Abstract
The WHO 2007 classification of tumors of the CNS distinguishes between diffuse astrocytoma WHO grade II (A IIWHO2007) and anaplastic astrocytoma WHO grade III (AA III WHO2007). Patients with A II WHO2007 are significantly younger and survive significantly longer than those with AA III WHO2007. So far, classification and grading relies on morphological grounds only and does not yet take into account IDH status, a molecular marker of prognostic relevance. We here demonstrate that WHO 2007 grading performs poorly in predicting prognosis when applied to astrocytoma carrying IDH mutations. Three independent series including a total of 1360 adult diffuse astrocytic gliomas with IDH mutation containing 683 A II IDHmut, 562 AA III IDHmut and 115 GBM IDHmut have been examined for age distribution and survival. In all three series patients with A II IDHmut and AA III IDHmut were of identical age at presentation of disease (36–37 years) and the difference in survival between grades was much less (10.9 years for A II IDHmut, 9.3 years for AA III IDHmut) than that reported for A II WHO2007 versus AA III WHO2007. Our analyses imply that the differences in age and survival between A II WHO2007 and AA III WHO2007 predominantly depend on the fraction of IDH-non-mutant astrocytomas in the cohort. This data poses a substantial challenge for the current practice of astrocytoma grading and risk stratification and is likely to have far-reaching consequences on the management of patients with IDH-mutant astrocytoma.
Introduction
Grading of adult diffuse astrocytomas and anaplastic astrocytomas according to WHO grades II (A II WHO2007) and III (AA III WHO2007) has been well established over the last 20 years. While the numbers for age and survival given by different studies vary, there is consensus that patients with A II WHO2007 are significantly younger and survive significantly longer than patients with AA III WHO2007. According to WHO 2007 patients with A II WHO2007 average 34 years and have a survival of 6–8 years, those with AA III WHO2007 average 45 years and survive approximately 2 years [10]. In an extensive population-based series patients with A II WHO2007 of the fibrillary subtype averaged 40 years and patients with AA III WHO2007 averaged 46 years and followed a markedly worse clinical course with an average survival of 1.6 years as opposed to 5.6 years in A II WHO2007 [13].
The current parameters for the discrimination between WHO grades II and III are cellular differentiation, presence or absence of early vascular changes and proliferation. Proliferation is of superior impact as otherwise well-differentiated astrocytomas with inconspicuous vessels but more than only very few mitoses are considered as WHO grade III with all clinical consequences attached to this diagnostic decision.
The identification of IDH mutations in the majority of astrocytomas [2, 7, 21, 25] and concurrent analyses revealed that among AA III WHO2007 the presence of these mutations was associated with a significant better clinical course [6, 17, 23], an observation not observed for A II WHO2007 [1, 20]. Numerous studies indicate the possibility that worse outcome of AA III WHO2007 compared to A II WHO2007 is strongly influenced by the inclusion of cases that indeed molecularly represent glioblastoma (GBM). Consequently, the inclusion of IDH mutational status into future astrocytoma classifications is discussed and has been proposed by the Haarlem international consensus meeting [11].
A point so far not sufficiently addressed is the feasibility of the current WHO criteria for grading IDH-mutated astrocytoma. We here provide evidence that application of the WHO2007 grading criteria on IDH-mutant astrocytoma lacks the power it had in distinguishing A II and AA III before exclusion of IDH wild-type tumors. We here present evidence that IDH-mutant diffuse astrocytoma graded according to the WHO2007 criteria (A II) and their anaplastic counterparts (AA IIIIDHmut) represent closely related groups which are virtually identical in patient age and that show only little difference in overall survival.
