Abstract
[Purpose] We compared the effects of acute aerobic exercise following overnight fasting and breakfast on energy substrate and hormone levels in obese male college students. [Subjects and Methods] This crossover study recruited 10 obese male college students with a body mass index >25 kg/m2 or >20% body fat. One week post-recruitment, the subjects exercised in the morning after an overnight fast. At 2 weeks, they exercised post-breakfast. Energy substrate (glucose, free fatty acid) and metabolic hormone (insulin, growth hormone, and cortisol) levels were measured immediately before and after exercise and at 60 min post-exercise. [Results] We observed interaction effects between the measurement time and exercise treatment for glucose; significant differences between measurement times and between exercise treatments for free fatty acids; interaction effects between the measurement time and exercise treatment for insulin and significant differences in the measurement time; significance differences between measurement times and between exercise treatments for growth hormone; and significant differences between measurement times and between exercise treatments for cortisol. [Conclusion] Morning exercise following an overnight fast can be more effective in reducing body fat than post-prandial exercise. However, increased cortisol levels following exercise after overnight fasting may negatively affect long-term weight loss in obese men.
Key words: Exercise, Energy substrates, Metabolic hormone
INTRODUCTION
In many countries, increased consumption of a westernized diet and lack of physical activity have caused an energy intake imbalance and resulted in an increased prevalence of obesity and chronic diseases, including hypertension, cardiovascular disorders, diabetes, and arthritis1). However, regular exercise and physical activity are reported to be effective methods for preventing obesity, its accompanying chronic lifestyle diseases, and premature death2). When performed on a regular, long-term basis, aerobic exercise can prevent or cure obesity, increase muscle volume, elevate oxygen levels and oxidizing enzymes in myocytes, and increase blood volume and fat oxidation3). In particular, aerobic exercise can improve the various metabolic risk factors involving blood pressure, fatty acids, and the cardiovascular system in obese and type 2 diabetic patients4,5,6). One study suggested that exercise in the morning on an empty stomach promotes the utilization of lipids in adipose tissue because the exercise is performed under low blood sugar conditions7). Because this exercise regime promotes increased utilization of lipids, it is thought to be more efficient for weight regulation9). However, findings from other studies have suggested that performing aerobic exercise in the morning before eating can induce neurotic symptoms and fatigue because low blood sugar levels result in stimulation of the hypothalamic-pituitary-adrenal (HPA) axis7, 8). This stimulation can increase the secretion of the steroid hormone cortisol7, 8). Elevated cortisol level can decrease protein synthesis in the muscle, thereby affecting carbohydrate, fat, and protein metabolism, and can also promote gluconeogenesis in the liver9, 10). However, increased cortisol levels have also been observed to result from moderate- to high-intensity aerobic exercise, which complicates the interpretation of these previous studies. The ability of exercise to prevent obesity and reduce body weight depends on the retention of internal nutrition before exercise and the secretion of hormones that affect carbohydrate, fat, and protein metabolism11). Many studies have evaluated energy substrate changes and hormone levels (e.g., cortisol, growth hormone, and insulin) in relation to different exercise parameters (e.g., intensity), but none have investigated the effects of exercise performed in the morning (when cortisol secretion is at its maximum) following an overnight fast or breakfast. Therefore, the present study aimed to compare the effects of exercise after overnight fasting and after breakfast on energy substrate changes and hormone levels in male university students with obesity.
SUBJECTS AND METHODS
The target group of the current investigation included 10 male students with obesity, a body mass index of >25 kg/m2, and body fat of >20%. Subjects were included if they were physically healthy and had no specific diseases based on a questionnaire survey and medical examination and had not taken any specific drugs for at least 6 months prior to the experiment. Informed consent was obtained from the subjects prior to their participation in the study. Kyungwoon University approved this study, which complies with the ethical standards of the Declaration of Helsinki. The physical characteristics of the subjects are shown in Table 1.
Table 1. Physical characteristics of the 10 study participants.
| Parameter | Mean ± standard deviation |
|---|---|
| Age | 20.2±1.9 |
| Height (cm) | 177.2±6.1 |
| Weight (kg) | 84.3±14.6 |
| BMI (kg/m2) | 26.7±4.1 |
| Body fat (%) | 23.8±4.9 |
| VO2max (ml/kg/min) | 45.2±8.5 |
BMI: body mass index; VO2max: maximal oxygen consumption
The experiment had a crossover design (Fig. 1) and was conducted over a period of 4 weeks. The subjects performed the first experiment (exercising after an overnight fast) after 1 week of testing and then performed the second experiment (exercising 2 h after breakfast) subsequent to a 2-week washout period from the first experiment. For both experiments, the subjects maintained an empty stomach after 10 P.M. on the day before exercising and then performed the exercise at 9 A.M. on the next day. For the second experiment, the subjects consumed a specified ratio of calories and nutrients 2 h before the exercise. Blood collection was performed immediately before and after exercise and also at 60 min after the exercise.
