Abstract
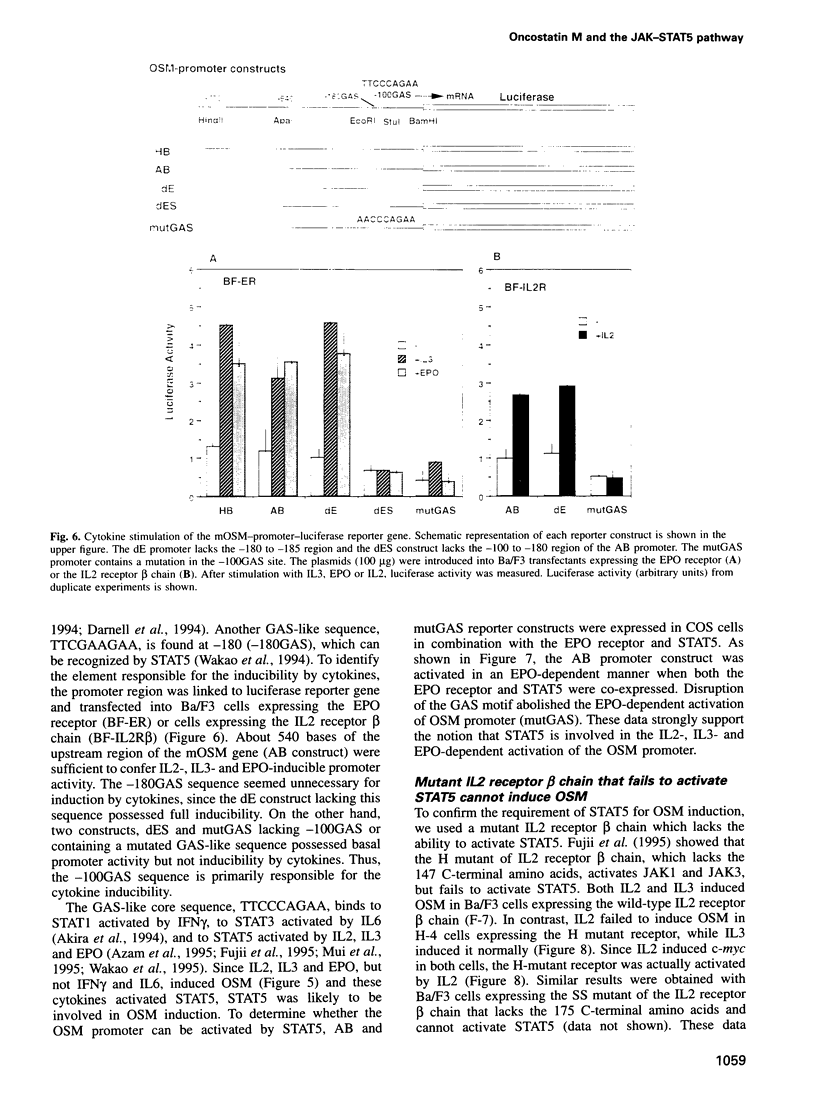
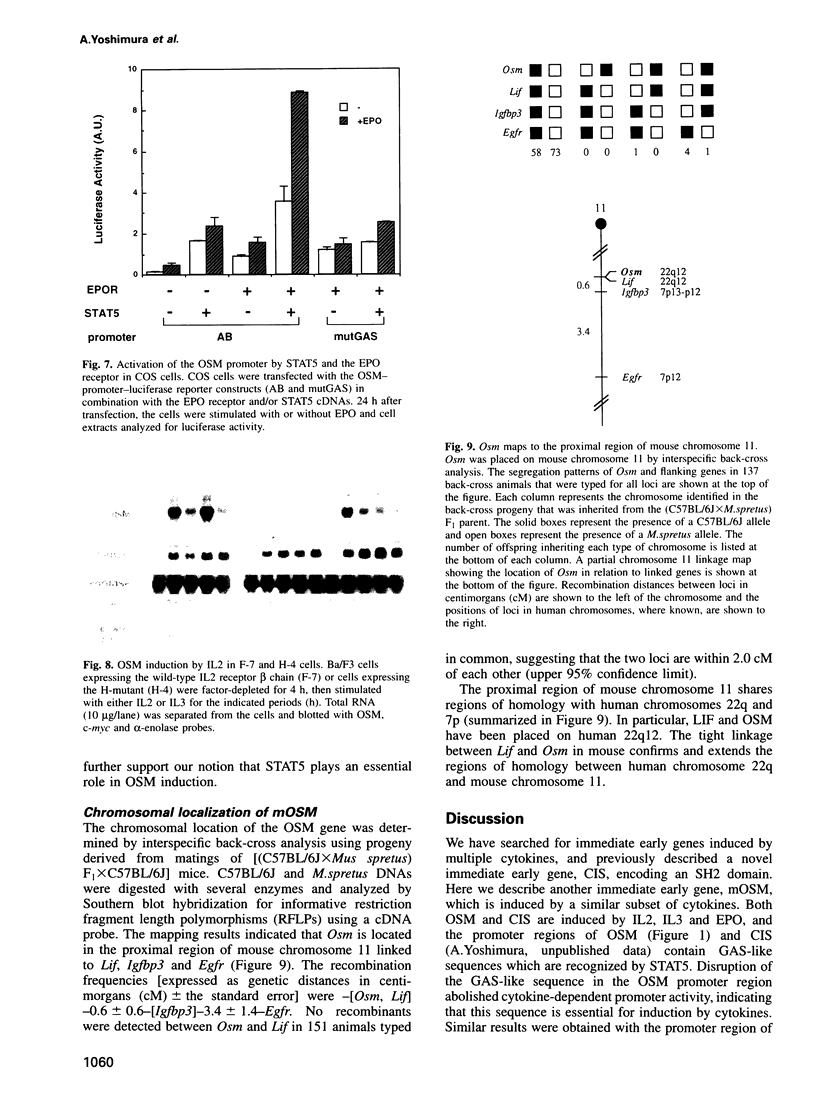
Oncostatin M (OSM) is a member of the interleukin-6 (IL6)-related cytokine subfamily that includes IL6, IL11, leukemia inhibitory factor (LIF), ciliary neurotrophic factor and cardiotrophin-1. While human OSM has been characterized and the bovine OSM gene was recently cloned, the murine counterpart had not been identified. Here we describe molecular cloning of murine OSM as an immediate early gene induced by a subset of cytokines including IL2, IL3 and erythropoietin (EPO) in myeloid and lymphoid cell lines. The induction kinetics of OSM are rapid and transient, reaching a maximal level within 30-60 min and decreasing thereafter. Induction of OSM depends on the signals generated by the membrane-proximal region of the EPO receptor as well as that of the beta chain of the IL3/GM-CSF receptor, which activate JAK2 and STAT5. About 100 bases upstream of the transcription initiation site of the OSM gene contains a possible STAT5 binding site which is essential for IL2, IL3 and EPO-dependent promoter activity of the OSM gene. Expression of STAT5 and the EPO receptor in COS cells conferred EPO-dependent activation of the OSM promoter. Moreover, the mutant IL2 receptor lacking the ability to activate STAT5 induced c-myc but failed to induce OSM. Thus OSM is one of the common targets of a subset of cytokines that activate STAT5. The murine OSM gene is located near to the LIF gene, expressed at high levels in bone marrow and possesses similar biological activity to human OSM. Identification of murine OSM as a cytokine-inducible immediate early gene provides a new insight into the physiological function of this unique cytokine.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Azam M., Erdjument-Bromage H., Kreider B. L., Xia M., Quelle F., Basu R., Saris C., Tempst P., Ihle J. N., Schindler C. Interleukin-3 signals through multiple isoforms of Stat5. EMBO J. 1995 Apr 3;14(7):1402–1411. doi: 10.1002/j.1460-2075.1995.tb07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. J., Rowe J. M., Liu J. W., Shoyab M. Regulation of IL-6 expression by oncostatin M. J Immunol. 1991 Oct 1;147(7):2175–2180. [PubMed] [Google Scholar]
