Abstract
[Purpose] This study evaluated the functional ability and kinesthetic sense of the hands of women with breast cancer-related lymphedema. [Subjects and Methods] Fifty-seven women experiencing lymphedema after breast surgery and adjuvant radiotherapy were included. The patients were divided into two groups: women with hand edema (HE+, n = 29) and without hand edema (HE−, n = 28) after breast cancer treatment. Arm edema severity, hand size, functional mobility and kinesthetic sense of the hand, and daily living skills were evaluated. [Results] The mean age of the patients was 55.8 years. In both groups, functional mobility, kinesthetic sense, and daily living skills decreased significantly with increasing edema severity. However, there was no significant difference between groups with respect to functional mobility or daily living skills. The kinesthetic sense of the hand was better in the HE− group than the HE+ group. There was a significant negative relationship between the severity of edema and hand function. [Conclusion] Breast cancer-related lymphedema can negatively impact women’s functional mobility and kinesthetic sense of the hands as well as daily living skills.
Key words: Breast cancer related lymphedema, Kinesthetic sense, Hand functions
INTRODUCTION
Breast cancer is one of the most common cancers affecting women, accounting for approximately 30% of all cancers among women worldwide1, 2). Breast cancer-related lymphedema (BCRL) is chronic swelling that can occur in the arms and/or hands, trunk, or breasts of patients who have received treatment for breast cancer. Lymphedema is characterized by the accumulation of interstitial fluid and tissue alterations due to insufficient lymph drainage1, 3). Removal of axillary lymph nodes is the primary risk factor, and the risk increases significantly when this is followed by postoperative radiation therapy4, 5). The overall reported incidence of post-mastectomy lymphedema ranges 5.5–56%6,7,8). BCRL usually develops gradually, and the swelling can range from mild to severe. Lymphedema may reduce the range of movement of the affected limb, and impair functional ability as well as cause physical disfigurement, pain, skin problems, anxiety, depression, and overall poorer quality of life1, 3, 4).
The clinical diagnosis of lymphedema is typically based on measurements of arm circumference or volume. Measuring circumference with a tape measure is the most frequently used technique in clinical practice9, 10). An alternative for measuring hand swelling is the figure-of-eight method11,12,13,14), which involves a simple measuring tape passed in a figure-of-eight configuration around the hand and wrist at specified points, particularly areas of the hand where swelling is known to accumulate, such as the dorsum11). The obvious advantage of this method is that only a simple measuring tape is required. The figure-of-eight method may also provide a clinically useful measure of hand size. Concordantly, Borthwick et al.15) report that the figure-of-eight method is a valid and reliable method of measuring hand swelling related to BCRL.
Hand edema that negatively affects daily activities and functional mobility is also present in 60–70% of upper-extremity lymphedema cases16,17,18,19). However, there is insufficient research related to the evaluation of hand function in upper-extremity lymphedema. Therefore, this study evaluated the functional mobility and kinesthetic sense of the hands as well as daily living skills in patients with upper-extremity lymphedema.
SUBJECTS AND METHODS
This study included 57 women with lymphedema after breast cancer treatment. All patients had completed breast cancer therapy (surgery, radiotherapy, or chemotherapy) at least 3 months before the beginning of the study. The inclusion criteria were as follows: (a) age over 18 years, (b) able to understand and speak the Turkish language, (c) unilateral lymphedema ranging from mild to severe, and (d) willingness and ability to participate in the study. Meanwhile, the exclusion criteria were as follows: previous contralateral breast disease, cancer recurrence, disorders related to muscles or joints, severe axillary pain, and conditions that would make participation difficult (e.g., dementia). All patients were informed of the purpose and procedures of the study, and signed an informed consent form in accordance with the guidelines approved by the university hospital ethical committee and the Declaration of Helsinki.
This study was a cross-sectional prospective study. All patients had lymphedema of the arm following treatment for breast cancer. The patients were divided into two groups according to the presence of hand edema: 29 patients with hand edema (HE+ group) and 28 patients without hand edema (HE− group). All measurements were made during one session lasting approximately 120 minutes. The lymphedema severity of the arms was assessed, and functional mobility, kinesthetic sense, daily living skills, and hand size were evaluated for both hands.