Materials and methods
Data collection
Data were derived from the files of the Department of Neuropathology Heidelberg (n = 866), a recently published series originating from the Department of Pathology MD Anderson Cancer Center and from the VU University Medical Center/The Netherlands (n = 263) [14], and a recently published series by the TCGA (n = 231) [3]. Therefore, the results here are in part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/. Level 1 450k methylation data and sequence data were downloaded from the open-source TCGA pages (https://tcga-data.nci.nih.gov/tcga/). Inclusion criteria were the diagnosis of a diffuse astrocytic glioma including diffuse astrocytoma, anaplastic astrocytoma, presence of an IDH mutation, absence of 1p/19q codeletion (1p/19q codel), information on age and gender available and patient age of 18 or older. TCGA and Heidelberg patients with GBM and IDH mutation were also included. For the TCGA series the presence of 450k methylation data were required. Cases included are listed in Table 1.
Table 1.
Clinical data of 1360 tumors with integrated diagnosis included
| Series | Integrated diagnosis | Number | Average age | Median age | Median OS |
p value A II versus AA III |
|
|---|---|---|---|---|---|---|---|
| MD Anderson | A II IDHmut | 132 | 37.6 | 36 | n = 263 | 4104 | 0.432 |
| AA III IDHmut | 131 | 37.1 | 35 | 3801 | |||
| Heidelberg | A II IDHmut | 437 | 38.8 | 37 | n = 68 | Not reached | 0.093 |
| AA III IDHmut | 324 | 39.1 | 37 | 5100 | |||
| GBM IDHmut | 105 | 42.0 | 40 | ||||
| TCGA | A II IDHmut | 114 | 37.5 | 36 | n = 221 | 2875 | 0.059 |
| AA III IDHmut | 107 | 38.2 | 36 | 2052 | |||
| GBM IDHmut | 10 | 40.9 | 42 |
Survival data for the Heidelberg series was only available from 68 patients with astrocytomas
OS overall survival in days
IDH and 1p/19q status
IDH and 1p/19q status in the MD Anderson series had been analyzed by IDH1-R132H immunohistochemistry, Sanger sequencing for IDH1 and IDH2 and by fluorescence in situ hybridization or microsatellite analysis for chromosomal arms 1p and 19q. A subset of samples was analyzed using low-pass whole genome sequencing for 1p/19q analysis [14]. The TCGA series was reanalyzed on basis of the Illumina Infinium HumanMethylation450 BeadChip (450k) array (Illumina, San Diego, USA). The array data were used to calculate a low-resolution copy number profile (CNP) as previously described [18]. The data were analyzed as previously described to allot the tumors to either a G-CIMP or a non-G-CIMP cluster [12, 24]. Furthermore, the TCGA exome sequencing data allowed to directly score IDH mutations. The cases of the Heidelberg series have been sequenced for IDH1 and IDH2 and the majority of the cases were analyzed by fluorescence in situ hybridization or microsatellite analysis for chromosomal arms 1p and 19q. In more recent cases the 1p/19 status has been determined by calculating a copy number profile on basis of 450k methylation data.
Classification and grading
An integrated diagnosis following the preliminary suggestions of the Haarlem consensus meeting [11, 16] was established with tumor classification as IDH-mutated astrocytoma depending on the absence of 1p/19q codel. Tumor grading was performed using the WHO 2007 grading parameters differentiation, cellularity, proliferation and extent of microvascular proliferation. IDH-mutated GBM exhibited additional necrosis in the absence of 1p/19q codel.
Statistics
Boxplots were generated using the statistical software R. Kaplan–Meier plots and log-rank tests were conducted by Partek GenomicsSuite software (Partek, St. Louis USA) and MedCalc software (Ostende, Belgium).
