Abstract
Introduction
Tumor invasion in lung adenocarcinoma is defined as infiltration of stroma, blood vessels, or pleura. Based on observation of tumor spread through air spaces (STAS), we considered whether this could represent new patterns of invasion and investigated whether it correlated with locoregional versus distant recurrence according to limited resection versus lobectomy.
Methods
We reviewed resected small (≤2 cm) stage I lung adenocarcinomas (n=411; 1995–2006). Tumor STAS was defined as tumor cells—micropapillary structures, solid nests, or single cells—spreading within air spaces in the lung parenchyma beyond the edge of the main tumor. Competing risks methods were used to estimate risk of disease recurrence and its associations with clinicopathological risk factors.
Results
STAS was observed in 155 cases (38%). In the limited resection group (n=120), the risk of any recurrence was significantly higher in patients with STAS-positive tumors than that of patients with STAS-negative tumors (5-year cumulative incidence of recurrence [CIR], 42.6% vs. 10.9%; P<0.001); the presence of STAS correlated with higher risk of distant (P=0.035) and locoregional recurrence (P=0.001). However, in the lobectomy group (n=291), presence of STAS was not associated with either any (P=0.50) or distant recurrence (P=0.76). In a multivariate analysis, presence of tumor STAS remained independently associated with the risk of developing recurrence (hazard ratio, 3.08; P=0.014).
Conclusion
Presence of STAS is a significant risk factor of recurrence in small lung adenocarcinomas treated with limited resection. These findings support our proposal that STAS should formally be recognized as a pattern of invasion in lung adenocarcinoma.
Keywords: lung, adenocarcinoma, invasion, spread through air spaces, recurrence
INTRODUCTION
Lung adenocarcinoma invasion is traditionally defined as: 1) presence of non-lepidic patterns such as acinar, papillary, solid, or micropapillary; 2) infiltration of stroma; and 3) infiltration of blood vessels or structures such as the visceral pleura.1 During our studies of the pathologic characteristics of lung adenocarcinoma,2–8 we noticed tumor cells spreading in air spaces into the lung parenchyma adjacent to the edge of the tumor. We named this phenomenon “spread through air spaces” (STAS) and define it as spread of lung cancer tumor cells into air spaces in the lung parenchyma adjacent to the main tumor.. Literature review revealed multiple studies of various cancers in the lung that have presented with this feature reported using different terms, some of which have shown associations with poor prognosis.9–12 Until now, this problem has received surprisingly little attention in the pathology literature, and the clinical implication of its presence in pathological specimens is not well appreciated. Therefore, using a large cohort of patients with resected small (≤2 cm) stage I lung adenocarcinoma, we investigated whether tumor STAS was a risk factor of disease recurrence according to types of surgical procedures (lobectomy or limited resection) and location of recurrence (locoregional or distant).
PATIENTS AND METHODS
Patient Cohorts
This retrospective study was approved by Memorial Sloan Kettering Cancer Center’s Institutional Review Board. Pathologic stage determination was based on the seventh edition of the American Joint Committee on Cancer Staging Manual.13 We reviewed patients with lung adenocarcinomas that had been surgically resected and diagnosed as small (≤2 cm), pathological stage I disease between 1995 and 2006. Cases with neoadjuvant therapy, multiple nodules, positive surgical margin, other lung cancer surgery within the past 2 years, other disease progression, and no available tumor slides for review were excluded from the study cohort. According to these criteria, we identified a total of 411 patients. Although a subset of these cases have been published in our previous publications,2–8 the medical records and database were reviewed in order to update patients’ follow-up as of March 2014. All recurrences were confirmed by clinical, radiological, or pathological assessment, and were classified into locoregional (local + regional) and distant recurrence.7, 14 Local recurrence was defined as evidence of a tumor in the same lobe or at the surgical margin of the original tumor. Regional recurrence was defined as evidence of a tumor in a second ipsilateral lobe, in the ipsilateral hilar lymph nodes, or in the ipsilateral mediastinal lymph nodes. Distant recurrence was defined by evidence of a tumor in the contralateral lung, in the contralateral mediastinal, in the ipsilateral supraclavicular lymph nodes, or outside the hemithorax.
Histologic Evaluation
Tumor slides from the internal training cohort were reviewed by 2 pathologists (K.K. and W.D.T.) who were blinded to patient clinical outcomes; they used an Olympus BX51 microscope (Olympus Optical Co. Ltd., Tokyo, Japan) with a standard 22-mm diameter eyepiece.