Before testing, a complete medical history was obtained from each patient, including demographic information (i.e., age, sex, height, weight, body mass index [BMI], profession, dominant hand, and affected hand) and disease characteristics (i.e., type and side of the operation, number of excised axillary lymph nodes, number of tumors and positive lymph nodes, radiotherapy technique used, adjuvant systemic treatment, lymphedema duration, and previous infection episodes).
Edema of the arm was assessed by circumference measurements taken with a standard 1-inch retractable fiberglass tape measure. For the arm circumference measurements, patients were in a supine position with their arms relaxed by their sides and elbows straight. Arm circumference was measured at 5-cm intervals beginning at the third phalanx nail fold and continuing 45 cm proximally. Both arms were assessed, and all measurements were recorded in centimeters. The severity of lymphedema was classified according to a modified version of the criteria described by the American Physical Therapy Association: circumference differences between arms <3, 3–5, and >5 cm were defined as mild, moderate, and severe lymphedema, respectively9, 20).
A standard 0.25-inch-wide retractable fiberglass tape measure was used to perform figure-of-eight measurements of hand size14). The testing position required the patient to sit with their arms abducted and externally rotated 90°, elbow flexed 90°, wrist neutral, fingers adducted and extended, and thumb abducted in the plane of the hand. A physiotherapist measured each patient’s hand twice. The same procedure was repeated for the other hand. All measurements were recorded in centimeters (± 0.1 cm, Fig. 1).
Fig. 1.
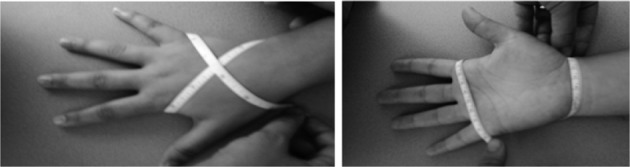
Figure-of-eight tape measure method for measuring hand size. (A) Dorsal view, (B) Palmar view.
Hand functional mobility was assessed by modified Kapandji index (MKI)21). The assessment was conducted twice by the physiotherapist with a 1-hour interval between trials. The MKI score was obtained by summing the scores of the following three tests. The first test assessed the opposition of the thumb, which was scored from 0 (impossible to do) to 10 (completely accomplished). The second test evaluated the flexion of each long finger, which was scored from 0 (impossible to do) to 5 (completely accomplished). The third test assessed finger extension, which was scored from 0 (impossible to do) to 5 (completely accomplished). Total scores for the scale of finger flexion (maximum 20 points), scale of finger extension (20 points), and total opposition test (10 points) were recorded. Higher scores reflect better functional mobility. The total score of the three tests (maximum 50 points) was calculated and used for analysis.
The kinesthetic sense of the hand was measured by examining the patients’ ability to copy hand positions22, 23). The test was conducted in a quiet well-lit room, with the patient comfortably seated using a table and chair of appropriate height. A masking box was placed on the table to occlude the patient’s vision while allowing the physiotherapist to have a full view of the patient’s hand. The box had an internal shelf that supported the forearm along predetermined lines drawn at an angle of 20° to the table edge; the hands hung freely over the edge of the self. The physiotherapist demonstrated a set of eight visually cued hand positions (Fig. 2), and the patient was instructed to produce mirror images of these gestures with the tested hand, which was hidden inside the masking box. The time taken to copy each test pattern was recorded in seconds rounded to two decimal places, and the accuracy of replication was graded using the criteria described by Lynch: 0, failure to move the hand from the resting position; 1, no resemblance to the test position; 2, incomplete replication, which may include use of the wrong fingers in the correct relationship, one finger out of place, inappropriate opposition, or a reversal of the gesture; 3, complete and accurate replication. Any position that could not be held by the non-test hand during kinesthetic cuing was also noted. The final score was reported as the mean replication accuracy in the tested hand for all eight test positions or the percent of kinesthetic sense lost with respect to the total possible score achievable.
Fig. 2.
