Abstract
OBJECTIVES
To review the known histopathologic findings and clinical behavior of mammary analogue secretory carcinoma (MASC).
DATA SOURCES
PubMed
REVIEW METHODS
Literature search using the terms “Mammary analogue secretory carcinoma,” “Mammary analog secretory carcinoma,” and “MASC” to identify all relevant publications.
RESULTS
MASC is an unusual and rare malignant salivary gland tumor first described in 2010. It shares histologic, immunohistochemical, and genetic features with secretory carcinoma of the breast. The clinical behavior of MASC ranges from slowly growing tumors that infrequently recur after surgical resection to aggressive tumors that cause widespread metastasis and death. Many cases of MASC were discovered in archived cases previously classified as acinic cell carcinoma, mucoepidermoid carcinoma, and adenocarcinoma not otherwise specified.
CONCLUSION
MASC is a newly recognized variant of salivary gland malignancy. Further research is needed to better delineate its overall prevalence and to define an appropriate treatment algorithm for this new clinical entity.
Keywords: salivary gland carcinoma, mammary analogue secretory carcinoma, head and neck surgery
CASE PRESENTATION
A 58-year old female presented with an 8-month history of right-sided face swelling that began after an upper respiratory tract infection. Magnetic resonance imaging (MRI) showed 3.2-cm well-circumscribed cystic and partially enhancing deep lobe mass in the right parotid gland (see Figure 1). Fine needle aspiration (FNA) was described as a high-grade carcinoma with extensive necrosis, with a differential diagnosis including salivary duct carcinoma, acinic cell carcinoma, mucoepidermoid carcinoma, and adenocarcinoma NOS, among others (see Figure 2). Positron-emission tomography-computed tomography (PET-CT) revealed FDG-avidity at the primary site. Right parotidectomy and right modified radical neck dissection were performed. No pathologic nodes were detected in the neck. The large parotid tumor was classified as T2N0 mammary analogue secretory carcinoma (MASC) of the salivary gland (see Figure 3), with subsequent FISH analysis confirming a translocation of the ETV6 gene on chromosome 12p13. Post-operative radiotherapy was not recommended. At 4 months follow-up, the patient remains without evidence of disease.
FIGURE 1. Magnetic resonance image (MRI) axial images.
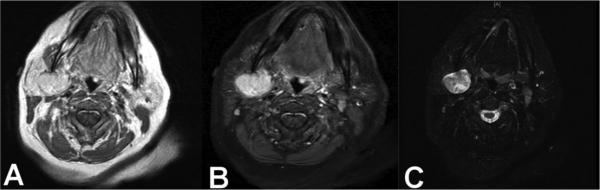
A) T1 sequence pre-Gadolinium demonstrates a well-circumscribed right parotid lesion hyperintense relative to muscle B) T1 sequence post-Gadolinium demonstrates partial enhancement C) T2 sequence demonstrates hyperintensity relative to parotid gland.
FIGURE 2. Fine needle aspirate.
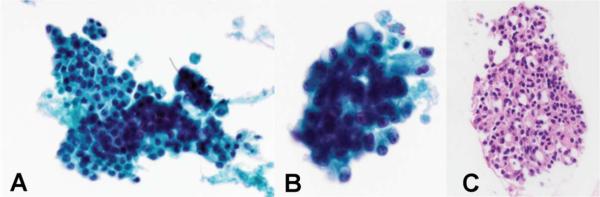
A-B) Papanicolaou stained ThinPrep slide showing tumor cells with crowding, enlarged nuclei with promiment nucleoli, and cytoplasmic vacuolization C) Hematoxylin and eosin stained cell block preparation showing cells with vacuolization of the eosinophilic cytoplasm and a microcystic architecture.
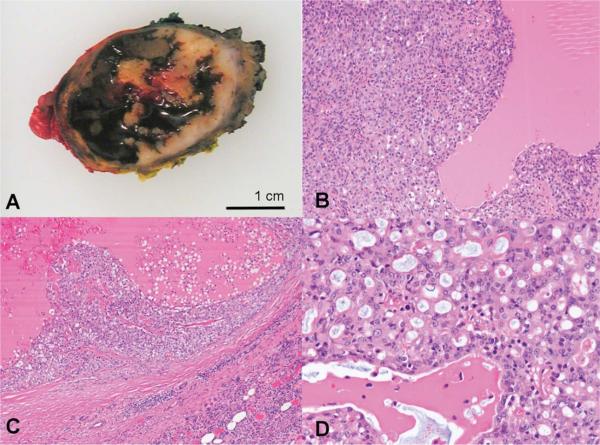
FIGURE 3. Gross and histologic pathology of the tumor.