Fig. 1.
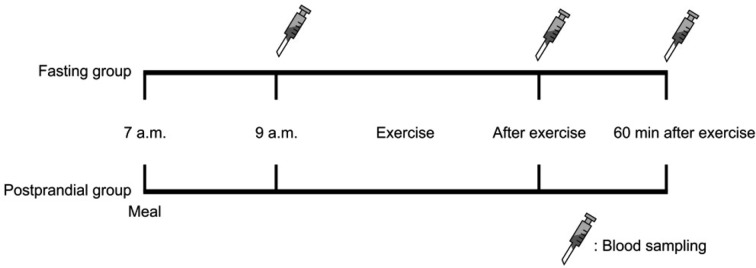
Schema of the study design and blood collection times
For both experiments, the subjects exercised on a treadmill at 75% of VO2max and burned up to 400 kcal. For controlling effects due to differences in exercise intensity and duration between the 2 exercise regimes, a regression equation derived from the data sheet for graded exercise test values (oxygen intake and heart rate per minute) was used to establish identical calorie consumption and exercise intensity between the experiments. The average target heart rate and exercise time were determined to be 155.80 ± 6.90 bpm and 31.70 ± 2.35 min, respectively. Meanwhile, to precisely monitor the individual caloric consumption, the time point at which the target heart rate and exercise time were reached was measured with an automated heart rate monitor (Polar Heart Monitor, Polar S610i, Polar Electro, Kempele, Finland). The VO2max was measured with an exercise load test (graded exercise test) using a treadmill (Medtrack ST 65; Quinton Instrument Co., Boston, MA, USA) and a respiratory gas analyzer (True-one; Quinton Instrument Co.). The Bruce protocol was used for the graded exercise test12). Blood was collected from the brachial vein (10 mL each time) immediately before and after exercise and at 60 min following exercise for both exercise regimes. The collected venous blood was maintained for 10 min at room temperature and then centrifuged at 3,000 rpm for 10 min. The upper phase was transferred to a microtube and stored at −80 °C until the analysis. Glucose level was measured immediately after blood collection using One-Touch (Lifescan Company, Mountain View, CA, USA), and serum insulin level was analyzed using a commercial insulin reagent kit (Human insulin ELISA-kit, DSL, TX, USA). Serum free fatty acid (FFA), cortisol, and growth hormone levels were assessed by Green Cross Inc., Youngin, Korea. The parameters measured immediately before and after exercise and at 60 min after exercise between the 2 exercise regimes were compared and analyzed by two-way analysis of variance with repeated measures. A significance level of p = 0.05 was used for the analyses. The statistics were performed with SPSS-PC version 20 (SPSS Inc., Chicago, IL, USA). The results are shown as the average ± standard deviation.
RESULTS
For minimizing the differences in the amount of exercise performed between the 2 experiments, energy consumption was set at 400 kcal. As a result, energy consumption did not differ between the exercise performed after an overnight fast and that performed after breakfast (426.05 ± 20.54 vs. 425.49 ± 19.00, t-value = 0.403, p = 0.692). Table 2 shows the energy substrate (glucose and FFA) and hormone levels (insulin, GH, and cortisol) immediately before and after exercise and at 60 min after exercise for both exercise treatments. Glucose showed significant interaction effects between the measurement time and the exercise treatment (p < 0.007), but no significant differences were observed between measurement times or between exercise treatments. A possible explanation for this interaction effect is that the glucose levels had not been restored to their post-breakfast exercise levels. Meanwhile, FFA showed no significant interaction between the measurement time and treatment. However, significant differences were observed between measurement times (p < 0.001) and between exercise treatments (p < 0.002). These results suggest that exercising in the morning before breakfast is more efficient than post-prandial exercise in terms of utilizing lipids as an energy source. Insulin showed a significant interaction effect between the measurement time and exercise treatment (p < 0.002; Table 2). Additionally, significant differences were observed between measurement times (p < 0.001) but not between exercise treatments. The interaction effect suggests that insulin was secreted in response to the high level of glucose resulting from the patient’s dietary intake. Meanwhile, neither growth hormone nor cortisol showed a significant interaction effect between measurement time and exercise treatment (p < 0.216 and p < 0.102, respectively). However, both hormone levels significantly differed between measurement times (p < 0.001 and p < 0.012, respectively) and between exercise treatments (p < 0.025 and p < 0.004, respectively).
