneously developing cohorts of genetically similar/identical individuals, exposure is simple and inexpensive, since volumes are low, and development is simple to observe in the clear embryos and larvae. Zebrafish eggs are fertilized outside of the mother, so embryonic exposure can be direct, the embryo hatches at 3 d and is free swimming with major organs formed at 5 d. The offspring are inexpensive to house, even when numerous. This is therefore a system that allows transgenerational observations of populations treated in a controlled fashion without variations in exposure, diet, and genetics.
As laboratory model species are developed, a key feature is reproducibility in response. Generally, inbred lines are developed so that changes in response can be confidently attributed to the controlled variable such as a drug or treatment rather than chance variations in genetics. This is useful but it often produces laboratory strains that have lost properties of the wild species. In some cases, whole gene networks or lost, such as lab yeast strains that have lost the ability to form pseudohyphae.39 Different zebrafish strains have clearly developed distinct properties, and crosses between strains can be used to create genetic diversity if desired.
Using Zebrafish to Study Effects of TCDD Exposure
Acute TCDD toxicity in zebrafish
Zebrafish embryos exposed to lethal doses of TCDD immediately after fertilization (1 h; 1 ppm) develop heart malformations and heart failure, yolk sac and pericardial edema, craniofacial malformations, impaired swim bladder development, growth arrest, and death.38,40-42 Zebrafish embryos are far more susceptible to TCDD-induced toxicity compared with adults.43 TCDD is therefore a developmental disruptor.
In adults, a lethal dose of TCDD produces wasting syndrome, and impaired reproduction. While TCDD produces catastrophic effects on the embryonic heart, at a lethal adult dose it has no effect on the adult heart.44
Sublethal toxicity
Pharmacological doses that kill the zebrafish by 7 d post-fertilization (dpf) do not allow study of lingering effects in the adults. King-Heiden reported that exposure to 50 pg/ml TCDD does not produce acute embryonic toxicity. As the exposed fish reached adulthood, subtle jaw malformations, reduced reproductive capacity, decreased cardiac output, and decreased survival were observed.14 In our recent study, zebrafish were exposed either as embryos at the time of fertilization, or as juveniles at 3 and again at 7 wk post-fertilization (wpf).13 In these experiments, the fish were incubated in water containing TCDD at 50 pg/ml (50 parts per trillion) for 1 h, and then rinsed and returned to aquaria. The TCDD is assumed to persist in the tissues for days after the exposure.45,46 The 3 and 7 wpf exposure coincides with the period of gonad specification and maturation.
The timing of exposure was critical. The early exposure produced few changes in the adult fish, while the exposure at 3 and 7 wpf had profound effects, despite not producing noticeable toxicity at the time of exposure.
Reproductive toxicity
As seen in other species, zebrafish exposed to TCDD at 3 and 7 wpf developed as a population with a higher than normal ratio of females to males.13,47,48 This feminization of the population was driven in part by a striking phenomenon, in which individuals with female secondary sex characteristics of body shape and color were found to carry testes rather than ovaries. It is noteworthy that control males consistently attempted to court these outwardly female fish, indicating that these fish appeared female to zebrafish as well as to the investigators.
When dissected, these fish had a rounded abdomen filled with fluid and only a strand of testes. We had previously assumed that the rounded shape characteristic of adult zebrafish females was due to the presence of a mass of eggs, distending the abdomen. We were therefore quite surprised to find that the shape was completely independent of eggs. This suggests that the rounded shape of the female is instead formed by muscle skin and bone structures that actually create a cavity for the eggs produced. This implies a specific female developmental program for the body that is distinct from that of the male.
These unusual fish were neither male nor female, but had characteristics of each: zebrafish carry no specific sex chromosome as in humans. However, we are intrigued by the parallel to a sex reversal syndrome in humans, termed campomelic dysplasia, in which XY chromosomal males develop as females. This is due to a deficiency in the sox9; a gene targeted for reduction by TCDD in zebrafish.40,49,50
Even discounting these individuals, the population had greater than the average female to male ratio. Ovarian development was disrupted in many of these females, with atretic follicles and disorganized oocyte progression. As might be imagined, reproductive success was diminished in these adults with decreased eggs released in spawns and decreased fertilization success.