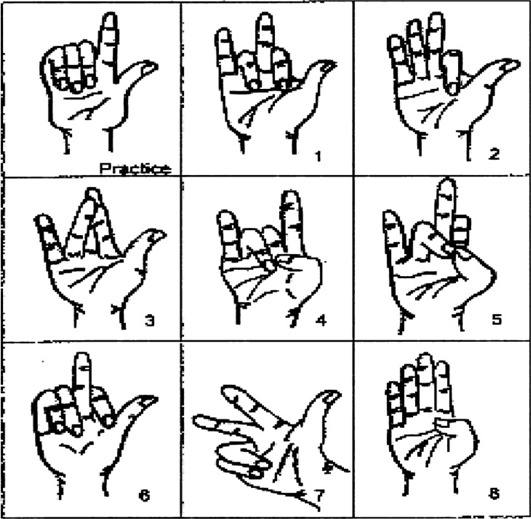
Hand positions
Daily living skills were assessed by the 62-item Hand Function Sort (HFS)24). This instrument is a self-rating of ability to perform the task by reviewing the item and judging one’s ability on a five-point scale (4, able; 3, slightly restricted; 2, moderately restricted; 1, very restricted; 0, unable). A total score for the items in each physical demand characteristic level was obtained along with an overall “rating of perceived capacity” score ranging 0–248. The items represent tasks with a broad range of physical demand, including activities of daily living. Test administration normally required 7–10 minutes.
All data were analyzed using SPSS version 20.0 for Windows (IBM Corp., Armonk, NY, USA). Descriptive statistics are summarized as frequencies and percentages for categorical variables. Normally distributed continuous variables are presented as means and standard deviations. The significance of differences between two groups was determined by the Mann-Whitney U-test. Intragroup comparisons were performed using the Wilcoxon signed-rank test. Pearson’s product-moment correlation coefficients were calculated to determine the relationships between measurement techniques. The level of significance was set at p < 0.05.
RESULTS
A total of 60 women diagnosed with BCRL were initially recruited. However, one patient withdrew because of infection, and two patients declined to participate, resulting in a final study group of 57 patients. Patients’ compliance during evaluation was good in both groups. The mean ages of the HE+ and HE− groups were 55.3 ± 11.1 years (range: 33–73 years) and 56.7 ± 10.6 years (range: 40–70 years), respectively; mean BMI was 26.9 ± 4.2 and 27.7 ± 3.8 kg/m2, respectively. There were no significant differences in the demographic characteristics between groups. The demographic characteristics and evaluation results of the 57 women are presented in Table 1.
Table 1. Patients characteristics.
| Lymphedema severity | HE+ group (n = 29) | HE− group (n = 28) | ||||
|---|---|---|---|---|---|---|
| Mild (n = 6) | Moderate (n = 15) | Severe (n = 8) | Mild (n = 5) | Moderate (n = 13) | Severe (n = 10) | |
| Age (years, mean ± SD (range) | 52.3 ± 10.03 | 54.7 ± 11.2 | 60.2 ± 9.7 | 54.8 ± 7.8 | 56.3 ± 11.8 | 58.2 ± 11.04 |
| (33–68) | (35–70) | (51–73) | (46–66) | (33–70) | (42–75) | |
| BMI (kg/m2, mean ± SD) | 24.1 ± 2.9 | 26.03 ± 3.5 | 31.0 ± 3.5 | 26.9 ± 1.7 | 26.4 ± 3.9 | 29.7 ± 3.9 |
| Dominant arm | ||||||
| Right | 5 | 12 | 6 | 5 | 11 | 7 |
| Left | 1 | 3 | 2 | - | 2 | 3 |
| Affected arm (%) | ||||||
| Dominant | 5 | 10 | 5 | 3 | 11 | 9 |
| Non-dominant | 1 | 5 | 3 | 2 | 2 | 1 |
| Duration of lymphedema (years, mean ± SD) | 2.7 ± 1.3 | 4.5 ± 3.4 | 5.7 ± 9.6 | 2.3 ± 0.5 | 5.9 ± 6.6 | 6.7 ± 4.8 |
| Lymph nodes removed (mean ± SD) | 5.8 ± 2.2 | 12 ± 8.9 | 22.7 ± 6.8 | 9.2 ± 3.5 | 15.6 ± 10.1 | 27.1 ± 11.2 |
| Treatments | ||||||
| ET+RT | 2 | 4 | 2 | 1 | 3 | 2 |
| CT+RT | 1 | 3 | - | - | 2 | 2 |