- Bruce A. G., Hoggatt I. H., Rose T. M. Oncostatin M is a differentiation factor for myeloid leukemia cells. J Immunol. 1992 Aug 15;149(4):1271–1275. [PubMed] [Google Scholar]
- Bruce A. G., Linsley P. S., Rose T. M. Oncostatin M. Prog Growth Factor Res. 1992;4(2):157–170. doi: 10.1016/0955-2235(92)90029-h. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 1991 Apr;7(4):113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Frohman M. A. Rapid amplification of complementary DNA ends for generation of full-length complementary DNAs: thermal RACE. Methods Enzymol. 1993;218:340–356. doi: 10.1016/0076-6879(93)18026-9. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Schindler C., Improta T., Aebersold R., Darnell J. E., Jr The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7840–7843. doi: 10.1073/pnas.89.16.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Nakagawa Y., Schindler U., Kawahara A., Mori H., Gouilleux F., Groner B., Ihle J. N., Minami Y., Miyazaki T. Activation of Stat5 by interleukin 2 requires a carboxyl-terminal region of the interleukin 2 receptor beta chain but is not essential for the proliferative signal transmission. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5482–5486. doi: 10.1073/pnas.92.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing D. P., Comeau M. R., Friend D. J., Gimpel S. D., Thut C. J., McGourty J., Brasher K. K., King J. A., Gillis S., Mosley B. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992 Mar 13;255(5050):1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- Giovannini M., Djabali M., McElligott D., Selleri L., Evans G. A. Tandem linkage of genes coding for leukemia inhibitory factor (LIF) and oncostatin M (OSM) on human chromosome 22. Cytogenet Cell Genet. 1993;64(3-4):240–244. doi: 10.1159/000133586. [DOI] [PubMed] [Google Scholar]
- Grove R. I., Eberhardt C., Abid S., Mazzucco C., Liu J., Kiener P., Todaro G., Shoyab M. Oncostatin M is a mitogen for rabbit vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):823–827. doi: 10.1073/pnas.90.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Harada N., Mitsui H., Miura T., Ishizaka T., Miyajima A. Characterization of cell phenotype by a novel cDNA library subtraction system: expression of CD8 alpha in a mast cell-derived interleukin-4-dependent cell line. Blood. 1994 Jul 1;84(1):189–199. [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Horn D., Fitzpatrick W. C., Gompper P. T., Ochs V., Bolton-Hansen M., Zarling J., Malik N., Todaro G. J., Linsley P. S. Regulation of cell growth by recombinant oncostatin M. Growth Factors. 1990;2(2-3):157–165. doi: 10.3109/08977199009071502. [DOI] [PubMed] [Google Scholar]
- Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994 Sep 16;265(5179):1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou K., James P. L., Clemmons D. R., Copeland N. G., Gilbert D. J., Jenkins N. A., Rotwein P. Identification of two clusters of mouse insulin-like growth factor binding protein genes on chromosomes 1 and 11. Genomics. 1994 Jun;21(3):653–655. doi: 10.1006/geno.1994.1329. [DOI] [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Kallestad J., Ochs V., Neubauer M. Cleavage of a hydrophilic C-terminal domain increases growth-inhibitory activity of oncostatin M. Mol Cell Biol. 1990 May;10(5):1882–1890. doi: 10.1128/mcb.10.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik N., Haugen H. S., Modrell B., Shoyab M., Clegg C. H. Developmental abnormalities in mice transgenic for bovine oncostatin M. Mol Cell Biol. 1995 May;15(5):2349–2358. doi: 10.1128/mcb.15.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik N., Kallestad J. C., Gunderson N. L., Austin S. D., Neubauer M. G., Ochs V., Marquardt H., Zarling J. M., Shoyab M., Wei C. M. Molecular cloning, sequence analysis, and functional expression of a novel growth regulator, oncostatin M. Mol Cell Biol. 1989 Jul;9(7):2847–2853. doi: 10.1128/mcb.9.7.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
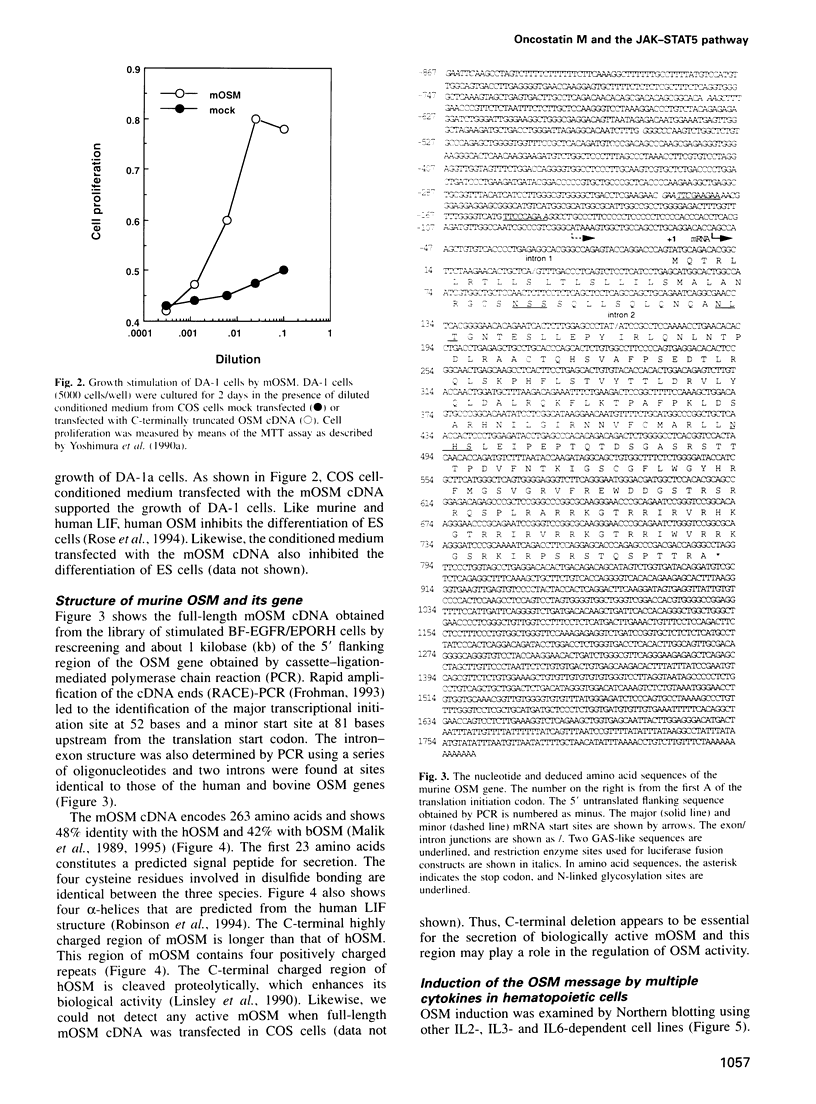
- Maruyama K., Miyata K., Yoshimura A. Proliferation and erythroid differentiation through the cytoplasmic domain of the erythropoietin receptor. J Biol Chem. 1994 Feb 25;269(8):5976–5980. [PubMed] [Google Scholar]
- Matsuda T., Hirano T., Kishimoto T. Establishment of an interleukin 6 (IL 6)/B cell stimulatory factor 2-dependent cell line and preparation of anti-IL 6 monoclonal antibodies. Eur J Immunol. 1988 Jun;18(6):951–956. doi: 10.1002/eji.1830180618. [DOI] [PubMed] [Google Scholar]
- Miles S. A., Martínez-Maza O., Rezai A., Magpantay L., Kishimoto T., Nakamura S., Radka S. F., Linsley P. S. Oncostatin M as a potent mitogen for AIDS-Kaposi's sarcoma-derived cells. Science. 1992 Mar 13;255(5050):1432–1434. doi: 10.1126/science.1542793. [DOI] [PubMed] [Google Scholar]