A) Gross appearance is that of a well-circumscribed partially cystic tumor B-C) Low-powered view demonstrates solid, microcystic and pseudopapillary architectural patterns with abundant eosinophilic, colloid-like secretions D) High-powered view reveals tumor cells with eosinophilic finely granular to vacuolated cytoplasm and round to oval nuclei with prominent nucleoli. (B-D, Hematoxylin and Eosin stains).
INTRODUCTION
Mammary analogue secretory carcinoma (MASC) is a rare salivary gland tumor that recapitulates the histology and genetics of an equally rare malignancy of the breast, secretory carcinoma (SC).1 SC is a slow-growing, low-grade ductal breast carcinoma that occurs in adolescent women and, occasionally, in elderly women.2,3 The tumor is defined by the t(12;15)(q13;q25) translocation, a fusion of the ETV6 gene from chromosome 12 and the NTRK3 gene from chromosome 15. The fusion results in a constitutively active chimeric tyrosine kinase and probable activation of the Ras-MAP kinase mitogenic pathway and the phosphatidyl inositol-3-kinase-AKT pathway.4,5 The same translocation was first detected in infantile fibrosarcoma and congenital mesoblastic nephroma.6,7
In 2010, Skalova et al reviewed the pathology of 16 salivary gland tumor cases previously classified as either acinic cell carcinoma (AciCC) or adenocarcinoma, not otherwise specified (ADC-NOS).1 The authors noted histological features--in particular the absence of zymogen granules, strong staining for mammaglobin, and the presence of abundant extracellular colloid-like material--that prevented easy classification by the existing pathological definitions of known salivary gland tumors. After noting features similar to SC of the breast, the authors found that 15 of these cases also tested positive for the ETV6-NTRK3 translocation via fluorescence in-situ hybridization (FISH). MASC became one of many salivary gland tumors with a breast tumor analog, including pleomorphic adenoma (PA), mucoepidermoid carcinoma (MEC), and AciCC.3 In the last two years, 90 cases of MASC have been published (see Table 1).1,8-24 Most are derived from retrospective review of institutional archives of other salivary gland tumor diagnoses. Bishop et al recently reported that 19% of parotid gland and 79% of extra-parotid gland tumors originally diagnosed as AciCC were re-classified as MASC.8
Table 1.
Case series and reports of mammary analogue secretory carcinoma.
| Series | Number of patients | Mean Age (years) (range) | Men/Women | Year | Location Parotid/Other | Mean Size (cm) | T stage | pN stage | Treatment | Survival |
|---|---|---|---|---|---|---|---|---|---|---|
| Bishop et al, 2013 | 5 | 52 (21 – 78) | 3/2 | 1992-2005 | 4/1 | 1.9 (0.8-4) | -- | -- | 5 Surgery | -- |
| Chiosea et al, 2012a* | 36 | 45.7 | 21/15 | 1956-2011 | 26/10 | -- | T1: 21 T2: 5 T3: 4 T4: 2 |
N0: 14 N1: 3 N2b: 1 |
36 Surgery 5 PORT 2 PORT+Chemo 18 ND |
5 LR 1DM 2DOD |
| Connor et al, 2012 | 7 | 40 (14-77) | 6/1 | 2012 | 2/5 | 1.8 (0.5-3) | -- | N0: 2 | 7 Surgery 2 PORT 2 ND |
|
| Fehr et al, 2011 | 4 | 52 (23-71) | 1/3 | 2011 | 2/2 | -- | -- | -- | -- | -- |
| Ito et al, 2012 | 1 | 37 | 0/1 | 2012 | 1/0 | 2.4 | -- | -- | -- | -- |
| Jung et al, 2013 | 13 | 46.4 (17-76) | 8/5 | 1990-2012 | 11/2 | 1.77 (0.7-2.5) | T1: 6 T2: 3 T3: 4 |
-- | 13 Surgery 2 PORT |
5 LR |
| Kratochvil et al, 2012 | 2 | 48, 52 | 1/1 | 2005-2012 | 0/2 | 1, 0.7 | -- | -- | 2 Surgery | 0 LR |
| Levine et al, 2012 | 1** | 34 | 0/1 | 2012 | 1/0 | 1 | T1: 1 | N0: 1 | -- | -- |