It is easy to suppose that changes in egg release are due to altered females. However, outcrossing experiments with control fish showed that TCDD-exposed males had low fertilization rates, and were less capable of eliciting egg release from control females. Such an effect must be due to the presence or absence of a signal, either behavioral or chemical that is transmitted to the female. These effects are reminiscent of previous experiments with rodents in which TCDD and PCBs feminized sexual behavior.12,36
Skeletal effects
TCDD exposure has long been associated with bone malformations. We found that as the zebrafish exposed at 3 and 7 wpf matured, they developed distinct kinks in their spines. These matched defects in the center of individual vertebral bodies, with adjacent vertebrae unaffected (Fig. 1A). Fish exposed earlier as embryos did not show this defect.
Figure 1.
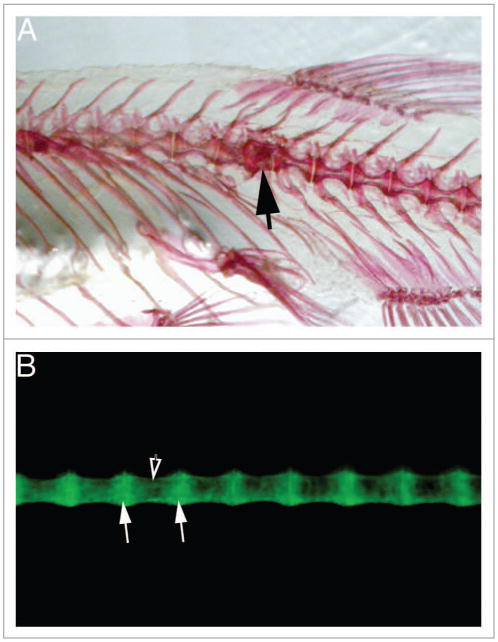
A sox9b:GFP reporter allows visualization of the developing spinal column. The figures show lateral views of adult zebrafish vertebral columns. Anterior is to the left. (A) Alizarin red stained skeleton treated as in.13 the black arrow indicates a malformed vertebra. (B) Spinal column of sox9b:GFP reporter fish viewed in a fluorescence microscope. White arrows indicate vertebral end plates; filled arrow indicates vertebral body.
Opportunities for Exploration
Questions
The kinks in the axial skeleton producing scoliosis are interesting: why a single vertebrae, rather than having all of them malformed?
One explanation is that individual vertebral bodies were malformed in a process that was somewhat stochastic, perhaps due to the timing of TCDD exposure as development of vertebrae progresses toward the tail. Another explanation is that these deformations represent a structural collapse in use, after the vertebrae formed. This model allows for a more uniform effect of TCDD, and the localized failure could be due to an accidental convergence of muscle stresses. These models are distinct but not mutually exclusive: a malformation at a specific vertebra could lead to mechanical failure.
A second question involves the outwardly female fish with testes. Did TCDD change the ovary in a female to a testis, or did TCDD change the body of a male to a female shape and color? In both cases the result is the same, but the TCDD targets are different. In normal development, a primitive ovary forms in all zebrafish. In females, this develops into a mature ovary, but in males the primitive ovary develops into testes. We know little if anything about how male and female body shapes are determined.
Sox9:eGFP
We have developed a transgenic zebrafish that may answer both of these questions. In both of our questions we have not been able to observe the malformations as they occur or develop. For example, the kink in the skeleton shown in Figure 1A is clearly visible in an adult skeleton. What is missing is observation of the kink as it forms. Figure 1B shows the vertebrae of a juvenile transgenic zebrafish carrying a sox9b:eGFP reporter. Sox9b is an important gene in chondrogenesis and bone formation and when the sox9b:eGFP reporter is inserted into the casper line of zebrafish lacking pigment, the vertebrae can be observed forming in live fish.51 This would allow continuous monitoring of vertebral development, without euthanizing the fish.