Results and discussion
Age distribution
The present analysis includes a total of 1360 adult diffuse astrocytic tumors with IDH mutation from three independent series (Table 1). Age distribution for IDH-mutant tumors with an integrated diagnosis that included both histological interpretation and molecular analysis [16] varied from those published for tumors classified according to WHO. Most notably, there was no significant difference in patient age between A II IDHmut and AA III IDHmut both averaging 38 years as opposed to an approximate 6–12 years difference between A II WHO2007 and AA III WHO2007 [10, 13]. Also, the age of GBM IDHmut averaging 42 years was only 4 years higher than that of A II IDHmut and AA III IDHmut. It is likely that the difference in age between patients with integrated diagnoses versus morphology-based WHO2007 diagnoses is due to the confounding by IDH wild-type tumors with morphologic characteristics of AA, which from a biologic point of view predominantly represent underdiagnosed GBM, since they generally exhibit classic molecular hallmarks of GBM, including loss of chromosome 10, gain of chromosome 7 and frequently amplification of EGFR [3]. The strong age effect of eliminating “IDH wild type anaplastic astrocytoma WHO grade III” raises the question on the effect of eliminating “IDH wild type diffuse astrocytoma WHO grade II”. A recent study found an age difference of 4 years between IDH-mutated and wild-type diffuse astrocytomas [1]. These tumors are presumed to represent a mixture of different tumor types [3]. We assume that this mixed bag likely contains patients with tumors having an higher average age (i.e., GBM) and others with a younger average age (i.e., DNT, pilocytic astrocytoma, ganglioglioma) than that of diffuse astrocytomas thus exerting less influence on the average age of WHO grade II astrocytomas.
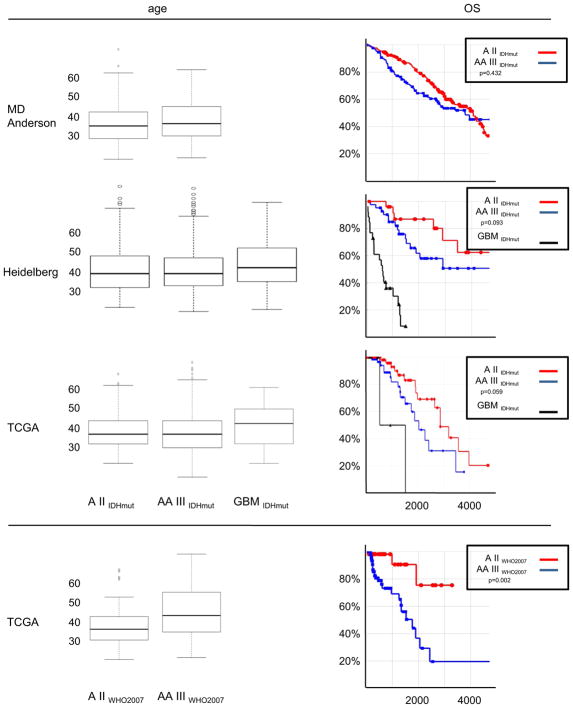
Box plots of patient ages with integrated diagnoses for the three studies are shown in the left panels of Fig. 1. Interestingly, not only average and median age of IDH-mutated astrocytomas are very similar, but also the age distributions among both grades are virtually identical (Fig. 2).
Fig. 1.
Box-plots (left panels) and survival statistics (right panels). A II IDHmut in red, AA III IDHmut in blue and GBM IDHmut in black. Three upper pairs depict the IDH-mutant only series from MD Anderson, Heidelberg and TCGA after receiving an integrated diagnosis. The lower pair depicts the TCGA A II WHO2007 and A III WHO2007 without exclusion of IDH wild-type cases. Survival on X-axis in right panels is given in days
Fig. 2.