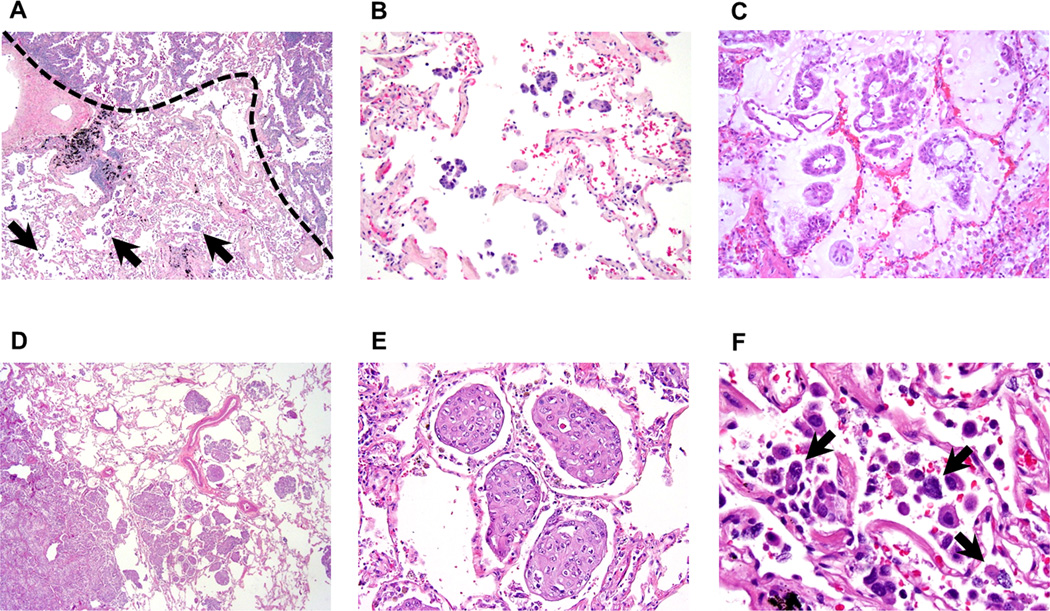
Tumor STAS was defined as tumor cells within air spaces in the lung parenchyma beyond the edge of the main tumor (Figure 1A and 1D) and was composed of 3 morphological patterns: 1) micropapillary structures consisting of papillary structures without central fibrovascular cores (Figure 1A and 1B),15, 16 which occasionally form ring-like structures within air spaces (Figure 1C); 2) solid nests or tumor islands consisting of solid collections of tumor cells filling air spaces (Figure 1D and 1E)17; and 3) single cells consisting of scattered discohesive single cells (Figure 1F). The edge of the main tumor was defined as the smooth surface of the tumor which is easily recognizable at gross or at low-power field examination as highlighted with the dotted line in Figure 1A. Tumor STAS was considered present when tumor STAS, as defined above, was identified beyond the edge of the main tumor even if it existed only in the first alveolar layer from the tumor edge. Lesions of STAS consist of tumor cells which morphologically appear to be situated within air spaces as micropapillary clusters, solid nests or single cells that are detached from alveolar walls. This differs from lepidic growth where tumor cells grow in a linear fashion along the surface of alveolar walls. Extent of air space filling by tumor cells varied from abundant cellular infiltrates to very inconspicuous single cells or micropapillary clusters that were sometimes difficult to distinguish from alveolar macrophages. In addition, distance between tumor surface and farthest STAS from tumor edge was measured by a ruler. Since lung specimens were not consistently inflated during processing, in order to account for artifactual atelectasis, we also measured according to the number of alveolar spaces.
Figure 1. Morphologic features of tumor spread through air spaces (STAS) pattern (original magnification: ×20 in A and D; ×200 in B, C and E; ×400 in F).
(A) Micropapillary pattern STAS (arrows) identified within air spaces in the lung parenchyma beyond the edge (a dotted line) of the main tumor. (B) Micropapillary pattern STAS consisting of papillary structures without central fibrovascular cores. (C) Micropapillary pattern STAS forming ring-like structures within air spaces. (D) Solid pattern STAS identified within air spaces in the lung parenchyma beyond the edge of the main tumor. (E) Solid type STAS consisting of solid collections of tumor cells filling air spaces. (F) Single cell pattern STAS consisting of scattered discohesive single cells (arrows).
Tumor cells of STAS were distinguished from alveolar macrophages using the following methods. Macrophages in smokers typically have cytoplasm containing faint brown pigment and black carbon granules while in nonsmokers the pigment is lacking and cytoplasm is sometimes foamy. Nuclei are small, uniform, and regular, without atypia. Nuclear folds are frequent and nucleoli are inconspicuous or absent. In contrast, tumor cells of STAS typically lack cytoplasmic pigment or foamy cytoplasm. They often grow in cohesive clusters and nuclei are atypical with hyperchromasia and frequent nucleoli. The distinction of STAS from artifacts was done in the following way. Tumor floaters were favored, by the presence of clusters of cells often randomly scattered over tissue and at the edges of the tissue section. Presence of jagged edges of tumor cell clusters suggested tumor fragmentation or edges of a knife cut during specimen processing rather than STAS. Linear strips of cells that were lifted off of alveolar walls also favored the presence of artifact. Identification of tumor cells distant from the main tumor was regarded as an artifact unless intraalveolar tumor cells could be demonstrated in a continuum of airspaces containing intraalveolar tumor cells back to the tumor edge.
According to the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society histological classification, the percentage of each histologic pattern—lepidic, acinar, papillary, solid, and micropapillary—was recorded in 5% increments and tumors were classified by their predominant pattern.1 Each histologic pattern was considered present in the tumor when it comprised ≥5% of the overall tumor.7 Presence of visceral pleural, lymphatic, and vascular invasion was also recorded.
Statistical Analysis
Associations between variables were analyzed using Fisher’s exact test (for categorical variables) and the Wilcoxon test (for continuous variables). The risk of developing disease recurrence was analyzed using competing risks methods. Cumulative incidence of recurrence (CIR) of any kind (locoregional or distant) was estimated using a cumulative incidence function that accounted for death without recurrence as a competing event.18, 19 In addition, for the analyses investigating the risk of disease recurrence according to the locations, distant or locoregional recurrence was considered as a second type of competing risk. Follow-up was calculated from date of surgery to date of disease recurrence, death from any cause, or last follow-up. Differences in CIR between groups were assessed using Gray’s test (for univariate nonparametric analysis) and Fine-Gray competing risk model (for multivariate analysis) after the adjustment for important potential confounders.19, 20 Overall survival (OS) was estimated using the Kaplan–Meier method, and nonparametric group comparisons were performed using the logrank test.
All P-values were based on 2-tailed statistical analysis and P<0.05 was considered statistically significance. Statistical analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC) and R (version 3.0.1; R Development Core Team), including the “survival” and “cmprsk” packages.