| Laco et al, 2013 | 2 | 34, 58 | 1/1 | 2013 | 1/1 | 1.5, 2 | T1: 2 | -- | 2 Surgery | 0 LR |
| Petersson et al, 2012 | 1 | 35 | 1/0 | 2012 | 1/0 | 2.5 | -- | N2b: 1 | 1 PORT 1 ND |
0 LR |
| Pisharodi et al, 2013 | 1 | 69 | 0/1 | 2011 | 1/0 | 3 | -- | -- | 1 Surgery | -- |
| Ratsatter et al, 2012 | 1 | 14 | 0/1 | 2011 | 1/0 | 3 | T2: 1 | N1: 1 | 1 Surgery | 0 LR |
| Skalova et al, 2010 | 16** | 46 (21-75) | 9/7 | 2010 | 13/3 | 2.1 (0.7-5.5) | T1: 8 T2: 3 T3: 3 T4: 1 |
N0: 14 N2: 1 |
16 Surgery 7 PORT 1 ND |
4 LR 1DM 2DOD |
| Our Series | 1 | 58 | 0/1 | 2013 | 1/0 | 3.2 | T2: 1 | N0: 1 | 1 Surgery 1 ND |
0 LR |
| Combined patients | 91 | 44.7 (14-77) | 51/40 | 1956-2013 | 64/27 | 2.1 (0.5-5.5) | T1: 38 T2: 13 T3: 11 T4: 3 |
-- | 86 Surgery 21 ND 17 PORT 2 PORT+Chemo |
PORT, post-operative radiotherapy; ND, neck dissection; LR, locoregional recurrence; DM, distant metastasis; DOD, dead of disease
Chiosea et al 2012 contains patients previously reported in Griffith et al 2012, Griffith et al 2011, Yu et al, 2011 and Chiosea et al, 2012b
One case in Levine et al and one case in Skalova et al were not tested for the ETV6-NTRK3 translocation
While MASC's histological features have been studied extensively in the pathology literature, its clinical behavior is largely uncharacterized. In this review, we seek to consolidate existing knowledge and encourage new research on the diagnosis and management of this increasingly important salivary gland malignancy.
METHODS
For this contemporary review, we conducted a PubMed search of the terms “mammary analogue secretory carcinoma,” “mammary analog secretory carcinoma” and “MASC.” We included both English and foreign-language publications, if available in translation. We identified 17 original case reports or case series and 1 review in a pathology journal.1,8-24 Two case series were from Japan and Korea16,17; the remaining publications were from the United States. Patients from all publications were combined in order to generate overall demographic descriptions of patients with this rare tumor; we did not double count patients reported in more than one publication.10,11,14,15,19 Survival meta-analysis was not attempted due to recent published attempt10 and variably reported and limited follow-up.
DISCUSSION
HISTOPATHOLOGY AND CYTOPATHOLOGY
On gross inspection, MASC is generally a solitary, well-circumscribed and often encapsulated mass that is brown or gray in color and rubbery in texture.1,9,17,18 The central histologic features include:
intraluminal and/or intracellular colloid-like secretions that stain positive for periodic acid Schiff (PAS) and are diastase-resistant 1,11-13,17,20,23,24
positive staining for mammaglobin, a uteroglobin protein identified in breast tissue, endometrial cancer and sweat gland carcinomas1,9-22
The defining cytogenetic characteristic of this lesion is the presence of the t(12;15)(q13;q25) ETV6-NTRK3 translocation, demonstrated by either FISH or PCR. 1,9-22
Although of limited use owing to significant overlap with other salivary gland tumors, multiple other immunohistochemical markers positively stain MASC tissue, including vimentin,1,11 HMWK,12 CK 7,1,13 CK 8,1,20 CK18,1 CK19,1,12 CAM 5.2,12 STAT5a,1,23 MUC-11,20 and MUC-4.1 GCDFP, or BRST-2, has variable staining strength; in Skalow et al, 8 of 11 samples were positive,1 while others have found positive GCDFP staining restricted to a minority of cases, and in positive cases, to a minority of tumor cells.17,20,21 Most studies have detected a low proliferation index by Ki67 staining (5-28%),1 and negative staining for calponin, CK15, smooth muscle actin, estrogen receptor, progesterone receptor, Herceptin receptor, CK 5 and 6.1,20 P63 is generally negative in tumor cells; however, Chiosa et al found that 28% of MASC cases had positive staining for peripherally located entrapped non-tumor basal cells, which could represent intraductal extension or possibly a ductal epithelial origin for this tumor.10,12