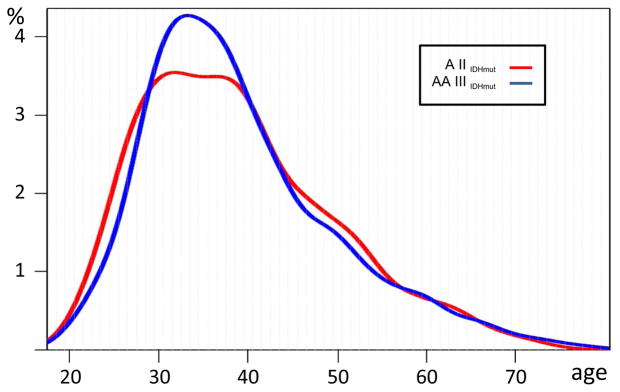
Age distribution of first tumor diagnosis of A II IDHmut (red) and AA III IDHmut (blue) of 1245 tumors of the combined MD Anderson, TCGA and Heidelberg series. Plots are normalized
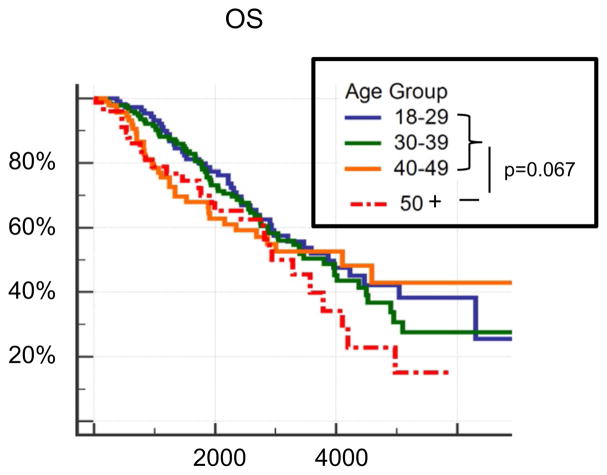
Age has been determined to be a strong prognostic factor for malignant glioma. Recent analysis of the effect of age revealed no significant impact of age on survival in A II IDHmut and AA III IDHmut; however, a significant impact was observed in IDH wild-type astrocytomas [14]. Analysis of the age impact on survival in the present three series revealed no difference. Stratification of patients in four age groups, 18–29, 30–39, 40–49 and over 50 demonstrated a trend (p = 0.067; log-rank test) for patients over 50 exhibiting a poorer outcome (Fig. 3). Whether this is a truly tumor inherent effect is questionable in light of the increased mortality from a spectrum of other causes of patients over the age of 50. Nevertheless, the effect of age on the survival in patients with IDH-mutant astrocytoma is lower than assumed from the previous series [4, 8, 9, 15] not stratified for this molecular hallmark.
Fig. 3.
Age-related survival (in days) in combined group of WHO II and III IDH-mutant from all three series
Clinical course
The natural clinical course of gliomas cannot be determined any more as nearly all patients diagnosed with anaplastic WHO grade III glioma will have received post-surgical adjuvant therapy and a considerable fraction of patients with WHO grade II glioma does so, too. Thus the groups compared here are very heterogeneous with respect to treatment and therefore comparisons of survival time need to ignore the potential beneficial effect of therapy.
The effect of grading on survival was detectable among IDH-mutated astrocytomas but not as substantial as would be expected from the experience with WHO2007 graded astrocytomas. In the MD Anderson series there was no significant difference (p = 0.432; log-rank test) in overall survival (OS) between patients receiving the integrated diagnoses A II IDHmut and AA III IDHmut. Cases from TCGA (p = 0.059; log-rank test) and those from the Heidelberg series (p = 0.093) with the integrated diagnoses of A II IDHmut tended to fare better than those AA III IDHmut; however, the difference was not significant in each cohort. Median OS for all three groups combined was 3978 days (10.9 years) for A II IDHmut and 3404 days (9.3 years) for AA III IDHmut and the difference was also not significant (p =0.055; log-rank test).
In fact, the difference in survival of A II IDHmut and AA III IDHmut in comparison to A II WHO2007 and AA III WHO2007 can be demonstrated on the TCGA series. While the effect of grading is not significant in the IDH-mutated astrocytomas (p = 0.059; log-rank test), the difference in A II WHO2007 and AA III WHO2007 (p = 0.002; log-rank test), including both IDH-mutant and IDH wild-type cases, is as marked as expected and matches well with other WHO graded series. Quite obvious is the initial strong drop in survivals of the AA III WHO2007 patients due to the tumors identified as GBMs by molecular means. As expected the slope of this survival curve approaches that for AA III IDHmut at longer survival times.