RESULTS
Patient Characteristics and Their Associations with Recurrence
Of all, 120 patients underwent limited resection (wedge resection [n=82] and sublobectomy [n=38]) and 291 underwent lobectomy. In limited resection group, 68 patients (57%) underwent lymph node dissection or sampling while, in lobectomy group, all patients underwent lymph node dissection or sampling. In the limited resection group, 28 (23%) experienced recurrence (locoregional [n=14] and distant [n=14]) and 37 (31%) died from any cause without documented recurrence; median follow-up for patients who did not experience recurrence was 88.4 months (range, 0.2–202.6 months). In the lobectomy group, 30 (10%) experienced recurrence (locoregional [n=6], and distant [n=24]) and 62 (21%) died from any cause without documented recurrence; median follow-up for patients who did not experience recurrence was 77.5 months (range, 0.6–190.6 months). Patient characteristics and their associations with CIR, according to types of surgery, are shown in Table 1. In the limited resection group, patient age (P=0.046), tumor size (P=0.004), lymphatic invasion (P=0.001), and vascular invasion (P=0.005) were risk factors for recurrence. In the lobectomy group, patient age (P=0.022), lymphatic invasion (P=0.003), vascular invasion (P=0.005), and predominant histologic subtypes (P=0.006) were risk factors for recurrence.
Table 1.
Associations between Clinicopathologic Factors and Tumor STAS
| Variables | All patients | STAS | P | ||||
|---|---|---|---|---|---|---|---|
| Absent | Present | ||||||
| N | % | N | % | N | % | ||
| Age, years | 0.090 | ||||||
| Median | 68 | 68 | 67 | ||||
| Range | 28 – 89 | 28 – 89 | 36 – 87 | ||||
| Gender | 0.22 | ||||||
| Female | 247 | 60 | 148 | 58 | 99 | 64 | |
| Male | 164 | 40 | 108 | 42 | 56 | 36 | |
| Smoking | 0.62 | ||||||
| Never | 52 | 13 | 34 | 13 | 18 | 12 | |
| Former/current | 359 | 87 | 222 | 87 | 137 | 88 | |
| Surgery | 0.87 | ||||||
| Lobectomy | 291 | 71 | 182 | 71 | 109 | 70 | |
| Limited resection | 120 | 29 | 74 | 29 | 46 | 30 | |
| Tumor size, cm | 0.71 | ||||||
| Median | 1.5 | 1.5 | 1.5 | ||||
| Range | 0.3–2.0 | 0.3–2.0 | 0.3–2.0 | ||||
| Pleural invasion | 0.47 | ||||||
| Absent | 364 | 89 | 229 | 89 | 135 | 87 | |
| Present | 47 | 11 | 27 | 11 | 20 | 13 | |
| Lymphatic invasion | 0.002 | ||||||
| Absent | 277 | 67 | 187 | 73 | 90 | 58 | |
| Present | 134 | 33 | 69 | 27 | 65 | 42 | |
| Vascular invasion | 0 | 0.043 | |||||
| Absent | 319 | 78 | 207 | 81 | 112 | 72 | |
| Present | 92 | 22 | 49 | 19 | 43 | 28 | |
| Lepidic pattern | <0.001 | ||||||
| Absent | 158 | 38 | 80 | 31 | 78 | 50 | |
| Present | 253 | 62 | 176 | 69 | 77 | 50 | |
| Acinar pattern | 0.15 | ||||||
| Absent | 15 | 4 | 12 | 5 | 3 | 2 | |
| Present | 396 | 96 | 244 | 95 | 152 | 98 | |
| Papillary pattern | <0.001 | ||||||
| Absent | 112 | 27 | 88 | 34 | 24 | 15 | |
| Present | 299 | 73 | 168 | 66 | 131 | 85 | |
| Micropapillary pattern | <0.001 | ||||||
| Absent | 205 | 50 | 178 | 70 | 27 | 17 | |
| Present | 206 | 50 | 78 | 30 | 128 | 83 | |
| Solid pattern | 0.001 | ||||||
| Absent | 263 | 64 | 179 | 70 | 84 | 54 | |
| Present | 148 | 36 | 77 | 30 | 71 | 46 | |
STAS, spread through air spaces
Significant P-values are shown in bold
Association between Clinicopathologic Factors and STAS
STAS was observed in 155 cases (38%). When classifying tumors according to predominant pattern of STAS, there were 94 tumors with micropapillary STAS, 53 with solid STAS, and 8 with single cell STAS. Associations between clinicopathologic factors and STAS are summarized in Table 1. Lymphovascular invasion and high-grade morphologic pattern in main tumors were more frequently identified in STAS-positive tumors than in STAS-negative tumors: lymphatic invasion (42% vs. 27%; P=0.002), vascular invasion (28% vs. 19%; P=0.043), micropapillary pattern (83% vs 30%; P<0.001) and solid pattern (46% vs. 30%; P=0.001). In contrast, lepidic pattern was less frequently identified in STAS-positive tumors than in STAS-negative tumors (50% vs. 69%; P<0.001).