Aggressive features are uncommon, though extracapsular/extraglandular extension and perineural invasion (PNI) have been reported in several cases.12 Jung et al, in 2013, have reported the only case of “dedifferentiation” or transition into high-grade histology; the authors described a sample with high-grade cellular and nuclear characteristics, solid and microcystic growth pattern, and comedonecrosis, all features reminiscent of high-grade de-differentiated acinic cell carcinoma.17
Cytopathology reveals similar features as histopathology, including moderately cellular sheets of S100-positive cells arranged in papillary, cystic, tubular and solid growth arrangements.9,15,20,21 Distinguishing between MASC and other common salivary gland tumors, or even normal salivary gland tissue, is more difficult in the context of aspirated tissue. Griffith et al use cellularity to distinguish between tumor tissue and normal tissue. The authors also identify cytoplasmic vacuolation, and acinar group size variations as key findings in aspirates of MASC.15 Levine et al found that MASC's characteristic cytological feature was the abundant extracellular material, which the authors describe as “bright metachromatic strings.”20 ETV6 FISH testing has been performed successfully on cell block material, including the case presented here, though one reported case did not have enough cells available for testing.15
The major differential diagnosis of MASC includes AciCC, MEC and ADC-NOS. In 2012, Chiosa et al reviewed 337 cases of salivary gland malignancy including AciCC, MEC, ADC-NOS, signet-ring adenocarcinoma, low-grade salivary duct carcinoma (LGCC), low-grade sinonasal non-intestinal type adenocarcinoma (non-ITAC), and polymorphous low-grade adenocarcinoma (PLGA) diagnosed at their institution between 1956 and 2011. The most common malignancies re-classified as MASC were ADC-NOS (14/37) and AciCC (11/89); MEC was surprisingly uncommon (1/165).10
The primary histologic and cytopathological features of MASC, AciCC and MEC are listed in Table 2. AciCC are histologically diverse: samples contain intercalated, clear and non-specific glandular cells arranged in solid, microcystic, papillary-cystic and follicular growth patterns.1,11 There is considerable overlap between AciCC and MASC, as both exhibit acinar differentiation and intercalated duct-type cells. A major point of distinction is the presence of blue-purple cytoplasmic zymogen granules, a sign of serous acinar differentiation, in AciCC.10,11 In 2012, Chiosa et al identified 17 “zymogen-poor” cases of AciCC from institutional archives; of these, 10 were reclassified as MASC. This has led some recent publications to suggest FISH testing on any ‘zymogen granule poor’ AciCC. In addition, MASC stains more reliably for S100, AciCC is not positive for mammaglobin, and intercalated ducts and mucin production are features in MASC but not AciCC.10-12,14,15 Finally, relative to AciCC, MASC is more likely to occur in men and at a location outside of the parotid gland.10
Table 2.
Major features of differential diagnosis for mammary analogue secretory carcinoma.