Kaplan–Meier diagrams for OS of patients with integrated diagnoses for the three studies and for WHO graded TCGA cases are shown in the right panels of Fig. 1.
Interestingly, a previous [5] as well as a more recent study by the German Glioma Net (GGN) also failed to see a significant difference in OS between IDH-mutated astrocytoma receiving WHO grades II and III [22]. The lack of grading impact on OS in those IDH-mutated astrocytomas nicely matches the observations by the MD Anderson, the Heidelberg and the TCGA series. Further, in a highly actual report based on exome sequencing of WHO grade II and WHO grade III patients from Japan as well as analysis of the low-grade glioma data provided by TCGA no substantial effect of WHO grade on overall survival in IDH-mutated gliomas was reported [19].
Implications for future diagnostic approach
Prior studies showing substantial survival difference between grade II and III astrocytoma included a mix of IDH-mutant and IDH wild-type cases, and in keeping with current thinking, these entities are best kept distinct, based on their biologic and clinical differences. Accordingly, the results clearly indicate that current WHO grading lacks power in prognosticating the clinical course of IDH-mutant astrocytoma into distinct patient risk groups. We believe that there is insufficient evidence for the use of current morphological criteria (mainly mitotic activity) as the basis for the diagnosis of anaplasia with all clinical consequences that follow from this designation. The MD Anderson series has previously been analyzed for the impact of mitotic count on survival and there was no survival difference between IDH-mutated astrocytomas with single mitoses and those with 4 or more mitoses per 1000 tumor cells [14]. Following WHO 2007 criteria astrocytomas with 4 or more mitoses per 10 high power fields would be graded WHO III and patients would receive aggressive postoperative treatment. It appears plausible that identifying factors that define survival risk groups will require different parameters as compared to those applied in WHO2007.
In fact, it was previously shown that GBM IDHmut exhibit clinical outcomes similar or slightly favorable to that of WHO grade III anaplastic astrocytomas without IDH mutation [6]. This raises the question whether IDH-mutant tumors with histologic features of GBM should receive a WHO grade III designation. It also raises the question whether the term GBM is appropriate for these tumors, which while conceivable from the historic point of view lacks a logical basis when looking at the genetics and the clinical outcome of these tumors.
Conclusions
The data show that IDH-mutant astrocytomas WHO grades II and III are much more similar in respect to age at presentation and overall survival than suggested by previous series not separated for IDH status. Our current grading algorithms are insufficient for differentiating between grades II and III in IDH-mutant astrocytoma.
Acknowledgments
AO was supported by the National Institutes of Health/National Cancer Institute (Training Grant No. 5T32CA163185). FS is a fellow of the Medical Faculty Heidelberg Physician-Scientist Program. PW and JCR were supported by grants of the Dutch Cancer Society (Grant No. VU 2009-4470) and Foundation ‘STOPHersentumoren.nl’. AvD was supported by the Bundesministerium fuer Bildung und Forschung (BMBF) program “SYS-GLIO”.
