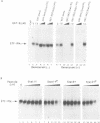
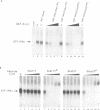
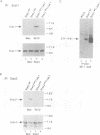
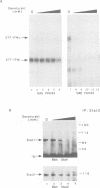
Abstract
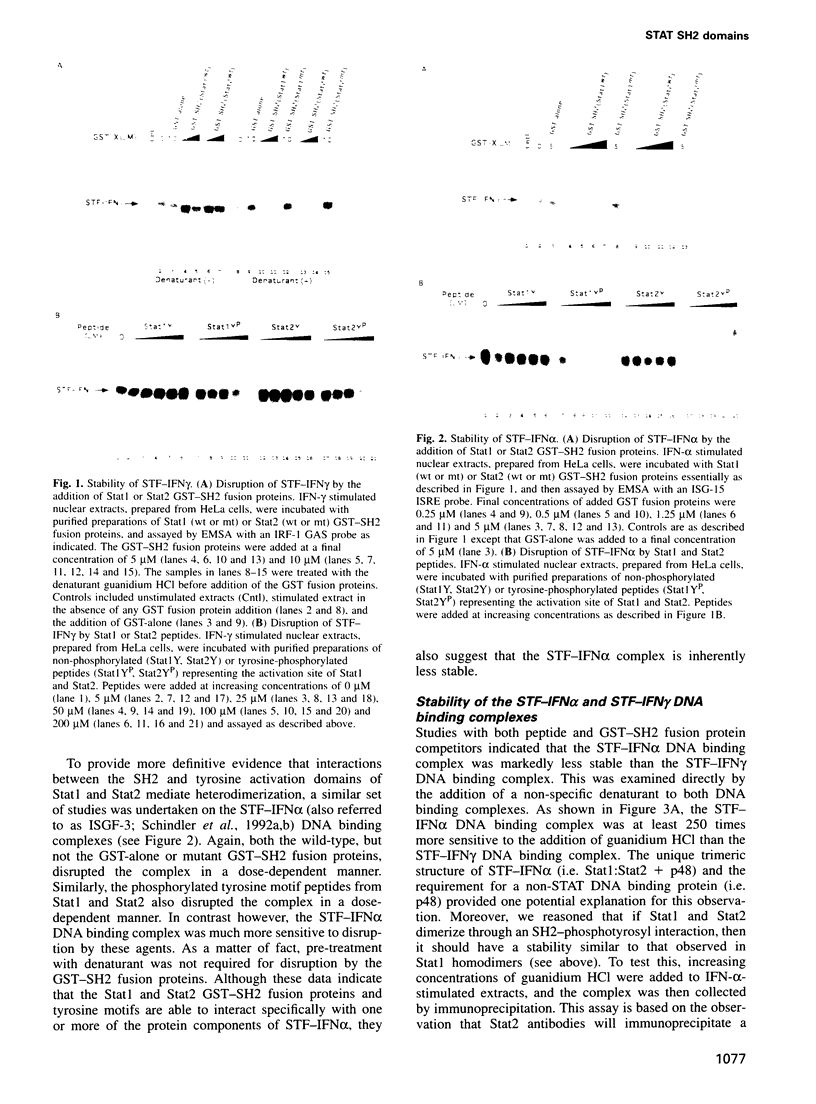
Analysis of the ability of IFN-alpha to rapidly stimulate genes has led to the identification of a new family of signal transducing factors (STFs). The STF activated by IFN-alpha consists of two members of the STAT (Signal Transducers and Activators of Transcription) family of signaling proteins, Stat1 and Stat2. Sequence comparison of these STATs has determined that they share several conserved domains, the most notable of which is an SH2 domain. Recently, studies have determined that these SH2 domains can mediate a specific association between a STAT and the cytoplasmic domain of the appropriate receptor. Once associated with the receptor, the STATs become activated by an associated tyrosine kinase, whereupon they dissociate from the receptor and dimerize to form active STFs. The SH2 domain has also been implicated in the formation of STAT dimers found in STFs, suggesting it may play multiple roles in signaling. We have carried out a detailed analysis on the role of the Stat1 and Stat2 SH2 domains in the mediation of IFN-alpha stimulated signals. These studies, which have determined that the SH2 domain of Stat1 and Stat2 can mediate homo- as well as heterodimerization, suggest that a single SH2 domain-phosphotyrosyl interaction is sufficient for dimerization. Moreover, they provide the first direct evidence that the target of the SH2 domain is the STAT tyrosine activation site. In addition, these studies implicate the SH2 domain in another step in the signaling cascade, namely mediation of the interaction between STATs and their activating kinases (i.e. the JAKs).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Azam M., Erdjument-Bromage H., Kreider B. L., Xia M., Quelle F., Basu R., Saris C., Tempst P., Ihle J. N., Schindler C. Interleukin-3 signals through multiple isoforms of Stat5. EMBO J. 1995 Apr 3;14(7):1402–1411. doi: 10.1002/j.1460-2075.1995.tb07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri G., Velazquez L., Scrobogna M., Fellous M., Pellegrini S. Activation of the protein tyrosine kinase tyk2 by interferon alpha/beta. Eur J Biochem. 1994 Jul 15;223(2):427–435. doi: 10.1111/j.1432-1033.1994.tb19010.x. [DOI] [PubMed] [Google Scholar]
- Bonni A., Frank D. A., Schindler C., Greenberg M. E. Characterization of a pathway for ciliary neurotrophic factor signaling to the nucleus. Science. 1993 Dec 3;262(5139):1575–1579. doi: 10.1126/science.7504325. [DOI] [PubMed] [Google Scholar]
- Colamonici O., Yan H., Domanski P., Handa R., Smalley D., Mullersman J., Witte M., Krishnan K., Krolewski J. Direct binding to and tyrosine phosphorylation of the alpha subunit of the type I interferon receptor by p135tyk2 tyrosine kinase. Mol Cell Biol. 1994 Dec;14(12):8133–8142. doi: 10.1128/mcb.14.12.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Decker T., Lew D. J., Cheng Y. S., Levy D. E., Darnell J. E., Jr Interactions of alpha- and gamma-interferon in the transcriptional regulation of the gene encoding a guanylate-binding protein. EMBO J. 1989 Jul;8(7):2009–2014. doi: 10.1002/j.1460-2075.1989.tb03608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Frangioni J. V., Neel B. G. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem. 1993 Apr;210(1):179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Schindler C., Improta T., Aebersold R., Darnell J. E., Jr The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7840–7843. doi: 10.1073/pnas.89.16.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri J. G., Ahdieh M., Eisenman J., Shanebeck K., Grabstein K., Kumaki S., Namen A., Park L. S., Cosman D., Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994 Jun 15;13(12):2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. M., Itoh N., Kitamura T., Schreurs J., Yonehara S., Yahara I., Arai K., Miyajima A. Cloning and expression of a gene encoding an interleukin 3 receptor-like protein: identification of another member of the cytokine receptor gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5459–5463. doi: 10.1073/pnas.87.14.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F., Pallard C., Dusanter-Fourt I., Wakao H., Haldosen L. A., Norstedt G., Levy D., Groner B. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J. 1995 May 1;14(9):2005–2013. doi: 10.1002/j.1460-2075.1995.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlund A. C., Farrar M. A., Viviano B. L., Schreiber R. D. Ligand-induced IFN gamma receptor tyrosine phosphorylation couples the receptor to its signal transduction system (p91). EMBO J. 1994 Apr 1;13(7):1591–1600. doi: 10.1002/j.1460-2075.1994.tb06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlund A. C., Morales M. O., Viviano B. L., Yan H., Krolewski J., Schreiber R. D. Stat recruitment by tyrosine-phosphorylated cytokine receptors: an ordered reversible affinity-driven process. Immunity. 1995 Jun;2(6):677–687. doi: 10.1016/1074-7613(95)90012-8. [DOI] [PubMed] [Google Scholar]
- Heim M. H., Kerr I. M., Stark G. R., Darnell J. E., Jr Contribution of STAT SH2 groups to specific interferon signaling by the Jak-STAT pathway. Science. 1995 Mar 3;267(5202):1347–1349. doi: 10.1126/science.7871432. [DOI] [PubMed] [Google Scholar]
- Ho A. S., Liu Y., Khan T. A., Hsu D. H., Bazan J. F., Moore K. W. A receptor for interleukin 10 is related to interferon receptors. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11267–11271. doi: 10.1073/pnas.90.23.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C. M., Wen Z., Darnell J. E., Jr A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 1995 Apr 15;9(8):984–994. doi: 10.1101/gad.9.8.984. [DOI] [PubMed] [Google Scholar]
- Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994 Sep 16;265(5179):1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995 Jan 27;80(2):225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Kessler D. S., Veals S. A., Fu X. Y., Levy D. E. Interferon-alpha regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes Dev. 1990 Oct;4(10):1753–1765. doi: 10.1101/gad.4.10.1753. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Kotanides H., Reich N. C. Requirement of tyrosine phosphorylation for rapid activation of a DNA binding factor by IL-4. Science. 1993 Nov 19;262(5137):1265–1267. doi: 10.1126/science.7694370. [DOI] [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Larose L., Gish G., Pawson T. Construction of an SH2 domain-binding site with mixed specificity. J Biol Chem. 1995 Feb 24;270(8):3858–3862. doi: 10.1074/jbc.270.8.3858. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Seegert D., Strehlow I., Schindler C., Lohmann-Matthes M. L., Decker T. IL-10-induced factors belonging to the p91 family of proteins bind to IFN-gamma-responsive promoter elements. J Immunol. 1994 Jul 1;153(1):165–172. [PubMed] [Google Scholar]