- Mui A. L., Wakao H., O'Farrell A. M., Harada N., Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995 Mar 15;14(6):1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B. C., DeVico A. L., Nakamura S., Copeland T. D., Chen Y., Patel A., O'Neil T., Oroszlan S., Gallo R. C., Sarngadharan M. G. Identification of a major growth factor for AIDS-Kaposi's sarcoma cells as oncostatin M. Science. 1992 Mar 13;255(5050):1430–1432. doi: 10.1126/science.1542792. [DOI] [PubMed] [Google Scholar]
- Ohashi H., Maruyama K., Liu Y. C., Yoshimura A. Ligand-induced activation of chimeric receptors between the erythropoietin receptor and receptor tyrosine kinases. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):158–162. doi: 10.1073/pnas.91.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallard C., Gouilleux F., Bénit L., Cocault L., Souyri M., Levy D., Groner B., Gisselbrecht S., Dusanter-Fourt I. Thrombopoietin activates a STAT5-like factor in hematopoietic cells. EMBO J. 1995 Jun 15;14(12):2847–2856. doi: 10.1002/j.1460-2075.1995.tb07284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., King K. L., Shaw K. J., Luis E., Rullamas J., Luoh S. M., Darbonne W. C., Knutzon D. S., Yen R., Chien K. R. Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1142–1146. doi: 10.1073/pnas.92.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose T. M., Weiford D. M., Gunderson N. L., Bruce A. G. Oncostatin M (OSM) inhibits the differentiation of pluripotent embryonic stem cells in vitro. Cytokine. 1994 Jan;6(1):48–54. doi: 10.1016/1043-4666(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Sato N., Sakamaki K., Terada N., Arai K., Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling. EMBO J. 1993 Nov;12(11):4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Stahl J., Gearing D. P., Willson T. A., Brown M. A., King J. A., Gough N. M. Structural organization of the genes for murine and human leukemia inhibitory factor. Evolutionary conservation of coding and non-coding regions. J Biol Chem. 1990 May 25;265(15):8833–8841. [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994 May 1;13(9):2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H., Harada