| ET+CT+RT | 3 | 9 | 6 | 4 | 8 | 6 |
| History of recurrent cellulitis (%) | - | 40 | 87.5 | - | 53.8 | 89.2 |
| Hand size (mean ± SD) | 36.1 ± 5.9 | 41.7 ± 7.2 | 52.9 ± 9.6 | 35.5 ± 7.7 | 36.8 ± 9.5 | 38.4 ± 10.8 |
| Kinesthetic sense (% lost) | 16.6 | 53.3 | 87.5 | - | 30.8 | 60 |
| Functional mobility score (mean ± SD) | 46.8 ± 13.5 | 39.6 ± 4.3 | 30.3 ± 4.9 | 49.1 ± 9.7 | 41.9 ± 8.2 | 34.9 ± 2.5 |
| HFS score (mean ± SD) | 214.0 ± 11.8 | 199.4 ± 16.6 | 178.4 ± 44.5 | 235.2 ± 1.09 | 217.5 ± 15.1 | 199.1 ± 51.3 |
HE: hand edema, ET: endocrine therapy, RT: radiotherapy, CT: chemotherapy, HFS: Hand Function Sort
According to arm circumference, 18 (31.58%), 28 (49.14%), and 11 (19.28%) patients had severe, moderate, and mild lymphedema, respectively.
Using the figure-of-eight method of hand size measurement, 10 (34.5%), 12 (41.4%), and 7 (24.1%) women in the HE+ group had severe, moderate, and mild hand lymphedema, respectively. Hand size differed significantly between the affected and unaffected hands in the HE+ group (p < 0.05) but not in the HE− group (p > 0.05, Table 2).
Table 2. Hand size, MKI scores, kinesthetic sense, and HFS score.
| HE+ group n = 29 |
HE− group n = 28 |
|
|---|---|---|
| Hand size (cm) | 43.5 ± 7.2 | 36.9 ± 9.3* |
| MKI score | 38.9 ± 11.5 | 41.3 ± 9.2 |
| Kinesthetic sense score | 1.8 ± 2.51 | 2.6 ± 1.78* |
| HFS score | 199 ± 10.2 | 217.7 ± 12.4 |
*p < 0.05; HE: hand edema, MKI: modified Kapandji index, HFS: Hand Function Sort
According to the MKI, the functional mobility of the hand decreased significantly with increasing hand edema severity (p < 0.05, Table 3). MKI scores tended to be higher in the HE− group than the HE+ group, but the difference was not significant (p > 0.05, Table 2). There was no significant difference regarding the functional mobility of the hand in patients whose dominant or non-dominant arm was affected (p > 0.05). Meanwhile, the functional mobility of the affected hand was significantly lower than that of the unaffected hand in the HE+ group (p < 0.05).
Table 3. Correlations among assessed clinical variables.
| MKI | HS | KS | HFS | |
|---|---|---|---|---|
| MKI | -- | −0.367** | 0.519*** | 0.468*** |
| HS | −0.367** | -- | −0.591*** | −0.472*** |
| KS | 0.519*** | −0.591*** | -- | 0.611*** |
| HFS | 0.468*** | −0.472*** | 0.611*** | -- |
MKI: modified Kapandji index, HS: Hand Size, KS: kinesthetic sense, HFS: Hand Function Sort, *p < 0.05, **p < 0.01, ***p < 0.001.
The HE− group exhibited significantly better kinesthetic sense of the hand than the HE+ group (p < 0.05, Table 2), and the kinesthetic sense of the hand decreased significantly with increasing edema severity (p < 0.05, Table 3). The kinesthetic sense of the affected hand was significantly lower than that of the unaffected hand (p < 0.05). In both groups, patients whose dominant hand was affected tended to have better kinesthetic sense than those whose non-dominant hand was affected, but the difference was not significant (p > 0.05).