Distance of Tumor STAS from Edge of Main Tumor
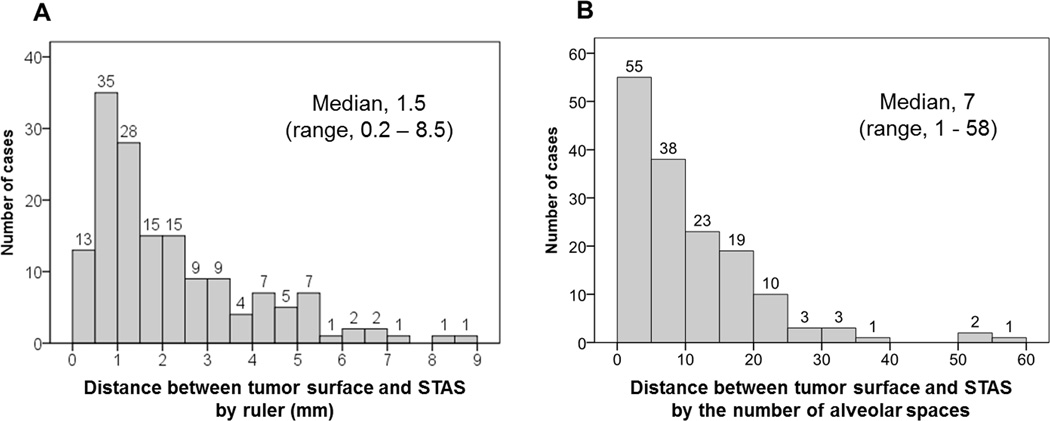
Distance between tumor surface and farthest STAS from the tumor edge was measured by a ruler with a median of 1.5 mm (range 0.2–8.5 mm) (Figure 2A). We also measured according to the number of alveolar spaces (median, 7; range, 1–58) (Figure 2B). Although we defined tumor STAS as tumor cells identified within air spaces beyond the tumor edge even if it existed only in the first alveolar layer, in 97% (n = 151) of the STAS-positive cases, tumor STAS was located beyond the first alveolar layer from the tumor edge (range, 2–58). Using an approximate alveolar size of 0.3 mm (range, 0.2–0.5 mm), we estimate a maximum distance of 1.7 cm for STAS found away from the tumor edge.
Figure 2. Distance of tumor spread through air spaces (STAS) from edge of main tumor.
(A) Distance between tumor surface and farthest STAS away from the tumor edge was measured by a ruler with a median of 1.5 mm (range 0.2–8.5 mm), and (B) according to number of alveolar spaces with a median of 7 (range 1–58).
Risk of Recurrence by Tumor STAS According to Types of Surgery and Locations of Recurrence
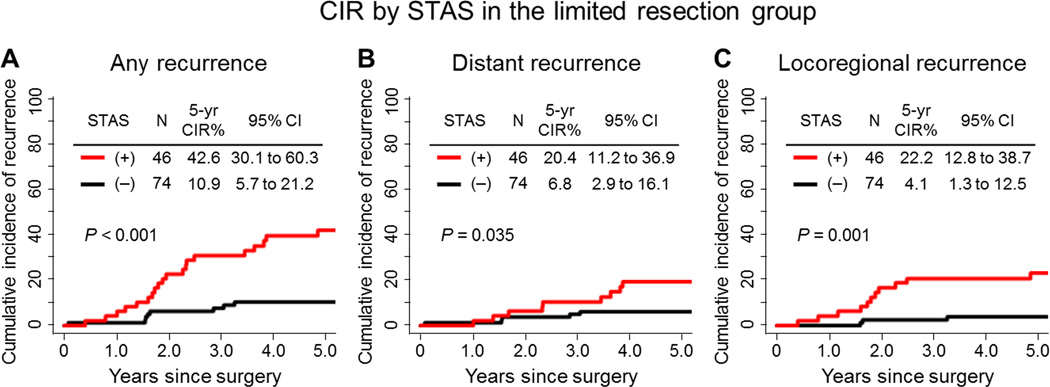
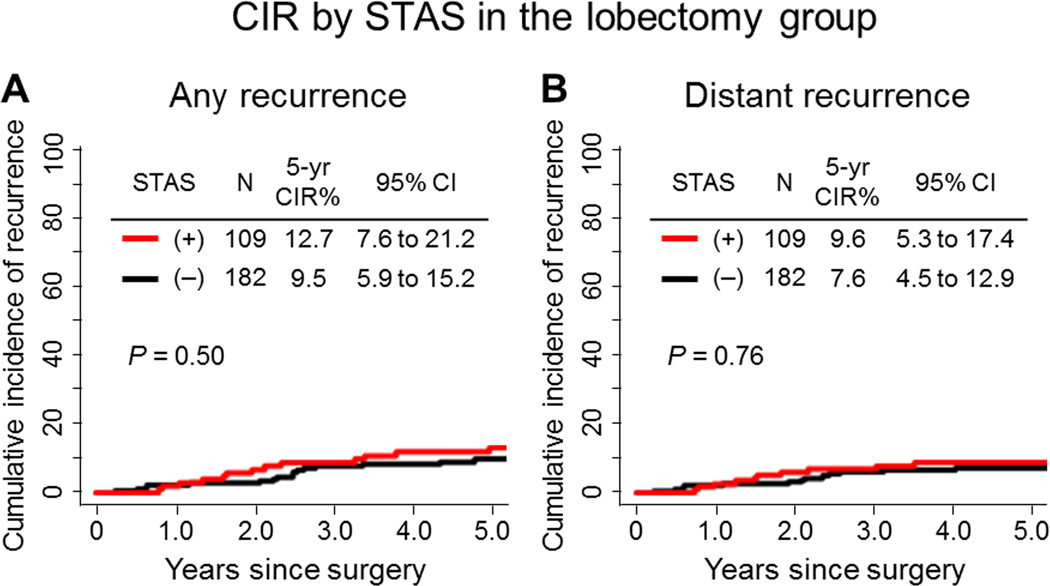
In limited resection group, the risk of developing any types (locoregional or distant) of recurrence was significantly higher in patients with STAS-positive tumors than in patients with STAS-negative tumors (5-year CIR, 42.6% vs. 10.9%; P<0.001) (Figure 3A). In multivariate analysis, presence of tumor STAS was an independent and the only risk factor of any recurrence (hazard ratio [HR], 3.08; P=0.014) (Table 3). However, in the lobectomy group, presence of tumor STAS was not associated with an increased risk of any recurrence, compared with absence of STAS (5-year CIR, 12.7% vs. 9.5%; P=0.50) (Figure 4A).
Figure 3. Cumulative incidence of recurrence (CIR) by spread through air spaces (STAS) in the limited resection group.
