Abstract
Background
About 15 000 persons receive the diagnosis of kidney cancer in Germany every year. Surgical resection is the standard treatment for locally confined tumors, but minimally invasive thermoablative techniques are increasingly being used as well.
Methods
This article is based on publications retrieved by a selective literature search in PubMed regarding the thermoablative techniques now used in clinical practice, with particular attention to radiofrequency ablation (RFA) and cryoablation (CA).
Results
RFA and CA are suitable for patients who cannot undergo surgery because of comorbid illnesses or who have contralateral recurrences or a hereditary precancerous condition. The primary technical success rate of these procedures ranges from 88% to 100%. More than 95% of tumors under 3 cm in diameter can be completely ablated. Reported complication rates range from 1% to 7%. New data on long-term outcomes reveal metastasis-free survival rates of 88% to 99% five years after ablation. A major advantage of these procedures is that thermoablation does not impair renal function to any relevant extent and is thus a good option for patients with limited renal function or a single kidney.
Conclusion
The thermoablative techniques are an important addition to the armamentarium of effective treatments for locally confined renal tumors. The guidelines of the American and European urological societies now list thermoablation with RFA or CA as an option for the treatment of small renal tumors with curative intent. Thermoablation of renal tumors has not yet been studied in randomized controlled trials; these will be needed so that the efficacy of tumor control, survival rates, complication rates, and quality of life after treatment can be reliably evaluated to provide definitive confirmation of the value of interstitial techniques.
About 15 000 new cases of kidney cancer are diagnosed in Germany annually, of which approximately 90% are renal cell carcinoma (RCC) and 10% transitional cell carcinoma (TCC) (1). The observed annual increase in the incidence of renal cell carcinoma of approximately 2% (2) can partly be attributed the rapid progress in tomographic imaging. The detection of the tumor in the early T1a stage (48–65%) (3, 4) as an incidental finding is advantageous as it allows curative treatment of small renal cell carcinomas in many cases (5).
Historically, radical nephrectomy was considered to be the treatment of choice even for small renal tumors (6). In the meantime, partial nephrectomy has replaced this approach as it preserves the organ while offering the same oncological efficacy with regard to tumor control (7, 8).
Criteria for resection surgery include, in addition to oncological guidelines, especially
the patient’s suitability for anesthesia, and
the anticipated reduction in renal function.
The growing interest in minimally invasive procedures has been triggered by the finding that the number of functional nephrons after tumor resection is directly related to patient survival (8).
Percutaneous techniques for interstitial tumor ablation have increasingly been used to treat solid tumors since the turn of the millennium. A variety of ablation techniques is available: While microwave ablation (MWA), high-intensity focused ultrasound (HIFU) and laser thermal ablation (LTA) are still in an experimental stage (9– 11), radiofrequency ablation (RFA) and cryoablation (CA) have become established modalities in the treatment of small renal tumors (12– 14). With both techniques, a needle-type probe (14–17 G) is placed in the center of the tumor.
The local heat produced by RFA induces a coagulation necrosis of the tumor tissue. By applying high-frequency alternating current (400–460 kHz), oscillation of ions is induced in the target tissue, creating temperatures of 60–90°C. The resulting protein denaturation is irreversible. The thermal energy applied also obliterates the tumor-supplying vessels (cauterization). The cytotoxic effect of RFA depends on the maximum temperature achieved and the duration of the ablation. To ensure complete tumor ablation, the entire tumor volume plus a 5–6 mm wide safety margin has to be heated up to a temperature of 60°C (15).
Most RFA systems verify the success of the ablation procedure by measuring the impedance of the target tissue. Impedance is the AC (alternating current) equivalent to the electrical resistance of the tissue; its rise during thermal ablation reflects the dehydration of the ablated tissue. Once the impedance threshold is reached, the ablation process is repeated to ensure complete coagulation necrosis of the tumor by a second exposure to thermal energy.