- Leung S., Qureshi S. A., Kerr I. M., Darnell J. E., Jr, Stark G. R. Role of STAT2 in the alpha interferon signaling pathway. Mol Cell Biol. 1995 Mar;15(3):1312–1317. doi: 10.1128/mcb.15.3.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Darnell J. E., Jr Cytoplasmic activation of ISGF3, the positive regulator of interferon-alpha-stimulated transcription, reconstituted in vitro. Genes Dev. 1989 Sep;3(9):1362–1371. doi: 10.1101/gad.3.9.1362. [DOI] [PubMed] [Google Scholar]
- Lütticken C., Wegenka U. M., Yuan J., Buschmann J., Schindler C., Ziemiecki A., Harpur A. G., Wilks A. F., Yasukawa K., Taga T. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994 Jan 7;263(5143):89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Jackson P. K., Van Etten R. A., Baltimore D. Point mutations in the abl SH2 domain coordinately impair phosphotyrosine binding in vitro and transforming activity in vivo. Mol Cell Biol. 1992 Feb;12(2):609–618. doi: 10.1128/mcb.12.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migone T. S., Lin J. X., Cereseto A., Mulloy J. C., O'Shea J. J., Franchini G., Leonard W. J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995 Jul 7;269(5220):79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- Mui A. L., Wakao H., O'Farrell A. M., Harada N., Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995 Mar 15;14(6):1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis A., Gupta S., Yopp J., Garfein E., Kashleva H., Schindler C., Rothman P. Gamma chain-associated cytokine receptors signal through distinct transducing factors. J Biol Chem. 1995 Jun 16;270(24):14517–14522. doi: 10.1074/jbc.270.24.14517. [DOI] [PubMed] [Google Scholar]
- Pine R., Canova A., Schindler C. Tyrosine phosphorylated p91 binds to a single element in the ISGF2/IRF-1 promoter to mediate induction by IFN alpha and IFN gamma, and is likely to autoregulate the p91 gene. EMBO J. 1994 Jan 1;13(1):158–167. doi: 10.1002/j.1460-2075.1994.tb06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle F. W., Thierfelder W., Witthuhn B. A., Tang B., Cohen S., Ihle J. N. Phosphorylation and activation of the DNA binding activity of purified Stat1 by the Janus protein-tyrosine kinases and the epidermal growth factor receptor. J Biol Chem. 1995 Sep 1;270(35):20775–20780. doi: 10.1074/jbc.270.35.20775. [DOI] [PubMed] [Google Scholar]
- Ralph S. J., Wines B. D., Payne M. J., Grubb D., Hatzinisiriou I., Linnane A. W., Devenish R. J. Resistance of melanoma cell lines to interferons correlates with reduction of IFN-induced tyrosine phosphorylation. Induction of the anti-viral state by IFN is prevented by tyrosine kinase inhibitors. J Immunol. 1995 Mar 1;154(5):2248–2256. [PubMed] [Google Scholar]