To answer the second question we can take advantage of the fact that the body shape matures somewhat after gonad determination is complete. This means we can in principle separate males from females at a very early stage, treat them with TCDD, and test whether any ovaries have switched, or if any of the fish initially carrying testes have developed female bodies. The problem is that we normally separate males and females by body shape: dissection to identify the gonads would be far too invasive.
Again, the casper line of zebrafish, carrying sox9b:eGFP, can answer the question because sox9b is strongly expressed in the ovaries, but not in the testes. Figure 2 shows a mature ovary, photographed through the body wall of a live adult zebrafish. The detail is remarkable: mature and developing follicles can be clearly discerned. The black arrows indicate examples of mature follicles, while the white indicates the shadow of a rib lying across the ovary.
Figure 2.
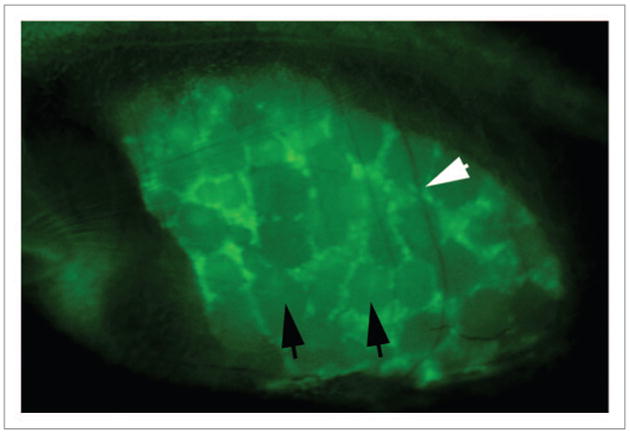
A sox9b:GFP reporter allows visualization of the developing ovary. Lateral view of an adult female zebrafish focused on the abdomen. Anterior is to the left. Black arrows point out examples of mature follicles, while the white arrow indicates the shadow of a rib lying over the ovary.
By being able to separate fish with ovaries from fish with testes before we add TCDD we will be able to determine whether our sex-revered fish stem from a change in the gonad or an effect on the skeleton.
Because TCDD has known effects on reproduction and on formation of the skeletal elements that determine body shape, there is no reason to choose one hypothesis over the other a priori. The only piece of data that we have is from our recent report in which we found that TCDD increased in the percentage of fish with ovaries.13 This is not consistent with an effect transforming ovaries into testes.
Future
These are exciting experiments to contemplate. Previous studies have shown effects in the F1 offspring of TCDD exposed zebrafish.14 We have also followed the offspring of the exposed F0 fish in the current study into the F1 and F2 generations. We find that many of the effects that we see in the adult F0 fish exposed as juveniles are also found into the F2 generations. In particular, we find skeletal abnormalities and male infertility as transgenerational effects of TCDD exposure.52 TCDD causes both transgenerational defects and alterations in developmental fate of cells. Both of these effects can be explained by epigenetic changes, in which transcriptional regulation of the genome becomes altered, even though the genetic code remains the same. This allows one set of cells to express a set of genes that the neighboring cells do not. The mechanisms underlying these changes, including altered DNA methylation, can also be passed on to the next generation through the gametes to produce transgenerational alterations.
Acknowledgments
We would like to thank Dorothy Nesbit, Maggie Shuda, and Ryan Adams for outstanding technical assistance.
Funding: This work was supported by (R01 ES012716) from the National Institute of Environmental Health Sciences (NIEHS) (to Heideman W and Peterson RE); the University of Wisconsin Sea Grant Institute, National Sea Grant College Program, National Oceanic and Atmospheric Administration, US. Department of Commerce (NA 16RG2257), Sea Grant Project R/BT-22 and 25 (to Heideman W and Peterson RE) and NIEHS support from T32 ES007015 and NCATS support from K01 OD010462 (to Baker T).