In contrast, CA relies on freezing the target tissue with a cryoprobe which is inserted into the tumor, lowering the temperature of the adjacent tissue by means of helium or argon down to -40 to -60°C. Repeated freeze-thaw cycles ultimately result in the destruction of cell organelles and membranes, as the created intracellular ice crystals cause irreversible hydropic cell damage.
The principle of physics underlying MWA is similar to that of RFA. By applying high-frequency alternating current in the microwave band (0.9–2.5 GHz) via an ablation probe, rapid agitation of the water molecules in the tissue surrounding the probe is induced; the resulting increase in temperature ultimately causes coagulation necrosis of the target tissue. As microwaves can be efficiently delivered to the target tissue and the ablation time required for tissue necrosis is very short (<10 min), it can be assumed that MWA also is a suitable treatment modality to effectively control small renal tumors; however, only very few case series with small patient populations have become available to date (16).
Indications and patient selection
Percutaneous thermal ablation is used to treat small renal tumors, especially in patients with a single kidney or impaired renal function, as it only slightly reduces the number of nephrons (thermal ablation 11% versus partial nephrectomy 35%) and does not require intraoperative ischemia, thus preventing the associated parenchymal injury (17). It is mostly indicated in patients with comorbidities who are not eligible for surgery because of their poor general condition. Thermal ablation techniques are suitable for patients with genetic disorders that increase the likelihood of multiple bilateral renal tumors (von Hippel-Lindau syndrome, tuberous sclerosis, Birt-Hogg-Dubé syndrome). In rare cases, patients refuse to consent to surgery. For these patients, too, minimally invasive percutaneous thermal ablation may be considered as an alternative to active surveillance.
Technique
Preparation and intervention guide
An important aspect of the preparation for the intervention is to determine the distance between the tumor and the renal hilum as the local thermal ablation effect may be reduced by heat loss via blood and urine flow close to the target tissue volume (heat sink effect) (18).