References
- 1.Ahmadi R, Stockhammer F, Becker N, Hohlen K, Misch M, Christians A, Dictus C, Herold-Mende C, Capper D, Unterberg A, et al. No prognostic value of IDH1 mutations in a series of 100 WHO grade II astrocytomas. J Neuro Oncol. 2012;109:15–22. doi: 10.1007/s11060-012-0863-y. [DOI] [PubMed] [Google Scholar]
- 2.Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 3.Brat D. Comprehensive, integrative genomic analysis of diffuse lower grade gliomas. N Engl J Med. doi: 10.1056/NEJMoa1402121. (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daumas-Duport C, Scheithauer B, O’Fallon J, Kelly P. Grading of astrocytomas. A simple and reproducible method. Cancer. 1988;62:2152–2165. doi: 10.1002/1097-0142(19881115)62:10<2152::aid-cncr2820621015>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Gorovets D, Kannan K, Shen R, Kastenhuber ER, Islamdoust N, Campos C, Pentsova E, Heguy A, Jhanwar SC, Mellinghoff IK, et al. IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res. 2012;18:2490–2501. doi: 10.1158/1078-0432.CCR-11-2977. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1 mutated glioblastomas and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120:707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 7.Ichimura K, Pearson DM, Kocialkowski S, Backlund LM, Chan R, Jones DT, Collins VP. IDH1 mutations are present in the majority of common adult gliomas but are rare in primary glioblastomas. Neuro Oncol. 2009;11:341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. (discussion 226–219) [DOI] [PubMed] [Google Scholar]
- 9.Laws ER, Jr, Taylor WF, Clifton MB, Okazaki H. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg. 1984;61:665–673. doi: 10.3171/jns.1984.61.4.0665. [DOI] [PubMed] [Google Scholar]
- 10.Louis D, Ohgaki H, Wiestler O, Cavenee W. World Health Organization Classification of tumours of the central nervous system. In: Bosman F, Jaffe E, Lakhani S, Ohgaki H, editors. World Health Organization Classification of tumours. 4. IARC Press; Lyon: 2007. [Google Scholar]
- 11.Louis DN, Perry A, Burger P, Ellison DW, Reifenberger G, von Deimling A, Aldape K, Brat D, Collins VP, Eberhart C, et al. Brain Pathol. City: 2014. International society of neuropathology-haarlem consensus guidelines, for nervous system tumor classification and grading; pp. 429–435. 2014/07/06 edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Pan F, Pelloski CE, Sulman EP, Bhat KP, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 14.Olar A, Wani KM, Alfaro-Munoz KD, Heathcock LE, van Thuijl HF, Gilbert MR, Armstrong TS, Sulman EP, Cahill DP, Vera-Bolanos E, et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathol. 2015;129:585–596. doi: 10.1007/s00401-015-1398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philippon JH, Clemenceau SH, Fauchon FH, Foncin JF. Supratentorial low-grade astrocytomas in adults. Neurosurgery. 1993;32:554–559. doi: 10.1227/00006123-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C, Schweizer L, Korshunov A, Jones DT, Hovestadt V, et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015;129:133–146. doi: 10.1007/s00401-014-1370-3. [DOI] [PubMed] [Google Scholar]
- 17.Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, Hallani SE, Boisselier B, Mokhtari K, Hoang-Xuan K, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 18.Sturm D, Witt H, Hovestadt V, Khuong Quang D-A, Jones D, Konermann C, Pfaff E, Tönjes M, Sill M, Bender S, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Aoki K, Chiba K, Sato Y, Shiozawa Y, Shiraishi Y, Shimamura T, Niida A, Motomura K, Ohka F, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47:458–468. doi: 10.1038/ng.3273. [DOI] [PubMed] [Google Scholar]
- 20.Thon N, Eigenbrod S, Kreth S, Lutz J, Tonn JC, Kretzschmar H, Peraud A, Kreth FW. IDH1 mutations in grade II astrocytomas are associated with unfavorable progression-free survival and prolonged postrecurrence survival. Cancer. 2012;118:452–460. doi: 10.1002/cncr.26298. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:653–656. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weller M, Weber RG, Willscher E, Riehmer V, Hentschel B, Kreuz M, Felsberg J, Beyer U, Loffler-Wirth H, Kaulich K, et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129:679–693. doi: 10.1007/s00401-015-1409-0. [DOI] [PubMed] [Google Scholar]
- 23.Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, Sabel M, Koeppen S, Ketter R, Meyermann R, et al. NOA-04 randomized phase III trial of sequential radio-chemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 24.Wiestler B, Capper D, Sill M, Jones DT, Hovestadt V, Sturm D, Koelsche C, Bertoni A, Schweizer L, Korshunov A, et al. Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol. 2014;128:561–571. doi: 10.1007/s00401-014-1315-x. [DOI] [PubMed] [Google Scholar]
- 25.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al. IDH1 and IDH2 Mutations in Gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]