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–84. doi: 10.1289/ehp.93101378. http://dx.doi.org/10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka T, Morita A, Kato M, Hirai T, Mizoue T, Terauchi Y, Watanabe S, Noda M SCOP Study Group. Congener-specific polychlorinated biphenyls and the prevalence of diabetes in the Saku Control Obesity Program (SCOP) Endocr J. 2011;58:589–96. doi: 10.1507/endocrj.k10e-361. http://dx.doi.org/10.1507/endocrj.K10E-361. [DOI] [PubMed] [Google Scholar]
- 3.Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol Endocrinol. 2010;24:526–39. doi: 10.1210/me.2009-0261. http://dx.doi.org/10.1210/me.2009-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White KL, Jr, Germolec DR, Booker CD, Hernendez DM, McCay JA, Delclos KB, Newbold RR, Weis C, Guo TL. Dietary methoxychlor exposure modulates splenic natural killer cell activity, antibody-forming cell response and phenotypic marker expression in F0 and F1 generations of Sprague Dawley rats. Toxicology. 2005;207:271–81. doi: 10.1016/j.tox.2004.09.011. http://dx.doi.org/10.1016/j.tox.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Palanza P, Parmigiani S, Liu H, vom Saal FS. Prenatal exposure to low doses of the estrogenic chemicals diethylstilbestrol and o,p′-DDT alters aggressive behavior of male and female house mice. Pharmacol Biochem Behav. 1999;64:665–72. doi: 10.1016/s0091-3057(99)00151-3. http://dx.doi.org/10.1016/S0091-3057(99)00151-3. [DOI] [PubMed] [Google Scholar]
- 6.Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med. 2000;224:61–8. doi: 10.1046/j.1525-1373.2000.22402.x. http://dx.doi.org/10.1046/j.1525-1373.2000.22402.x. [DOI] [PubMed] [Google Scholar]
- 7.Hermsen SA, Larsson S, Arima A, Muneoka A, Ihara T, Sumida H, Fukusato T, Kubota S, Yasuda M, Lind PM. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) affects bone tissue in rhesus monkeys. Toxicology. 2008;253:147–52. doi: 10.1016/j.tox.2008.09.005. http://dx.doi.org/10.1016/j.tox.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Gray LE, Ostby JS, Kelce WR. A dose-response analysis of the reproductive effects of a single gestational dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male Long Evans Hooded rat offspring. Toxicol Appl Pharmacol. 1997;146:11–20. doi: 10.1006/taap.1997.8223. http://dx.doi.org/10.1006/taap.1997.8223. [DOI] [PubMed] [Google Scholar]
- 9.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–61. doi: 10.1073/pnas.0703739104. http://dx.doi.org/10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clayton EM, Todd M, Dowd JB, Aiello AE. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003-2006. Environ Health Perspect. 2011;119:390–6. doi: 10.1289/ehp.1002883. http://dx.doi.org/10.1289/ehp.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147(Suppl):S11–7. doi: 10.1210/en.2005-1164. http://dx.doi.org/10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- 12.Colciago A, Casati L, Mornati O, Vergoni AV, Santagostino A, Celotti F, Negri-Cesi P. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat Part 2: Effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the offspring. Toxicol Appl Pharmacol. 2009;239:46–54. doi: 10.1016/j.taap.2009.04.023. http://dx.doi.org/10.1016/j.taap.