| Mammary analogue secretory carcinoma (MASC) | Acinic cell carcinoma (AciCC) | Low-grade mucoepidermoid carcinoma (MEC) | |
|---|---|---|---|
| HISTOLOGY | • Cystic, papillary and solid architecture • Bland, low-grade nuclei • Vacuolated eosinophilic cytoplasm • Intracytoplasmic and intraluminal mucin • Colloid-like secretions |
• solid, microcystic, papillary, or follicular architecture • cytoplasmic PAS-positive, diastase-resistant zymogen granules • frequent lymphoid infiltrate • usually mucin negative |
• variably cystic and solid architecture • mucous, intermediate, and epidermoid cells • inflammation and fibrosis common |
| POSITIVE STAINING OF TUMOR CELLS | S-100 Mammoglobin |
Amylase S-100 DOG-1 |
P63 Cytokeratins |
| NEGATIVE STAINING OF TUMOR CELLS | P63 | P63 | S100 |
| GENETICS | ETV6-NTRK3 | None | CRTC1-MAML2; CRT3-MAML2 |
Mucin production and occasional differentiation into serous cells may lead to diagnostic confusion between MASC and MEC. However, MASC does not contain the combination of goblet-type mucous cells, intermediate and squamoid/epidermoid cells characteristic of MEC.8,10,15,19,20 In addition, MEC contains its own set of distinctive translocations: EWSR1-POUF1, CRTC1-MAML2 and/or CRT3-MAML2.10,19,20,25 While MEC is nearly always positive for p63, S100 staining is uncommon.15,26
CLINICAL PRESENTATION AND DIAGNOSIS
Ninety-one cases of MASC, including the case presented at the beginning of this review, have been reported in the literature. Although several case series have noted a male predominance,1,10,11 reported cases are divided fairly evenly by gender; fifty-one (56%) are male, and 40 (44%) are female. The average age was 44.7 years (range, 14 to 77). Four patients were under the age of 18 years. The majority (70%) of tumors were located in the parotid gland, while the remainder occurred in a large diversity of locations, including the submandibular gland (7%), soft palate, buccal mucosa, base of tongue, and lips. In the 9 series that reported tumor dimensions, the average tumor size was 2.11 cm (range, 0.5 to 5.5 cm).1,9,12,16-18,20-24
Presenting symptoms have been described in 59 patients, including the case at the beginning of this review.1,10,12,16,20-24 The most common presentation is a slowly enlarging and painless nodule, often detected incidentally on physical examination. Only one patient presented with facial paralysis from a bulky parotid gland tumor.10 Combining the 23 patients with detailed symptom timing, the median duration of symptoms is 4.25 years (range, 2 months to 30 years).1,12,16,20-22 Although the disease follows an indolent course in most patients, one reported patient presented after rapid growth of a 5.5 cm parotid gland nodule within 2 months; the patient, who had regional metastases to cervical nodes at diagnosis, went on to develop distant metastases and died of the disease.
The appearance on imaging has been described in 3 patients. On ultrasound, the lesion appeared hypoechoic and solid in 2 patients.20,21 On CT with contrast, a single patients’ lesion was well-circumscribed and mildly enhancing. On MRI, an intra-parotid lesion exhibited hyperintensity relative to muscle on T1, and hypointensity relative to the parotid gland on T2.16 In our case, we found a similarly hyperintense and enhancing T1 appearance, but we also detected hyperintensity in T2 (see Figure 1).
Fine needle aspiration is an appropriate adjunct to imaging, as in most salivary gland tumors. Although the cytopathological findings on FNA may not be distinctive, recent reports have described preparation of cell blocks that can undergo immunohistochemistry and FISH analysis for the ETV6-NTRK3 translocation. 9,15 Of the 12 fine needle aspirate cases described in the literature, only one received the initial diagnosis of MASC.9,15,20 Surgical extirpation may be required for definitive diagnosis.
PROGRESSION AND SURVIVAL
Reported cases of MASC encompass a broad range of clinical behaviors, from indolent to aggressive. In the largest estimate of the risk of progression and death, Chiosa et al followed 14 patients from the authors’ institutional cohort, and 14 additional patients culled from the literature.1 The mean disease-free survival, using death or recurrence as the end-point, was 92 months (95% CI, 71-115). In contrast, among 38 cases with conventional AciCC, the mean survival was 121 months (95% CI, 92-149); the difference was not statistically significant (p=0.43). Jung et al followed 9 patients.17 Of these, 3 developed local recurrence at a median time of 44 months (range, 10-101), and 2 developed metastases to lymph nodes at 62 months and 70 months after diagnosis. Overall, among all 91 reported cases, only 4 cases of death from disease have been reported, though survival data is variably reported, and follow-up is minimal. Two of those cases followed the only two reports of distant metastasis. The third case followed multiple locoregional recurrences, and the fourth case followed unspecified recurrence. 1,10
Patients with MASC may be more likely to have a higher T stage at diagnosis than patients with AciCC, though no statistical comparison has been published to support this observation.10 Of the 65 patients with known T stage reported in the literature, 38 (58%) were T1, 13 (20%) were T2, 11 (17%) were T3 and 3 (5%) were T4.1,10,17,20,22 The risk for regional nodal disease at diagnosis may also be higher in patients with MASC than in patients with AciCC. In an observation that has been widely cited, Chiosa et al found that 4 of 18 (22%) patients with MASC who underwent neck dissection were found to have disease involvement of nodes.10 In contrast, only 3 of 38 (7.9%) of patients with AciCC who underwent neck dissection were found to have disease involvement of nodes. While this difference was statistically significant, the significance was lost when Chiosa et al included seventeen additional cases with known nodal status from previous reports, though the proportion of MASC patients with nodal involvement (6/34 or 17.6%) remained higher than AciCC.