Table 2. Comparison of energy substrate and hormone changes according to exercise treatment.
| Variable | Fasting exercise | Post-prandial exercise | ||||
|---|---|---|---|---|---|---|
| BE | AE | 60 min AE | BE | AE | 60 min AE | |
| Glucose (mg/dl) | 98.7±9.7 | 102.8±8.8 | 105.4±9.4 | 109.7±10.8 | 100.1±10.3 | 102.3±4.1‡ |
| FFA (μEq/l) | 502.7±231.4 | 683.8±428.9 | 707.5±328.7 | 115.1±46.2 | 518.9±165.0 | 378.1±190.5*,† |
| Insulin (uIU/ml) | 13.99±11.15 | 5.27±13.09 | 14.74±17.43 | 40.22±24.44 | 4.28±7.64 | 10.00±8.26*,‡ |
| GH (ng/ml) | 1.07±1.85 | 11.02±7.03 | 1.55±1.32 | 0.07±0.37 | 5.73±3.75 | 0.44±0.41*,† |
| Cortisol (μg/dl) | 28.57±6.74 | 24.93±9.16 | 20.92±9.25 | 15.49±5.73 | 20.06±8.22 | 13.29±5.19*,† |
Values are shown as means ± standard deviation *p < 0.05. significant main effect for time. †p < 0.05. significant main effect for group. ‡p < 0.05. significant interaction between time and group. BE; before exercise, AE; after exercise, 60 min AE; 60 min after exercise.
DISCUSSION
In the current study, glucose and insulin showed a significant interaction effect between the measured time and treatment. One likely explanation is that their levels were high due to the dietary intake 2 hours prior to the exercise. In addition, there were significant differences in insulin level between the measurement time points, independent of treatment, indicating that the insulin levels decreased immediately after exercise due to the increased energy demand13) and then stabilized 60 min after the exercise. Increased physical exercise improves glucose homeostasis and increases sensitivity to insulin, and acute exercise can increase the sensitivity of tissues or organs such as the skeletal muscles, fat, liver, and hypothalamus to insulin14,15,16,17,18,19). Further, acute exercise has been shown to result in improved glucose homeostasis in an animal model of obesity16, 18). When blood sugar levels are low, cAMP level increases20) and activates fat disassembly in the adipocytes21). In addition, cortisol levels are higher in the morning22). These factors imply that the morning is the most efficient time of day to reduce body fat through exercise, particularly since blood sugar levels are at their lowest. Dreave et al.7) reported that the fat oxidation rate is high during morning exercise after overnight fasting as compared to that during post-prandial exercise, and they concluded that the former exercise regime is efficient for body fat reduction. Further, in the present study, growth hormone levels were higher at all evaluated time points after overnight fasting as compared to the levels after breakfast before exercise, and these levels could be related to the higher FFA levels observed after an overnight fast. According to these results, the high levels of growth hormone after overnight fasting increased the transport of fatty acids from adipocytes into the blood, thereby acting as a source of energy for muscles during exercise in the absence of carbohydrates.
Growth hormone and cortisol were previously reported to be simultaneously secreted during fasting23, 24) or exercise25, 26) and were found to increase FFA and glycerol levels in the blood27). In the current study, the FFA concentrations were high at all measured time points, which is consistent with the results of previous studies with respect to overnight fasting and morning exercise. In these previous studies, morning exercise was considered to be efficient for decreasing weight and body fat in those with obesity, particularly when performed on an empty stomach. However, in the current study, high cortisol concentrations were also observed at all the accessed time points for both treatment groups. During exercise, cortisol is secreted by the adrenal cortex in response to stress. Cortisol decreases protein synthesis in the muscles; increases catabolism, affecting the metabolism of carbohydrates, fats, and proteins; and stimulates gluconeogenesis in the liver28). In the current study, the cortisol concentration was higher after overnight fasting than it was after dietary intake. These results indicate that glucose and insulin levels were high before exercise following dietary intake because the cortisol levels were too low for the hormone to play a strong antagonistic role29). Moreover, these results suggest that a greater amount of muscle wasting for use as an energy source during exercise after a morning fast, as compared to exercise after breakfast, presumably resulted in a decreased improvement in the fat-free mass. However, because the current study only examined the effects of acute exercise, a long-term study will be needed for further validation. In addition, persistently high cortisol levels resulting from regular exercise after overnight fasting can negatively affect physical health. In particular, previous studies have indicated that these persistently high cortisol levels can promote fat accumulation in the abdomen, decrease insulin sensitivity30), increase the risk of osteoporosis31) and hypertension32), and decrease immune function and muscle mass, thereby negatively affecting the patient’s overall physical health. In the present study, we were unable to draw clear conclusions on the effect of performing exercise after overnight fasting on physical health. However, the fundamental results reported here provide the basis for a long-term study to more clearly address this issue.