HFS scores revealed that increasing edema severity was significantly correlated with diminished ability to perform activities of daily living (p < 0.05, Table 3). The HFS score was significantly higher in the HE− group than the HE+ group (p < 0.05, Table 2). Within each group, the score of the affected hand was significantly lower than that of the unaffected hand (p < 0.05). In both groups, daily living skills were significantly better in women with lymphedema of the dominant arm than the non-dominant arm (p < 0.05).
DISCUSSION
The present study assessed the functionality and kinesthetic sense of the hand in women who developed BCRL. In both groups, hand function decreased with increasing arm edema. Furthermore, the kinesthetic sense of the hand was significantly better in patients without hand edema than those with hand edema. This result may indicate stronger interactions between edema severity and reductions in hand function and kinesthetic sense. This also demonstrates arm lymphedema negatively affects kinesthetic sense and hand function.
Lymphedema is a serious complication in the treatment of breast cancer. It can cause substantial functional deficiency, especially for women during reproductive age. Lymphedema results in pain, loss of sensation, muscular weakness, lack of movement in articulations, and impairment of upper extremity functional skills25,26,27). The present results corroborate the literature in this respect. Most studies used standard circumference measurement and volumetric measuring to assess edema severity9, 28, 29). Borthwick et al.15) demonstrated that the figure-of-eight method, the primary assessment used in the present study, is a valid and reliable technique for measuring hand swelling related to BCRL. The present results also show the results of the figure-of-eight method are strongly correlated with those of the circumference measurement method.
It was reported in previous studies that the hand area was affected in 60–70% of cases of upper-extremity lymphedema. Hand edema negatively impacts daily activities and functional mobility17,18,19, 30). However, no studies have evaluated how functional mobility of the hand is affected by lymphedema occurring after breast cancer treatment. Lefevre-Calau et al.21) report that the MKI is a valid and reliable assessment method for evaluating the functional mobility of the hand in patients with rheumatoid arthritis. Meanwhile, we examined the effect of upper-extremity lymphedema on the functional mobility of the hand using the MKI. The results show the functional mobility of the hand decreased with increasing edema severity in the affected arm of both groups. The functional mobility of the hands in the HE− group tended to be better than in the HE+ group, although the difference was not significant. Furthermore, there was no difference in the functional mobility of the hand between cases in which the dominant and non-dominant arm was affected. This could be attributable to the small number of cases in which the non-dominant arm was affected in the present study.
Loss of sensation in the upper extremity due to surgery is another post-treatment problem that is exacerbated by edema31,32,33). Bosompra et al.32) and Maunsell et al.33) report that 63% and 61% of patients with lymphedema experience loss of sensation, respectively. Hwang et al.22) hypothesize that a loss of kinesthetic sense of the hand results in inability to perform many functional skills of the hand, consequently causing difficulty performing activities of daily living. The present findings indicate that a loss of kinesthetic sense is correlated with hand edema severity. Loss of kinesthetic sense was observed in 65.3% of patients, who also had impaired daily activity skills. These findings directly support the hypothesis proposed by Hwang et al.
Patients who develop lymphedema during the post-treatment period of breast cancer experience a sense of heaviness in the arm, muscle weakness, restricted shoulder mobility, difficulty performing activities of daily living, and overall diminished quality of life5, 17,18,19). Gosselink et al.34) evaluated postoperative upper-extremity function in breast cancer patients and found that restriction of the movement of the upper and lower extremities negatively affects patients’ ability to perform activities of daily living, necessitating immediate physiotherapy. Voogd et al.19) evaluated the effect of upper-extremity lymphedema on quality of life and found it caused a loss of daily functional skills, energy and motivation; they conclude that such factors lead to diminished quality of life.
As mentioned in the above examples, most previous studies focused on the functional impact of lymphedema of the shoulder and upper extremities. However, the hand plays a significant role in upper-extremity function32, 33). Thus, it is surprising how few studies have employed activities of daily living questionnaires to assess the functional skill of the hands in lymphedema patients. The present study examined the effect of upper-extremity lymphedema on hand function by using the HFS questionnaire. The results indicate that daily life activity skills decreased with increasing edema severity in the affected hand; this relationship was even stronger when the dominant arm was affected. Our study has various strengths and limitations. This study is valuable in that few studies have evaluated hand function in upper-extremity lymphedema. However, the relatively small number of patients may limit the study’s power. Further studies on larger samples are needed to support the findings.