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Baker TR, Peterson RE, Heideman W. Early dioxin exposure causes toxic effects in adult zebrafish. Toxicol Sci. 2013;135:241–50. doi: 10.1093/toxsci/kft144. http://dx.doi.org/10.1093/toxsci/kft144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King Heiden TC, Spitsbergen J, Heideman W, Peterson RE. Persistent adverse effects on health and reproduction caused by exposure of zebrafish to 2,3,7,8-tetrachlorodibenzo-p-dioxin during early development and gonad differentiation. Toxicol Sci. 2009;109:75–87. doi: 10.1093/toxsci/kfp048. http://dx.doi.org/10.1093/toxsci/kfp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anway MD, Rekow SS, Skinner MK. Comparative anti-androgenic actions of vinclozolin and flutamide on transgenerational adult onset disease and spermatogenesis. Reprod Toxicol. 2008;26:100–6. doi: 10.1016/j.reprotox.2008.07.008. http://dx.doi.org/10.1016/j.reprotox.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7. doi: 10.1111/j.1365-2796.2007.01809.x. http://dx.doi.org/10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 17.Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56:311–7. doi: 10.1203/01.PDR.0000135998.08025.FB. http://dx.doi.org/10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- 18.Bern H. The fragile fetus. In: Colborn T, Clement C, editors. Chemically-Induced Alterations in Sexual and Functioal Development: The Wildlife/Human Connection. Princeton: Princeton Scientific Publishing Co; 1992. [Google Scholar]
- 19.Safe SH. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu Rev Pharmacol Toxicol. 1986;26:371–99. doi: 10.1146/annurev.pa.26.040186.002103. http://dx.doi.org/10.1146/annurev.pa.26.040186.002103. [DOI] [PubMed] [Google Scholar]
- 20.Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–54. doi: 10.1146/annurev.pa.22.040182.002505. http://dx.doi.org/10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 21.NAS-IOM. Veterans and Agent Orange: Update 2010. The National Academies Press; 2011. [Google Scholar]
- 22.Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, et al. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. doi: 10.1242/dev.123.1.293. [DOI] [PubMed] [Google Scholar]
- 23.Eskenazi B, Mocarelli P, Warner M, Needham L, Patterson DG, Jr, Samuels S, Turner W, Gerthoux PM, Brambilla P. Relationship of serum TCDD concentrations and age at exposure of female residents of Seveso, Italy. Environ Health Perspect. 2004;112:22–7. doi: 10.1289/ehp.6573. http://dx.doi.org/10.1289/ehp.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Samad R, Zalzali H, Rammah C, Giraud J, Naudin C, Dupasquier S, Poulat F, Boizet-Bonhoure B, Lumbroso S, Mouzat K, et al. MiniSOX9, a dominant-negative variant in colon cancer cells. Oncogene. 2011;30:2493–503. doi: 10.1038/onc.2010.621. http://dx.doi.org/10.1038/onc.2010.621. [DOI] [PubMed] [Google Scholar]
- 25.Guo YL, Hsu PC, Hsu CC, Lambert GH. Semen quality after prenatal exposure to polychlorinated biphenyls and dibenzofurans. Lancet. 2000;356:1240–1. doi: 10.1016/S0140-6736(00)02792-6. http://dx.doi.org/10.1016/S0140-6736(00)02792-6. [DOI] [PubMed] [Google Scholar]
- 26.Mocarelli P, Gerthoux PM, Patterson DG, Jr, Milani S, Limonta G, Bertona M, Signorini S, Tramacere P, Colombo L, Crespi C, et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116:70–7. doi: 10.1289/ehp.10399. http://dx.doi.org/10.1289/ehp.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelclová D, Urban P, Preiss J, Lukás E, Fenclová Z, Navrátil T, Dubská Z, Senholdová Z. Adverse health effects in humans exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Rev Environ Health. 2006;21:119–38. doi: 10.1515/reveh.2006.21.2.119. http://dx.doi.org/10.1515/REVEH.2006.21.2.119. [DOI] [PubMed] [Google Scholar]