Disease outcomes in large cohorts of AciCC patients are well-known, but complicated by the probable presence of a sub-set of patients with MASC.27-29 In 1991, Lewis et al followed 90 patients with AciCC and found a five year overall survival (OS) rate of 90%, 10 year OS of 83% and 20 year OS of 67%.28 By crude calculation, 44% suffered local recurrence, 19% developed metastases and 25% died of disease. Patients with high grade AciCC faced a very poor prognosis; the majority developed nodal and distant disease and die of disease within 5 years. In 1983, Ellis et al followed 244 patients with AciCC, and found a recurrence rate of 12%, metastatic rate of 7.8% and death rate of 6.1%.27 While histology did not have prognostic significance, the presence of a prominent intercalated duct component, a feature suggestive of MASC19, was the most common histology pattern observed in cases with metastatic disease.
TREATMENT
Of the 86 patients with known treatment details, all underwent varying degrees of surgical resection. At least 21 patients (26%) underwent neck dissections (ND). Seventeen patients (20%) received post-operative radiotherapy (PORT), and two patients received PORT and chemotherapy (agents unspecified) (2%). No reported patients have received RT without prior surgical resection.
The value of PORT is unclear due to the paucity of treatment-specific survival data. Skalova et al, in their initial report of 16 patients, found that, of the seven patients who received PORT, one patient died of metastatic disease, one died after multiple local recurrences, three have no evidence of disease after locoregional recurrences and two did not experience any disease progression.1 In comparison, of the nine patients who did not receive RT, one patient suffered a local recurrence, one was lost to follow-up and seven patients did not experience any disease progression. These two groups differed significantly, however, in the size of local disease, which makes comparison difficult. In addition, RT dose and field data were not reported.
Standard of care for low-grade malignant salivary gland tumors is radical surgical resection. PORT is reserved for close (<5mm) margins/incomplete resection, perineural invasion and all T3-T4 tumors.30,31 Doses between 60-66 Gy are delivered in fractions of 1.8 to 2 Gy for between five to six treatments per week. In 2005, Terhaard et al reported outcomes after PORT in 386 patients, the largest cohort of mixed salivary gland tumor histologies, including AciCC, MEC and ADC-NOS.31 While PORT significant improved local control (LC) at 10 years (PORT vs. Surgery alone; 91% vs. 76%; p=0.0005), patients with close or incomplete resection and advanced T stage benefited the most. Patients not meeting these criteria experienced adequate disease control (LC 95% at 5 years) with surgery alone. Primary RT alone, delivered to 70 Gy in fractions of 1.8 to 2 Gy, is given to tumors with unresectable or distant disease; in Terhaard et al, tumors that received ≥66 Gy had a LC of 50% at 5 years. 31
There are no straightforward indications for ND, though the risk of nodal disease is known to depend on T stage, histology (least likely for AciCC, adenoid cystic carcinoma and carcinoma ex-pleomorphic adenoma; most likely for squamous cell and undifferentiated carcinoma) and location (most likely for submandibular gland).31 Terhaard et al and Al-Mamgani et al recommend ND for all high-risk patients (all T3-T4, stage T2 MEC, Stage T1-T2 squamous cell/undifferentiated carcinoma) and for patients with clinical nodal disease.30,31 PORT to the neck, delivered to doses between 50 and 54 Gy in fractions of 1.8 to 2 Gy, is reserved for patients with confirmed pathological nodal disease.30,31
Overall, there is no conclusive evidence that MASC should be treated any differently than other low-grade malignant salivary gland cancers. Surgeons may be more likely to consider neck dissection and/or neck radiotherapy in patients with MASC given limited available data. However, the increased propensity for regional lymph node metastases is based on retrospective review of a very small group of patients managed with variable methods.10
CONCLUSION
MASC is a newly recognized malignant salivary gland tumor distinct from acinic cell carcinoma that mimics the histology and genetics of SC of the breast. Although it appears to follow an indolent course in most patients, certain cases appear predisposed to distant metastasis and increased mortality. Currently there is no way to predict which tumors will behave aggressively. There is a growing body of data on this disease in the pathology literature, however it is not a commonly described entity in the otolaryngology community. Further research is needed to understand the clinical behavior and prognostic significance of MASC. Indeed, large-scale retrospective analysis of existing salivary gland carcinoma tissue may help to answer this question.