In conclusion, the functional mobility, kinesthetic sense, and daily living skills of the hand can be negatively affected in upper-extremity lymphedema. Therefore, greater emphasis should be placed on addressing the long-term post-treatment complications of lymphedema in patients with breast cancer, such as sensation loss and functional impairment of the hand, which significantly impact quality of life.
REFERENCES
- 1.Fu MR: Breast cancer-related lymphedema: symptoms, diagnosis, risk reduction, and management. World J Clin Oncol, 2014, 5: 241–247Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paramanandam VS, Roberts D: Weight training is not harmful for women with breast cancer-related lymphoedema: a systematic review. J Physiother, 2014, 60: 136–143. [DOI] [PubMed] [Google Scholar]
- 3.Taghian NR, Miller CL, Jammallo LS, et al. : Lymphedema following breast cancer treatment and impact on quality of life: a review. Crit Rev Oncol Hematol, 2014, 92: 227–234. [DOI] [PubMed] [Google Scholar]
- 4.Sherman KA, Miller SM, Roussi P, Sherman KA, Miller SM, Roussi P, et al. : Factors predicting adherence to risk management behaviors of women at increased risk for developing lymphedema. Support Care Cancer, 2015, 23: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monleon S, Murta-Nascimento C, Bascuas I, et al. : Lymphedema predictor factors after breast cancer surgery: a survival analysis. Lymphat Res Biol, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda K, Ogawa Y, Kajino C, et al. : The influence of axillary reverse mapping related factors on lymphedema in breast cancer patients. Eur J Surg Oncol, 2014, 40: 818–823. [DOI] [PubMed] [Google Scholar]
- 7.DiSipio T, Rye S, Newman B, et al. : Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol, 2013, 14: 500–515. [DOI] [PubMed] [Google Scholar]
- 8.Letellier ME, Towers A, Shimony A, et al. : Breast cancer-related lymphedema: a randomized controlled pilot and feasibility study. Am J Phys Med Rehabil, 2014, 93: 751–759, quiz 760–761. [DOI] [PubMed] [Google Scholar]
- 9.Karges JR, Mark BE, Stikeleather SJ, et al. : Concurrent validity of upper-extremity volume estimates: comparison of calculated volume derived from girth measurements and water displacement volume. Phys Ther, 2003, 83: 134–145. [PubMed] [Google Scholar]
- 10.Choi YH, Seo KS: Correlation among bioimpedance analysis, sonographic and circumferential measurement in assessment of breast cancer-related arm lymphedema. Lymphology, 2014, 47: 123–133. [PubMed] [Google Scholar]
- 11.Maihafer GC, Llewellyn MA, Pillar WJ, Jr, et al. : A comparison of the figure-of-eight method and water volumetry in measurement of hand and wrist size. J Hand Ther, 2003, 16: 305–310. [DOI] [PubMed] [Google Scholar]
- 12.Pellecchia GL: Figure-of-eight method of measuring hand size: reliability and concurrent validity. J Hand Ther, 2003, 16: 300–304. [DOI] [PubMed] [Google Scholar]
- 13.Leard JS, Breglio L, Fraga L, et al. : Reliability and concurrent validity of the figure-of-eight method of measuring hand size in patients with hand pathology. J Orthop Sports Phys Ther, 2004, 34: 335–340. [DOI] [PubMed] [Google Scholar]
- 14.Dewey WS, Hedman TL, Chapman TT, et al. : The reliability and concurrent validity of the figure-of-eight method of measuring hand edema in patients with burns. J Burn Care Res, 2007, 28: 157–162. [DOI] [PubMed] [Google Scholar]
- 15.Borthwick Y, Paul L, Sneddon M, et al. : Reliability and validity of the figure-of-eight method of measuring hand size in patients with breast cancer-related lymphoedema. Eur J Cancer Care (Engl), 2013, 22: 196–201. [DOI] [PubMed] [Google Scholar]