(A) CIR for any recurrence of patients with STAS-positive tumors was significantly higher than for patients with STAS-negative tumors (5-year CIR, 42.6% vs. 10.9%; P<0.001). (B) CIR for distant recurrence of patients with STAS-positive tumors was significantly higher than for patients with STAS-negative tumors (5-year CIR, 20.4% vs. 6.8%; P=0.035). (C) CIR for locoregional recurrence of patients with STAS-positive tumors was significantly higher than for patients with STAS-negative tumors (5-year CIR, 22.2% vs. 4.1%; P=0.001).
Table 3.
Multivariate Analysis for Recurrence in the Limited Resection Group
| Variables | HR | 95% CI | P | |
|---|---|---|---|---|
| Age, years | ≥65 vs. <65 | 0.50 | 0.24 to 1.05 | 0.068 |
| Tumor size, cm | >1 vs. ≤1 | 4.00 | 0.90 to 17.80 | 0.069 |
| Vascular invasion | present vs. absent | 2.10 | 0.97 to 4.56 | 0.061 |
| STAS | present vs. absent | 3.08 | 1.26 to 7.56 | 0.014 |
HR, hazard ratio; CI, confidence interval; STAS, spread through air spaces
Significant P-values are shown in bold.
Figure 4. Cumulative incidence of recurrence (CIR) by spread through air spaces (STAS) in the lobectomy group.
(A) Presence of tumor STAS was not associated with risk of any recurrence compared with absence of STAS (5-year CIR, 12.7% vs. 9.5%; P=0.50). (B) Presence of tumor STAS was not associated with risk of distant recurrence compared with absence of STAS (5-year CIR, 9.6% vs. 7.6%; P=0.76).
Among patients who underwent limited resection, patients with STAS-positive tumors had a significantly increased risk of developing distant recurrences, compared to patients with STAS-negative tumors (5-year CIR, 20.4% vs. 6.8%; P=0.035) (Figure 3B). In contrast, in the lobectomy group presence of tumor STAS was not associated with an increased risk of distant recurrence, compared with absence of STAS (5-year CIR, 9.6% vs. 7.6%; P=0.76) (Figure 4B).
Similarly, in the limited resection group, patients with STAS-positive tumors had a significantly increased risk of developing locoregional recurrences, compared to patients with STAS-negative tumors (5-year CIR, 22.2% vs. 4.1%; P=0.001) (Figure 3C).
OS by Tumor STAS According to Types of Surgery
Among patients who underwent limited resection, STAS-positive tumors was significantly associated with worse OS, compared to STAS-negative tumors (5-year OS, 59.3% [95%CI: 46.4 – 75.8] vs. 75.1% [95%CI: 65.7 – 85.8]; P=0.001). Among patients who underwent limited resection, although the association of OS with STAS (presence vs. absence) was statistically significant (P=0.045), the difference of 5-year OS rates between STAS-positive and STAS-negative patients was minimal (83.9% [95%CI: 76.9 – 91.5] vs. 88.6% [95%CI: 83.9 – 93.6]).
DISCUSSION
In this study, we have shown that STAS was a significant prognostic factor for distant and locoregional recurrence in patients undergoing limited resection. We found this in almost 40% of resected lung adenocarcinomas and observed tumor cells over 1 cm away from the edge of the tumor. This observation is the primary basis for our proposal that STAS be formally recognized as a pattern of invasion in lung adenocarcinoma. STAS is an insidious pattern of invasion because it is not visible to pathologists on gross exam and to surgeons on external examination of the tumor specimen at the time of surgery, and we are unaware of any method of radiologically detecting it.
There were several reasons why this pattern has not been previously accepted as a form of invasion. First, this pattern of invasion is unique to the lung compared with other organs because lung anatomy differs due to presence of air spaces, which normally contain air but also provide a path through which tumor cells can spread. Second, STAS is easily dismissed as an artifact where cells within air spaces were regarded as floaters or carry over due to contamination during processing. Because pathologists are not trained to look for these cells in the lung parenchyma beyond the edge of lung adenocarcinomas, STAS is mostly overlooked on microscopic review. Furthermore, due to paucity of data, there has been little emphasis on the clinical significance of this finding, and pathologists have not been compelled to look for this routinely.
Review of the literature revealed several sources of data that also support our proposal. First, studies of angiogenesis in lung cancer have classified tumors according to several different patterns of growth including alveolar patterns that correlated significantly with poor outcome.9 In context of investigations primarily focused on lung cancer angiogenesis, it was shown that alveolar pattern of spread was non-angiogenic in contrast with other patterns of invasion which were angiogenic.21 Second, Onozato et al studied the solid nest form of STAS (calling them “tumor islands”) with 3D reconstruction using an automated tissue sectioning machine. They demonstrated that tumor islands were interconnected with each other and connected to solid areas in the adjacent main tumor.22 This study was followed by a clinical study that indicated a worse prognosis for patients whose tumors had tumor islands.17 Third, radiation therapists have been interested in “microscopic extension” in lung cancers and have emphasized the importance of clinical target volume or areas of subclinical involvement around gross tumor volume, as they design accurate radiation dose delivery.23–26 They pointed out that these microscopic extensions spread in increasing amounts up to over 1 cm beyond the grossly visible edge of tumor, very similar to our observations.23–26 In several articles on the topic of “microscopic extension”, pathology figures illustrated typical examples of what we proposed to label STAS.23, 24 Fourth, the adverse impact of the STAS-like pattern has not only been demonstrated in lung cancer, but also in colon cancer metastatic to the lung where patients whose metastatic tumors showed that this feature had worse prognosis compared with those that did not have this feature.10, 11, 27 Finally, although the historical term aerogenous spread could be considered for STAS, it was used in the context of the old concept of bronchioloalveolar carcinoma, most of those cases consisted of lepidic growth, which is different from the intraalveolar localization of STAS.12
Identification of tumor cells within air spaces and detached from the adjacent alveolar walls in histologic sections raised important issues in the differential diagnosis. First, one can ask whether tumor cells were completely detached and floating within the air space or if they had an attachment to an alveolar wall. Onozato et al showed that “tumor islands” were interconnected with each other and with the main tumor using reconstructed 3D images of serial-sectioned slides, thereby suggesting tumor islands may have been another pattern of tumor infiltration into the lung parenchyma.17 Whether this is true for the micropapillary or single cell patterns of STAS, particularly for tumor cells found over 1 cm away from the tumor edge is not clear. Rare cases of lung adenocarcinomas with tumor cells in air spaces within the main tumor have been reported noting a pattern resembling desquamative interstitial pneumonia (DIP).28, 29 However, this pattern differs from STAS which is present in airspaces outside the main tumor rather than within it. The reports of a DIP pattern resemble the single cell discohesive pattern of STAS that occurred outside the main tumor, and raised the differential diagnosis of between the tumor cells of STAS and alveolar macrophages. Key points on how to distinguish STAS from artifacts and alveolar macrophages were outlined in our methods. If this distinction is difficult based on morphological criteria, immunohistochemistry for keratin and a macrophage marker such as CD68 may be helpful. The finding that STAS is an independent predictor of recurrence suggests this is clinically important and it does not represent an artifact.