- 28.Warner M, Mocarelli P, Samuels S, Needham L, Brambilla P, Eskenazi B. Dioxin exposure and cancer risk in the Seveso Women's Health Study. Environ Health Perspect. 2011;119:1700–5. doi: 10.1289/ehp.1103720. http://dx.doi.org/10.1289/ehp.1103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadeu JC, Hughes CL, Agarwal S, Foster WG. Alcohol, drugs, caffeine, tobacco, and environmental contaminant exposure: reproductive health consequences and clinical implications. Crit Rev Toxicol. 2010;40:633–52. doi: 10.3109/10408444.2010.493552. http://dx.doi.org/10.3109/10408444.2010.493552. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho PS, Noltie DB, Tillitt DE. Intra-strain dioxin sensitivity and morphometric effects in swim-up rainbow trout (Oncorhynchus mykiss) Comp Biochem Physiol C Toxicol Pharmacol. 2004;137:133–42. doi: 10.1016/j.cca.2003.12.005. http://dx.doi.org/10.1016/j.cca.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Hauser R, Sokol R. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult male. Fertil Steril. 2008;89(Suppl):e59–65. doi: 10.1016/j.fertnstert.2007.12.033. http://dx.doi.org/10.1016/j.fertnstert.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Roeleveld N, Bretveld R. The impact of pesticides on male fertility. Curr Opin Obstet Gynecol. 2008;20:229–33. doi: 10.1097/GCO.0b013e3282fcc334. http://dx.doi.org/10.1097/GCO.0b013e3282fcc334. [DOI] [PubMed] [Google Scholar]
- 33.Schnorr TM, Lawson CC, Whelan EA, Dankovic DA, Deddens JA, Piacitelli LA, Reefhuis J, Sweeney MH, Connally LB, Fingerhut MA. Spontaneous abortion, sex ratio, and paternal occupational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environ Health Perspect. 2001;109:1127–32. doi: 10.1289/ehp.011091127. http://dx.doi.org/10.1289/ehp.011091127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eskenazi B, Warner M, Marks AR, Samuels S, Needham L, Brambilla P, Mocarelli P. Serum dioxin concentrations and time to pregnancy. Epidemiology. 2010;21:224–31. doi: 10.1097/EDE.0b013e3181cb8b95. http://dx.doi.org/10.1097/EDE.0b013e3181cb8b95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roman BL, Peterson RE. In utero and lactational exposure of the male rat to 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs prostate development. 1. Effects on gene expression. Toxicol Appl Pharmacol. 1998;150:240–53. doi: 10.1006/taap.1997.8362. http://dx.doi.org/10.1006/taap.1997.8362. [DOI] [PubMed] [Google Scholar]
- 36.Mably TA, Bjerke DL, Moore RW, Gendron-Fitzpatrick A, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 3. Effects on spermatogenesis and reproductive capability. Toxicol Appl Pharmacol. 1992;114:118–26. doi: 10.1016/0041-008x(92)90103-y. http://dx.doi.org/10.1016/0041-008X(92)90103-Y. [DOI] [PubMed] [Google Scholar]
- 37.Alworth LC, Howdeshell KL, Ruhlen RL, Day JK, Lubahn DB, Huang TH, Besch-Williford CL, vom Saal FS. Uterine responsiveness to estradiol and DNA methylation are altered by fetal exposure to diethylstilbestrol and methoxychlor in CD-1 mice: effects of low versus high doses. Toxicol Appl Pharmacol. 2002;183:10–22. doi: 10.1006/taap.2002.9459. http://dx.doi.org/10.1006/taap.2002.9459. [DOI] [PubMed] [Google Scholar]