Recently, a number of studies have been carried out in the attempt to develop exercise methodologies for maintaining and improving physical health to reduce the prevalence of obesity. The effect of exercise timing and pre-exercise nutritional state has emerged as an important consideration. In the current study, it should be emphasized that energy substrate and metabolic hormone levels differed between the treatment groups. Compared to the post-prandial exercise, the exercise performed after overnight fasting showed low glucose and insulin levels but high cortisol, growth hormone, and FFA levels before exercise. Hence, the latter treatment could have a long-term positive effect on body fat reduction but negatively affect physical health because of the high concentration of cortisol.
REFERENCES
- 1.Nicklas BJ, Penninx BW, Ryan AS, et al. : Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care, 2003, 26: 1413–1420. [DOI] [PubMed] [Google Scholar]
- 2.Williams DM: Exercise, affect, and adherence: an integrated model and a case for self-paced exercise. J Sport Exerc Psychol, 2008, 30: 471–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagenmakers AJ, van Riel NA, Frenneaux MP, et al. : Integration of the metabolic and cardiovascular effects of exercise. Essays Biochem, 2006, 42: 193–210. [DOI] [PubMed] [Google Scholar]
- 4.Karoline de Morais P, Sales MM, Alves de Almeida J, et al. : Effects of aerobic exercise intensity on 24-h ambulatory blood pressure in individuals with type 2 diabetes and prehypertension. J Phys Ther Sci, 2015, 27: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DY, Jung SY, Seo BD: Effect of exercise intervention on changes in free Fatty Acid levels and metabolic risk factors in stroke patients. J Phys Ther Sci, 2014, 26: 275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DY, Jung SY: Effect of aerobic exercise on risk factors of cardiovascular disease and the apolipoprotein B / apolipoprotein a-1 ratio in obese woman. J Phys Ther Sci, 2014, 26: 1825–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derave W, Mertens A, Muls E, et al. : Effects of post-absorptive and postprandial exercise on glucoregulation in metabolic syndrome. Obesity (Silver Spring), 2007, 15: 704–711. [DOI] [PubMed] [Google Scholar]
- 8.Raastad T, Bjøro T, Hallén J: Hormonal responses to high- and moderate-intensity strength exercise. Eur J Appl Physiol, 2000, 82: 121–128. [DOI] [PubMed] [Google Scholar]
- 9.Hässig A, Wen-Xi L, Stampfli K: Stress-induced suppression of the cellular immune reactions: on the neuroendocrine control of the immune system. Med Hypotheses, 1996, 46: 551–555. [DOI] [PubMed] [Google Scholar]
- 10.Orth DN: Corticotropin-releasing hormone in humans. Endocr Rev, 1992, 13: 164–191. [DOI] [PubMed] [Google Scholar]
- 11.Layman DK, Evans E, Baum JI, et al. : Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr, 2005, 135: 1903–1910. [DOI] [PubMed] [Google Scholar]
- 12.Bruce RA: Multi-stage treadmill tests of maximal and submaximal exercise. In exercise testing and training of apparently healthy individuals: A handbook for physicians. Ney York: Amercian Heart Association, 1972, pp 32–34. [Google Scholar]
- 13.Borer KT: Exercise endocrinology. Champaign: Human Kinetics, 2003. [Google Scholar]
- 14.Luciano E, Carneiro EM, Carvalho CR, et al. : Endurance training improves responsiveness to insulin and modulates insulin signal transduction through the phosphatidylinositol 3-kinase/Akt-1 pathway. Eur J Endocrinol, 2002, 147: 149–157. [DOI] [PubMed] [Google Scholar]