In this study, we identified that the tumor STAS pattern was independently associated with high risk of recurrence in patients undergoing limited resection but not in those undergoing lobectomy. More interestingly, the STAS pattern was a significant risk factor for locoregional recurrence, suggesting these occult tumor cells, which are invisible grossly after limited resection, may result in positive surgical margins after limited resection. However, since this was a retrospective review of cases, it was very difficult to evaluate the status of tumor STAS in the surgical margins. We also had insufficient data to calculate the significance of differing amounts of STAS. We measured the distance from the tumor surface to the farthest tumor STAS; however, our specimens were not consistently inflated making these measurements challenging. Therefore, further investigation is warranted to study the clinical impact of confirming whether surgical margins are microscopically free of the tumor STAS pattern in limited resection specimens and whether the tumor STAS pattern can be detected on frozen sections during surgery. This may help thoracic surgeons make clinical decisions on additional treatments, intraoperatively and postoperatively. In addition, further studies are required to determine whether or not the extent of tumor STAS, in terms of circumference involved or distance from the tumor edge, is associated with disease recurrence. Defining the edge of the tumor may be difficult in some cases particularly with a significant micropapillary component; however, in most cases this can be achieved. Tumor cells identified as STAS beyond the border of the tumor should not be included in the comprehensive histologic subtyping of lung adenocarcinomas as they represent a pattern of invasion. It is possible that one of the reasons why small percentages (5%) of a micropapillary pattern in lung adenocarcinomas have been reported to correlate with poor prognosis may be because these actually represented micropapillary STAS.7, 16 This also needs to be investigated in future studies.
The most important clinical implication of finding STAS in a resection specimen is the possibility of a positive or close margin that might easily be missed by pathologists who do not specifically look for this lesion. Our data suggested that presence of STAS was particularly important in patients who had undergone limited resections for lung adenocarcinoma. Lobectomy with hilar and mediastinal lymph node dissection was the current gold standard for treatment of early-stage lung cancers, whereas multiple studies have suggested that peripheral small (≤2 cm) lung cancers could be treated by limited resection alone; this technique may be as effective as a lobectomy with the added advantage of preserving lung function.30–32 With recent randomized trials assessing low-dose computed tomography screening for lung cancers,33–35 it is anticipated that an increasing number of patients will be diagnosed with early-stage small lung adenocarcinomas. Despite this, there have been no established criteria for choosing limited resection over lobectomy for the treatment of lung adenocarcinomas to date.
The micropapillary pattern has been reported to be a high-grade morphologic pattern in lung adenocarcinoma and was associated with lymphovascular invasion.15, 16, 36, 37 In our study, STAS was more frequently identified in cases that had a micropapillary pattern in the main tumor. However, for adenocarcinoma subtyping the micropapillary pattern is assessed only within the borders of the tumor edge while STAS represents a pattern of invasion within surrounding airspaces beyond the edge of the tumor. Although the STAS pattern was also positively associated with presence of lymphovascular invasion, which has been considered a significant poor prognostic factor, tumor STAS was an independent risk factor of recurrence in the limited resection group while vascular invasion was not. Our group has recently demonstrated that presence (≥5%) of micropapillary pattern identified a high-risk group for recurrence among patients who underwent limited resection but not those who underwent lobectomy,7 thereby suggesting that patients whose tumors were pathologically diagnosed as having micropapillary pattern after limited resection may benefit from completion lobectomy.
In conclusion, we have shown that STAS pattern is a significant risk factor of disease recurrence in small (≤2 cm) stage I lung adenocarcinoma treated with limited resection. This supports our proposal that STAS be formally recognized as a pattern of invasion in lung adenocarcinoma. Although we did not study STAS in other histologic types of lung cancer, we have seen it in our clinical work and prior literature suggests that it occurs in other histologies such as squamous cell carcinoma.25 Furthermore, based on this observation, in the future it is possible for patients who undergo limited resection that the identification of STAS may help clinicians make better clinical decisions on postoperative therapies, such as adjuvant chemotherapy and completion of lobectomy with lymph node dissection. Hopefully, our findings will encourage further investigations to determine whether the tumor STAS pattern is microscopically recognizable on frozen sections of lung adenocarcinoma, which may help thoracic surgeons choose lobectomy over limited resection for appropriate patients intraoperatively. In addition, the issues in surgical margin by tumor STAS, especially in limited resection cases, should be investigated and resolved in the prospective studies in the future. Further work is also needed at the molecular level to better understand what are the genetic and biological characteristics of tumor cells that make them prone to loss of cohesiveness and spread through air spaces.
Table 2.