Acknowledgments
Funding: This work was supported by a grant from the Doris Duke Charitable Foundation to Harvard Medical School to fund Clinical Research Fellow R.S.
Footnotes
Conflicts: No authors have conflicts of interest to disclose.
REFERENCES
- 1.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 2.Ozguroglu M, Tascilar K, Ilvan S, Soybir G, Celik V. Secretory carcinoma of the breast. Case report and review of the literature. Oncology. 2005;68:263–268. doi: 10.1159/000086782. [DOI] [PubMed] [Google Scholar]
- 3.Pia-Foschini M, Reis-Filho JS, Eusebi V, Lakhani SR. Salivary gland-like tumours of the breast: surgical and molecular pathology. J Clin Pathol. 2003;56:497–506. doi: 10.1136/jcp.56.7.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/s1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 5.Wai DH, Knezevich SR, Lucas T, Jansen B, Kay RJ, Sorensen PH. The ETV6-NTRK3 gene fusion encodes a chimeric protein tyrosine kinase that transforms NIH3T3 cells. Oncogene. 2000;19:906–915. doi: 10.1038/sj.onc.1203396. [DOI] [PubMed] [Google Scholar]
- 6.Knezevich SR, Garnett MJ, Pysher TJ, Beckwith JB, Grundy PE, Sorensen PH. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. 1998;58:5046–5048. [PubMed] [Google Scholar]
- 7.Bourgeois JM, Knezevich SR, Mathers JA, Sorensen PH. Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am J Surg Pathol. 2000;24:937–946. doi: 10.1097/00000478-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bishop JA. Unmasking MASC: Bringing to Light the Unique Morphologic, Immunohistochemical and Genetic Features of the Newly Recognized Mammary Analogue Secretory Carcinoma of Salivary Glands. Head Neck Pathol. 2013;7:35–39. doi: 10.1007/s12105-013-0429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop JA, Yonescu R, Batista DA, Westra WH, Ali SZ. Cytopathologic features of mammary analogue secretory carcinoma. Cancer Cytopathol. 2012 doi: 10.1002/cncy.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiosea SI, Griffith C, Assaad A, Seethala RR. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology. 2012;61:387–394. doi: 10.1111/j.1365-2559.2012.04232.x. [DOI] [PubMed] [Google Scholar]
- 11.Chiosea SI, Griffith C, Assaad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol. 2012;36:343–350. doi: 10.1097/PAS.0b013e318242a5b0. [DOI] [PubMed] [Google Scholar]
- 12.Connor A, Perez-Ordonez B, Shago M, Skalova A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012;36:27–34. doi: 10.1097/PAS.0b013e318231542a. [DOI] [PubMed] [Google Scholar]
- 13.Fehr A, Loning T, Stenman G. Mammary analogue secretory carcinoma of the salivary glands with ETV6-NTRK3 gene fusion. Am J Surg Pathol. 2011;35:1600–1602. doi: 10.1097/PAS.0b013e31822832c7. [DOI] [PubMed] [Google Scholar]
- 14.Griffith C, Seethala R, Chiosea SI. Mammary analogue secretory carcinoma: a new twist to the diagnostic dilemma of zymogen granule poor acinic cell carcinoma. Virchows Arch. 2011;459:117–118. doi: 10.1007/s00428-011-1098-6. [DOI] [PubMed] [Google Scholar]
- 15.Griffith CC, Stelow EB, Saqi A, et al. The cytological features of mammary analogue secretory carcinoma: A series of 6 molecularly confirmed cases. Cancer Cytopathol. 2012 doi: 10.1002/cncy.21249. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Ishida E, Skalova A, Matsuura K, Kumamoto H, Sato I. Case report of Mammary Analog Secretory Carcinoma of the parotid gland. Pathol Int. 2012;62:149–152. doi: 10.1111/j.1440-1827.2011.02759.x. [DOI] [PubMed] [Google Scholar]