- 38.Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol Pharmacol. 2006;70:549–61. doi: 10.1124/mol.106.025304. http://dx.doi.org/10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- 39.Winzeler EA, Castillo-Davis CI, Oshiro G, Liang D, Richards DR, Zhou Y, Hartl DL. Genetic diversity in yeast assessed with whole-genome oligonucleotide arrays. Genetics. 2003;163:79–89. doi: 10.1093/genetics/163.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor-mediated down-regulation of sox9b causes jaw malformation in zebrafish embryos. Mol Pharmacol. 2008;74:1544–53. doi: 10.1124/mol.108.050435. http://dx.doi.org/10.1124/mol.108.050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol Sci. 2005;84:368–77. doi: 10.1093/toxsci/kfi073. http://dx.doi.org/10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- 42.Henry EC, Kent TA, Gasiewicz TA. DNA binding and transcriptional enhancement by purified TCDD. Ah receptor complex. Arch Biochem Biophys. 1997;339:305–14. doi: 10.1006/abbi.1996.9873. http://dx.doi.org/10.1006/abbi.1996.9873. [DOI] [PubMed] [Google Scholar]
- 43.Lanham KA, Peterson RE, Heideman W. Sensitivity to dioxin decreases as zebrafish mature. Toxicol Sci. 2012;127:360–70. doi: 10.1093/toxsci/kfs103. http://dx.doi.org/10.1093/toxsci/kfs103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofsteen P, Mehta V, Kim MS, Peterson RE, Heideman W. TCDD inhibits heart regeneration in adult zebrafish. Toxicol Sci. 2013;132:211–21. doi: 10.1093/toxsci/kfs329. http://dx.doi.org/10.1093/toxsci/kfs329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brambilla G, Dellatte E, Fochi I, Iacovella N, Miniero R, di Domenico A. Depletion of selected polychlorinated biphenyl, dibenzodioxin, and dibenzofuran congeners in farmed rainbow trout (Oncorhynchus mykiss): a hint for safer fish farming. Chemosphere. 2007;66:1019–30. doi: 10.1016/j.chemosphere.2006.07.033. http://dx.doi.org/10.1016/j.chemosphere.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 46.Philips BH, Susman TC, Powell WH. Developmental differences in elimination of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) during Xenopus laevis development. Mar Environ Res. 2006;62(Suppl):S34–7. doi: 10.1016/j.marenvres.2006.04.027. http://dx.doi.org/10.1016/j.marenvres.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 47.Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, Needham L, Patterson D, Brambilla P. Seveso Women's Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40:1247–53. doi: 10.1016/s0045-6535(99)00376-8. http://dx.doi.org/10.1016/S0045-6535(99)00376-8. [DOI] [PubMed] [Google Scholar]
- 48.Ikeda M, Mitsui T, Setani K, Tamura M, Kakeyama M, Sone H, Tohyama C, Tomita T. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats disrupts brain sexual differentiation. Toxicol Appl Pharmacol. 2005;205:98–105. doi: 10.1016/j.taap.2004.09.010. http://dx.doi.org/10.1016/j.taap.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Schafer AJ, Foster JW, Kwok C, Weller PA, Guioli S, Goodfellow PN. Campomelic dysplasia with XY sex reversal: diverse phenotypes resulting from mutations in a single gene. Ann N Y Acad Sci. 1996;785:137–49. doi: 10.1111/j.1749-6632.1996.tb56252.x. http://dx.doi.org/10.1111/j.1749-6632.1996.tb56252.x. [DOI] [PubMed] [Google Scholar]
- 50.Hofsteen P, Plavicki J, Johnson SD, Peterson RE, Heideman W. Sox9b is required for epicardium formation and plays a role in TCDD-induced heart malformation in zebrafish. Mol Pharmacol. 2013;84:353–60. doi: 10.1124/mol.113.086413. http://dx.doi.org/10.1124/mol.113.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wenner M. The most transparent research. Nat Med. 2009;15:1106–9. doi: 10.1038/nm1009-1106. http://dx.doi.org/10.1038/nm1009-1106. [DOI] [PubMed] [Google Scholar]
- 52.Baker TR, Peterson RE, Heideman W. Using zebrafish as a model system for studying the transgenerational effects of dioxin. Toxicol Sci. 2014 doi: 10.1093/toxsci/kfu006. In press. http://dx.doi.org/10.1093/toxsci/kfu006. [DOI] [PMC free article] [PubMed]