- 15.Aoi W, Ichiishi E, Sakamoto N, et al. : Effect of exercise on hepatic gene expression in rats: a microarray analysis. Life Sci, 2004, 75: 3117–3128. [DOI] [PubMed] [Google Scholar]
- 16.Peres SB, de Moraes SM, Costa CE, et al. : Endurance exercise training increases insulin responsiveness in isolated adipocytes through IRS/PI3-kinase/Akt pathway. J Appl Physiol 1985, 2005, 98: 1037–1043. [DOI] [PubMed] [Google Scholar]
- 17.Flores MB, Fernandes MF, Ropelle ER, et al. : Exercise improves insulin and leptin sensitivity in hypothalamus of Wistar rats. Diabetes, 2006, 55: 2554–2561. [DOI] [PubMed] [Google Scholar]
- 18.Ropelle ER, Pauli JR, Cintra DE, et al. : Acute exercise modulates the Foxo1/PGC-1alpha pathway in the liver of diet-induced obesity rats. J Physiol, 2009, 587: 2069–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pauli JR, Ropelle ER, Cintra DE, et al. : Acute physical exercise reverses S-nitrosation of the insulin receptor, insulin receptor substrate 1 and protein kinase B/Akt in diet-induced obese Wistar rats. J Physiol, 2008, 586: 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dachicourt N, Serradas P, Giroix MH, et al. : Decreased glucose-induced cAMP and insulin release in islets of diabetic rats: reversal by IBMX, glucagon, GIP. Am J Physiol, 1996, 271: E725–E732. [DOI] [PubMed] [Google Scholar]
- 21.Carmen GY, Víctor SM: Signalling mechanisms regulating lipolysis. Cell Signal, 2006, 18: 401–408. [DOI] [PubMed] [Google Scholar]
- 22.Guyton AC, Hall JE: Textbook of medical physiology, 11th ed. 2002, pp 956. [Google Scholar]
- 23.Bergendahl M, Vance ML, Iranmanesh A, et al. : Fasting as a metabolic stress paradigm selectively amplifies cortisol secretory burst mass and delays the time of maximal nyctohemeral cortisol concentrations in healthy men. J Clin Endocrinol Metab, 1996, 81: 692–699. [DOI] [PubMed] [Google Scholar]
- 24.Møller N, Nørrelund H: The role of growth hormone in the regulation of protein metabolism with particular reference to conditions of fasting. Horm Res, 2003, 59: 62–68. [DOI] [PubMed] [Google Scholar]
- 25.Duclos M, Gouarne C, Bonnemaison D: Acute and chronic effects of exercise on tissue sensitivity to glucocorticoids. J Appl Physiol 1985, 2003, 94: 869–875. [DOI] [PubMed] [Google Scholar]
- 26.Ehrnborg C, Lange KH, Dall R, et al. GH-2000 Study Group: The growth hormone/insulin-like growth factor-I axis hormones and bone markers in elite athletes in response to a maximum exercise test. J Clin Endocrinol Metab, 2003, 88: 394–401. [DOI] [PubMed] [Google Scholar]
- 27.Djurhuus CB, Gravholt CH, Nielsen S, et al. : Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. Am J Physiol Endocrinol Metab, 2004, 286: E488–E494. [DOI] [PubMed] [Google Scholar]
- 28.Jefferies WM: Cortisol and immunity. Med Hypotheses, 1991, 34: 198–208. [DOI] [PubMed] [Google Scholar]
- 29.Shih KC, Hsieh SH, Kwok CF, et al. : Effect of growth hormone on dawn phenomenon in patients with type 2 diabetes. Growth Factors, 2013, 31: 66–73. [DOI] [PubMed] [Google Scholar]
- 30.Purnell JQ, Kahn SE, Samuels MH, et al. : Enhanced cortisol production rates, free cortisol, and 11beta-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. Am J Physiol Endocrinol Metab, 2009, 296: E351–E357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alesci S, De Martino MU, Ilias I, et al. : Glucocorticoid-induced osteoporosis: from basic mechanisms to clinical aspects. Neuroimmunomodulation, 2005, 12: 1–19. [DOI] [PubMed] [Google Scholar]
- 32.Kelly JJ, Mangos G, Williamson PM, et al. : Cortisol and hypertension. Clin Exp Pharmacol Physiol, 1998, 25: S51–S56. [DOI] [PubMed] [Google Scholar]