- Rothman P., Kreider B., Azam M., Levy D., Wegenka U., Eilers A., Decker T., Horn F., Kashleva H., Ihle J. Cytokines and growth factors signal through tyrosine phosphorylation of a family of related transcription factors. Immunity. 1994 Sep;1(6):457–468. doi: 10.1016/1074-7613(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Schindler C., Darnell J. E., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- Schindler C., Fu X. Y., Improta T., Aebersold R., Darnell J. E., Jr Proteins of transcription factor ISGF-3: one gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Kashleva H., Pernis A., Pine R., Rothman P. STF-IL-4: a novel IL-4-induced signal transducing factor. EMBO J. 1994 Mar 15;13(6):1350–1356. doi: 10.1002/j.1460-2075.1994.tb06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Shuai K., Horvath C. M., Huang L. H., Qureshi S. A., Cowburn D., Darnell J. E., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994 Mar 11;76(5):821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Schindler C., Schlessinger J., Levy D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993 Sep 24;261(5129):1736–1739. doi: 10.1126/science.8378775. [DOI] [PubMed] [Google Scholar]
- Sliva D., Wood T. J., Schindler C., Lobie P. E., Norstedt G. Growth hormone specifically regulates serine protease inhibitor gene transcription via gamma-activated sequence-like DNA elements. J Biol Chem. 1994 Oct 21;269(42):26208–26214. [PubMed] [Google Scholar]
- Songyang Z., Carraway K. L., 3rd, Eck M. J., Harrison S. C., Feldman R. A., Mohammadi M., Schlessinger J., Hubbard S. R., Smith D. P., Eng C. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1995 Feb 9;373(6514):536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Stahl N., Farruggella T. J., Boulton T. G., Zhong Z., Darnell J. E., Jr, Yancopoulos G. D. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995 Mar 3;267(5202):1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- Velazquez L., Mogensen K. E., Barbieri G., Fellous M., Uzé G., Pellegrini S. Distinct domains of the protein tyrosine kinase tyk2 required for binding of interferon-alpha/beta and for signal transduction. J Biol Chem. 1995 Feb 17;270(7):3327–3334. doi: 10.1074/jbc.270.7.3327. [DOI] [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994 May 1;13(9):2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman G., Kominos D., Robertson S. C., Pant N., Baltimore D., Birge R. B., Cowburn D., Hanafusa H., Mayer B. J., Overduin M. Crystal structure of the phosphotyrosine recognition domain SH2 of v-src complexed with tyrosine-phosphorylated peptides. Nature. 1992 Aug 20;358(6388):646–653. doi: 10.1038/358646a0. [DOI] [PubMed] [Google Scholar]
- Yan H., Krishnan K., Greenlund A. C., Gupta S., Lim J. T., Schreiber R. D., Schindler C. W., Krolewski J. J. Phosphorylated interferon-alpha receptor 1 subunit (IFNaR1) acts as a docking site for the latent form of the 113 kDa STAT2 protein. EMBO J. 1996 Mar 1;15(5):1064–1074. [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Blenis J., Li H. C., Schindler C., Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995 Mar 31;267(5206):1990–1994. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wagner F., Frank S. J., Kraft A. S. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J Biol Chem. 1995 Jun 9;270(23):13814–13818. doi: 10.1074/jbc.270.23.13814. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4806–4810. doi: 10.1073/pnas.91.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]