N., Kitamura T., Mui A. L., Miyajima A. Interleukin 2 and erythropoietin activate STAT5/MGF via distinct pathways. EMBO J. 1995 Jun 1;14(11):2527–2535. doi: 10.1002/j.1460-2075.1995.tb07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring P. M., Waring L. J., Billington T., Metcalf D. Leukemia inhibitory factor protects against experimental lethal Escherichia coli septic shock in mice. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1337–1341. doi: 10.1073/pnas.92.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Zhong Z., Darnell J. E., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995 Jul 28;82(2):241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Quelle F. W., Thierfelder W. E., Kreider B. L., Gilbert D. J., Jenkins N. A., Copeland N. G., Silvennoinen O., Ihle J. N. Stat4, a novel gamma interferon activation site-binding protein expressed in early myeloid differentiation. Mol Cell Biol. 1994 Jul;14(7):4342–4349. doi: 10.1128/mcb.14.7.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., D'Andrea A. D., Lodish H. F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Longmore G., Lodish H. F. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990 Dec 13;348(6302):647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Ohkubo T., Kiguchi T., Jenkins N. A., Gilbert D. J., Copeland N. G., Hara T., Miyajima A. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995 Jun 15;14(12):2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Zimmers T., Neumann D., Longmore G., Yoshimura Y., Lodish H. F. Mutations in the Trp-Ser-X-Trp-Ser motif of the erythropoietin receptor abolish processing, ligand binding, and activation of the receptor. J Biol Chem. 1992 Jun 5;267(16):11619–11625. [PubMed] [Google Scholar]
- Zarling J. M., Shoyab M., Marquardt H., Hanson M. B., Lioubin M. N., Todaro G. J. Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9739–9743. doi: 10.1073/pnas.83.24.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Blenis J., Li H. C., Schindler C., Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995 Mar 31;267(5206):1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4806–4810. doi: 10.1073/pnas.91.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994 Apr 1;264(5155):95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]