Clinicopathologic Associations with Recurrence According to Types of Surgery
| Variables | Limited Resection Group (n = 120) | Lobectomy Resection Group (n = 291) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | 5-yr CIR% | 95% CI | P | N | % | 5-yr CIR% | 95% CI | P | |
| Age, years | 0.046 | 0.022 | ||||||||
| <65 | 41 | 34 | 34.2 | 22.2 to 52.7 | 127 | 44 | 15.3 | 10.0 to 23.5 | ||
| ≥65 | 79 | 66 | 17.1 | 10.4 to 28.1 | 164 | 56 | 7.1 | 4.0 to 12.6 | ||
| Gender | 0.40 | 0.22 | ||||||||
| Female | 73 | 61 | 19.8 | 12.3 to 31.8 | 174 | 60 | 8.7 | 5.2 to 14.3 | ||
| Male | 47 | 39 | 27.8 | 17.4 to 44.5 | 117 | 40 | 13.6 | 8.5 to 21.9 | ||
| Smoking | 0.79 | 0.25 | ||||||||
| Never | 15 | 13 | 20.6 | 7.2 to 59.1 | 37 | 13 | 5.7 | 1.4 to 22.3 | ||
| Former/Current | 105 | 88 | 23.3 | 16.4 to 33.2 | 254 | 87 | 11.5 | 8.0 to 16.4 | ||
| Tumor size | 0.004 | 0.64 | ||||||||
| ≤1.0 cm | 35 | 29 | 5.9 | 1.5 to 23.1 | 50 | 17 | 12.9 | 6.0 to 27.6 | ||
| >1.0 cm | 85 | 71 | 29.9 | 21.4 to 41.6 | 241 | 83 | 10.2 | 6.9 to 15.1 | ||
| Pleural invasion | 0.87 | 0.13 | ||||||||
| Absent | 102 | 85 | 23.1 | 16.1 to 33.2 | 262 | 90 | 9.9 | 6.7 to 14.4 | ||
| Present | 18 | 15 | 22.2 | 9.1 to 54.4 | 29 | 10 | 17.4 | 7.7 to 39.2 | ||
| Lymphatic invasion | 0.001 | 0.003 | ||||||||
| Absent | 75 | 63 | 13.7 | 7.6 to 24.5 | 202 | 69 | 7.0 | 4.1 to 11.8 | ||
| Present | 45 | 38 | 38.6 | 26.5 to 56.4 | 89 | 31 | 19.2 | 12.3 to 29.9 | ||
| Vascular invasion | 0.005 | 0.005 | ||||||||
| Absent | 96 | 80 | 17.1 | 10.9 to 26.8 | 223 | 77 | 7.8 | 4.9 to 12.5 | ||
| Present | 24 | 20 | 45.8 | 29.2 to 72.0 | 68 | 23 | 20.0 | 12.2 to 32.8 | ||
| Histologic subtype | 0.22 | 0.006 | ||||||||
| AIS + MIA | 3 | 3 | 0.0 | NA | 6 | 2 | 0.0 | NA | ||
| Lepidic | 18 | 15 | 6.4 | 0.9 to 45.7 | 20 | 7 | 5.0 | 0.7 to 35.5 | ||
| Acinar | 49 | 41 | 31.2 | 20.4 to 47.8 | 145 | 50 | 8.2 | 4.6 to 14.5 | ||
| Papillary | 24 | 20 | 29.2 | 15.3 to 55.5 | 66 | 23 | 8.3 | 3.6 to 19.4 | ||
| Micropapillary | 8 | 7 | 25.0 | 6.9 to 90.9 | 10 | 3 | 20.0 | 5.3 to 75.4 | ||
| Solid | 16 | 13 | 13.0 | 3.4 to 50.5 | 36 | 12 | 30.2 | 17.7 to 51.4 | ||
| Others* | 2 | 2 | NA | NA | 8 | 3 | NA | NA | ||
CIR, cumulative incidence of recurrence; CI, confidence interval; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; NA, not applicable
Others include invasive mucinous and colloid predominant adenocarcinomas
Significant P-values are shown in bold
ACKNOWLEDGMENTS
We thank Joe Dycoco of the Thoracic Surgery Service for assisting with the Thoracic Service of the Department of Surgery’s lung cancer database and Alex Torres of the Thoracic Surgery Service for his editorial assistance.