- 16.Rhee H, Yu J, Kim S: Influence of compression types on hand function: a preliminary investigation. J Phys Ther Sci, 2011, 23: 477–480. [Google Scholar]
- 17.Caffo O, Amichetti M, Ferro A, et al. : Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat, 2003, 80: 39–48. [DOI] [PubMed] [Google Scholar]
- 18.Engel J, Kerr J, Schlesinger-Raab A, et al. : Axilla surgery severely affects quality of life: results of a 5-year prospective study in breast cancer patients. Breast Cancer Res Treat, 2003, 79: 47–57. [DOI] [PubMed] [Google Scholar]
- 19.Voogd AC, Ververs JM, Vingerhoets AJ, et al. : Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg, 2003, 90: 76–81. [DOI] [PubMed] [Google Scholar]
- 20.Tretbar LL: Structure and Function of the Lymphatic System In: Tretbar LL, Morgan CL, Lee BB, et al., Lymphedema: Diagnosis and Treatment. London: Springer, 2008, pp 21–31. [Google Scholar]
- 21.Lefevre-Colau MM, Poiraudeau S, Oberlin C, et al. : Reliability, validity, and responsiveness of the modified Kapandji index for assessment of functional mobility of the rheumatoid hand. Arch Phys Med Rehabil, 2003, 84: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 22.Hwang R, Kentish M, Burns Y: Hand positioning sense in children with spina bifida myelomeningocele. Aust J Physiother, 2002, 48: 17–22. [DOI] [PubMed] [Google Scholar]
- 23.Kara B, Yildirim Y, Karadýbak D, et al. : Evaluation of the kinesthetic sense and function of the hand in early period in operated cervical disc hernia. Eur Spine J, 2006, 15: 992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matheson LN, Kaskutas VK, Mada D: Development and construct validation of the hand function sort. J Occup Rehabil, 2001, 11: 75–86. [DOI] [PubMed] [Google Scholar]
- 25.Rietman JS, Dijkstra PU, Debreczeni R, et al. : Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. Disabil Rehabil, 2004, 26: 78–84. [DOI] [PubMed] [Google Scholar]
- 26.Dawes DJ, Meterissian S, Goldberg M, et al. : Impact of lymphoedema on arm function and health-related quality of life in women following breast cancer surgery. J Rehabil Med, 2008, 40: 651–658. [DOI] [PubMed] [Google Scholar]
- 27.Togawa K, Ma H, Sullivan-Halley J, et al. : Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Res, 2014, 16: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto N, Yamamoto T, Hayashi N, et al. : Arm volumetry versus upper extremity lymphedema index: validity of upper extremity lymphedema index for body-type corrected arm volume evaluation. Ann Plast Surg, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Brorson H, Höijer P: Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J Plast Surg Hand Surg, 2012, 46: 410–415. [DOI] [PubMed] [Google Scholar]
- 30.Stanton AW, Svensson WE, Mellor RH, et al. : Differences in lymph drainage between swollen and non-swollen regions in arms with breast-cancer-related lymphoedema. Clin Sci (Lond), 2001, 101: 131–140. [PubMed] [Google Scholar]
- 31.Hayes SC, Johansson K, Stout NL, et al. : Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer, 2012, 118: 2237–2249. [DOI] [PubMed] [Google Scholar]
- 32.Bosompra K, Ashikaga T, O’Brien PJ, et al. : Swelling, numbness, pain, and their relationship to arm function among breast cancer survivors: a disablement process model perspective. Breast J, 2002, 8: 338–348. [DOI] [PubMed] [Google Scholar]
- 33.Maunsell E, Brisson J, Deschênes L: Arm problems and psychological distress after surgery for breast cancer. Can J Surg, 1993, 36: 315–320. [PubMed] [Google Scholar]
- 34.Gosselink R, Rouffaer L, Vanhelden P, et al. : Recovery of upper limb function after axillary dissection. J Surg Oncol, 2003, 83: 204–211. [DOI] [PubMed] [Google Scholar]