- 17.Jung MJ, Song JS, Kim SY, et al. Finding and characterizing mammary analogue secretory carcinoma of the salivary gland. Korean J Pathol. 2013;47:36–43. doi: 10.4132/KoreanJPathol.2013.47.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kratochvil FJ, 3rd, Stewart JC, Moore SR. Mammary analog secretory carcinoma of salivary glands: a report of 2 cases in the lips. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:630–635. doi: 10.1016/j.oooo.2012.07.480. [DOI] [PubMed] [Google Scholar]
- 19.Lei Y, Chiosea SI. Re-evaluating historic cohort of salivary acinic cell carcinoma with new diagnostic tools. Head Neck Pathol. 2012;6:166–170. doi: 10.1007/s12105-011-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine P, Fried K, Krevitt LD, Wang B, Wenig BM. Aspiration biopsy of mammary analogue secretory carcinoma of accessory parotid gland: Another diagnostic dilemma in matrix-containing tumors of the salivary glands. Diagn Cytopathol. 2012 doi: 10.1002/dc.22886. [DOI] [PubMed] [Google Scholar]
- 21.Pisharodi L. Mammary analog secretory carcinoma of salivary gland: Cytologic diagnosis and differential diagnosis of an unreported entity. Diagn Cytopathol. 2013;41:239–241. doi: 10.1002/dc.21766. [DOI] [PubMed] [Google Scholar]
- 22.Rastatter JC, Jatana KR, Jennings LJ, Melin-Aldana H. Mammary analogue secretory carcinoma of the parotid gland in a pediatric patient. Otolaryngol Head Neck Surg. 2012;146:514–515. doi: 10.1177/0194599811419044. [DOI] [PubMed] [Google Scholar]
- 23.Laco J, Svajdler M, Jr., Andrejs J, et al. Mammary analog secretory carcinoma of salivary glands: A report of 2 cases with expression of basal/myoepithelial markers (calponin, CD10 and p63 protein). Pathol Res Pract. 2013;209:167–172. doi: 10.1016/j.prp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Petersson F, Lian D, Chau YP, Yan B. Mammary analogue secretory carcinoma: the first submandibular case reported including findings on fine needle aspiration cytology. Head Neck Pathol. 2012;6:135–139. doi: 10.1007/s12105-011-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiosea SI, Dacic S, Nikiforova MN, Seethala RR. Prospective testing of mucoepidermoid carcinoma for the MAML2 translocation: clinical implications. Laryngoscope. 2012;122:1690–1694. doi: 10.1002/lary.22419. [DOI] [PubMed] [Google Scholar]
- 26.Sams RN, Gnepp DR. P63 expression can be used in differential diagnosis of salivary gland acinic cell and mucoepidermoid carcinomas. Head Neck Pathol. 2013;7:64–68. doi: 10.1007/s12105-012-0403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis GL, Corio RL. Acinic cell adenocarcinoma. A clinicopathologic analysis of 294 cases. Cancer. 1983;52:542–549. doi: 10.1002/1097-0142(19830801)52:3<542::aid-cncr2820520326>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 28.Lewis JE, Olsen KD, Weiland LH. Acinic cell carcinoma. Clinicopathologic review. Cancer. 1991;67:172–179. doi: 10.1002/1097-0142(19910101)67:1<172::aid-cncr2820670129>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Timon CI, Dardick I, Panzarella T, et al. Acinic cell carcinoma of salivary glands. Prognostic relevance of DNA flow cytometry and nucleolar organizer regions. Arch Otolaryngol Head Neck Surg. 1994;120:727–733. doi: 10.1001/archotol.1994.01880310033007. [DOI] [PubMed] [Google Scholar]
- 30.Al-Mamgani A, van Rooij P, Verduijn GM, Meeuwis CA, Levendag PC. Long-term outcomes and quality of life of 186 patients with primary parotid carcinoma treated with surgery and radiotherapy at the Daniel den Hoed Cancer Center. Int J Radiat Oncol Biol Phys. 2012;84:189–195. doi: 10.1016/j.ijrobp.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 31.Terhaard CH, Lubsen H, Rasch CR, et al. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol Biol Phys. 2005;61:103–111. doi: 10.1016/j.ijrobp.2004.03.018. [DOI] [PubMed] [Google Scholar]