Funding Support: This work was supported by grants from the National Institutes of Health (R21 CA164568-01A1, R21 CA164585-01A1, R01 CA136705-06, U54 CA137788, P30 CA008748, and P50 CA086438-13), the U.S. Department of Defense (PR101053 and LC110202), and the Mr. William H. Goodwin and Mrs. Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25(8):1117–1127. doi: 10.1038/modpathol.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol. 2014;38(4):448–460. doi: 10.1097/PAS.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadota K, Yeh YC, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol. 2014;27(5):690–700. doi: 10.1038/modpathol.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadota K, Yeh Y-C, D’Angelo SP, et al. Associations between mutations and histologic patterns of mucin in lung adenocarcinoma: Invasive mucinous pattern and extracellular mucin are associated with KRAS mutation. Am J Surg Pathol. 2014;38(8):1118–1127. doi: 10.1097/PAS.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadota K, Nitadori J, Sarkaria IS, et al. Thyroid transcription factor-1 expression is an independent predictor of recurrence and correlates with the IASLC/ATS/ERS histologic classification in patients with stage I lung adenocarcinoma. Cancer. 2013;119(5):931–938. doi: 10.1002/cncr.27863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013;105(16):1212–1220. doi: 10.1093/jnci/djt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki K, Kadota K, Sima CS, et al. Clinical Impact of Immune Microenvironment in Stage I Lung Adenocarcinoma: Tumor Interleukin-12 Receptor beta2 (IL-12Rbeta2), IL-7R, and Stromal FoxP3/CD3 Ratio Are Independent Predictors of Recurrence. J Clin Oncol. 2013;31(4):490–498. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sardari Nia P, Colpaert C, Vermeulen P, et al. Different growth patterns of non-small cell lung cancer represent distinct biologic subtypes. Ann Thorac Surg. 2008;85(2):395–405. doi: 10.1016/j.athoracsur.2007.08.054. [DOI] [PubMed] [Google Scholar]
- 10.Welter S, Jacobs J, Krbek T, Krebs B, Stamatis G. Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg. 2007;84(1):203–210. doi: 10.1016/j.athoracsur.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Shiono S, Ishii G, Nagai K, et al. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg. 2005;79(1):278–282. doi: 10.1016/j.athoracsur.2004.06.096. [DOI] [PubMed] [Google Scholar]
- 12.Clayton F. Bronchioloalveolar carcinomas. Cell types, patterns of growth, and prognostic correlates. Cancer. 1986;57(8):1555–1564. doi: 10.1002/1097-0142(19860415)57:8<1555::aid-cncr2820570820>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009. pp. 253–270. [Google Scholar]
- 14.Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest. 2012;142(6):1620–1635. doi: 10.1378/chest.12-0790. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsumida H, Nomoto M, Goto M, et al. A micropapillary pattern is predictive of a poor prognosis in lung adenocarcinoma, and reduced surfactant apoprotein A expression in the micropapillary pattern is an excellent indicator of a poor prognosis. Mod Pathol. 2007;20(6):638–647. doi: 10.1038/modpathol.3800780. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol. 2003;27(1):101–109. doi: 10.1097/00000478-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Onozato ML, Kovach AE, Yeap BY, et al. Tumor Islands in Resected Early-stage Lung Adenocarcinomas are Associated With Unique Clinicopathologic and Molecular Characteristics and Worse Prognosis. Am J Surg Pathol. 2013;37(2):287–294. doi: 10.1097/PAS.0b013e31826885fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappell R. Competing Risk Analyses: How Are They Different and Why Should You Care? Clin Cancer Res. 2012;18(8):2127–2129. doi: 10.1158/1078-0432.CCR-12-0455. [DOI] [PubMed] [Google Scholar]
- 19.Dignam JJ, Zhang Q, Kocherginsky M. The Use and Interpretation of Competing Risks Regression Models. Clin Cancer Res. 2012;18(8):2301–2308. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 21.Pezzella F, Pastorino U, Tagliabue E, et al. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol. 1997;151(5):1417–1423. [PMC free article] [PubMed] [Google Scholar]
- 22.Onozato ML, Klepeis VE, Yagi Y, Mino-Kenudson M. A role of three-dimensional (3D) reconstruction in the classification of lung adenocarcinoma. Stud Health Technol Inform. 2012;179:250–256. [PubMed] [Google Scholar]
- 23.Meng X, Sun X, Mu D, et al. Noninvasive evaluation of microscopic tumor extensions using standardized uptake value and metabolic tumor volume in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):960–966. doi: 10.1016/j.ijrobp.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 24.Giraud P, Antoine M, Larrouy A, et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys. 2000;48(4):1015–1024. doi: 10.1016/s0360-3016(00)00750-1. [DOI] [PubMed] [Google Scholar]
- 25.van Loon J, Siedschlag C, Stroom J, et al. Microscopic disease extension in three dimensions for non-small-cell lung cancer: development of a prediction model using pathology-validated positron emission tomography and computed tomography features. Int J Radiat Oncol Biol Phys. 2012;82(1):448–456. doi: 10.1016/j.ijrobp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Grills IS, Fitch DL, Goldstein NS, et al. Clinicopathologic analysis of microscopic extension in lung adenocarcinoma: defining clinical target volume for radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(2):334–341. doi: 10.1016/j.ijrobp.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Shiono S, Ishii G, Nagai K, et al. Predictive factors for local recurrence of resected colorectal lung metastases. Ann Thorac Surg. 2005;80(3):1040–1045. doi: 10.1016/j.athoracsur.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 28.Raparia K, Ketterer J, Dalurzo ML, et al. Lung tumors masquerading as desquamative interstitial pneumonia (DIP): report of 7 cases and review of the literature. Am J Surg Pathol. 2014;38(7):921–924. doi: 10.1097/PAS.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 29.Mutton AE, Hasleton PS, Curry A, et al. Differentiation of desquamative interstitial pneumonia (DIP) from pulmonary adenocarcinoma by immunocytochemistry. Histopathology. 1998;33(2):129–135. doi: 10.1046/j.1365-2559.1998.00463.x. [DOI] [PubMed] [Google Scholar]
- 30.Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg. 2005;129(1):87–93. doi: 10.1016/j.jtcvs.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 31.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg. 2010;251(3):550–554. doi: 10.1097/SLA.0b013e3181c0e5f3. [DOI] [PubMed] [Google Scholar]
- 32.Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer <=1 cm in size: a review of SEER data. Chest. 2011;139(3):491–496. doi: 10.1378/chest.09-2547. [DOI] [PubMed] [Google Scholar]
- 33.National Lung Screening Trial Research T. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: Selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON) Int J Cancer. 2007;120(4):868–874. doi: 10.1002/ijc.22134. [DOI] [PubMed] [Google Scholar]
- 36.Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol. 2008;21(8):992–1001. doi: 10.1038/modpathol.2008.79. [DOI] [PubMed] [Google Scholar]
- 37.Makimoto Y, Nabeshima K, Iwasaki H, et al. Micropapillary pattern: a distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral lung adenocarcinoma (</=20 mm) with mixed bronchioloalveolar and invasive subtypes (Noguchi's type C tumours) Histopathology. 2005;46(6):677–684. doi: 10.1111/j.1365-2559.2005.02126.x. [DOI] [PubMed] [Google Scholar]