RFA and CA are typically performed under sedation and analgesia. The ablation probe is inserted percutaneously into the target lesion under tomographic guidance. Where bowel is located immediately adjacent to the tumor, a safety distance to the ablation probe can be created by means of hydrodissection. This is achieved by advancing a 20G tap cannula between the tumor and the bowel and then injecting 100–500 mL of 5% dextrose solution or air (Figure 1) to create a protective thermal insulation zone (19, 20).
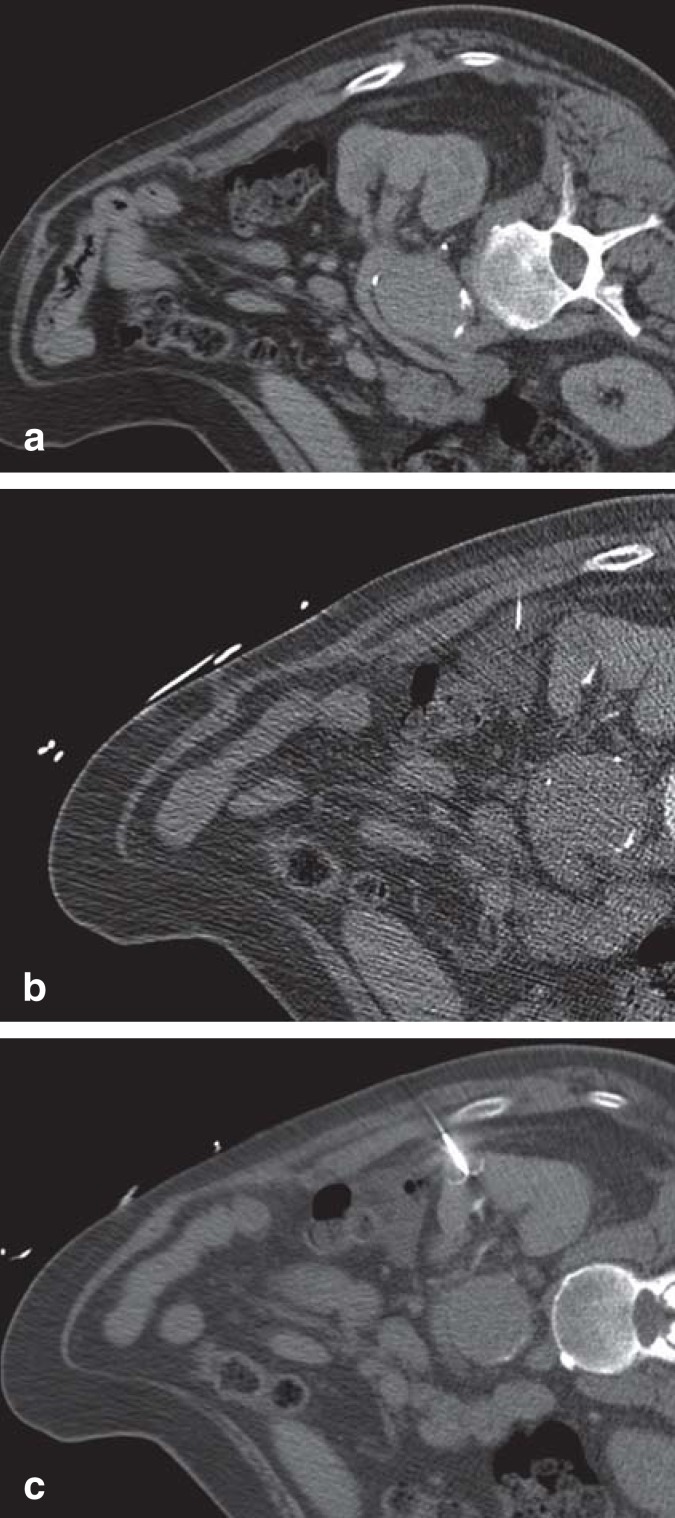
Figure 1.
CT-guided radiofrequency ablation of a renal cell carcinoma after hydrodissection.
a) The pre-interventional CT showed a space-occupying lesion in the left kidney. The descending colon is located immediately adjacent to the lesion.
b) Using a 20-G needle, 250 mL of 5% dextrose solution was injected between kidney and colon.
c) The umbrella-shaped RFA electrode was placed in the tumor core and RF ablation of the lesion was performed without complications. RFA, radiofrequency ablation; RF, radiofrequency
With RFA, the ablation process of 5–20 min duration is considered to be successfully completed once the impedance threshold of the target tissue has been reached twice by incrementally increasing the generator output.
With CA, a 10-minute freezing phase is followed by an 8-minute active thawing phase which in turn is followed by a 10-minute re-freezing phase.
Complications
A key advantage of the interstitial treatment techniques is their comparatively low complication rate (21, 22). The risk of major complication requiring intervention is 1–7% (23, 24), while the ablation-associated mortality rate is below 1% (23, 25). Table 1 provides an overview of the complications and their incidences after thermal ablation.
Table 1. Summary of potential complications of radiofrequency ablation and cryoablation as well as their incidences*.
| Complications | Method | Incidence rate |
|---|---|---|
| Post-ablation syndrome (myalgia, fever, nausea <48 h) (e5) | RFA & CA | 22% |
| Self-limiting hematuria (e6) | RFA & CA | 20% |
| Temporary cloudiness of urine due to chyluria (e7) | RFA & CA | 18% |
| Self-limiting perirenal hematoma (13, e6) | RFA | 9% |
| Self-limiting perirenal hematoma (13, e6) | CA | 2–5% |
| Perirenal hematoma requiring treatment (e7) | RFA | 2% |
| Perirenal hematoma requiring treatment (e8) | CA | 5% |
| Temporary nerve injury (intercostal or genitofemoral) (19, 23) | RFA & CA | 7% |
| Uretral stenosis (13, e9) | RFA | 1.5% |
| Skin burns at the neutral electrode (19) | RFA | 0.8–2% |
| Urinary retention due to occluding blood clot (e6) | RFA & CA | <1% |
| Pneumoperitoneum (13, 19, e6) | RFA & CA | <1% |
| Measurable loss of renal function immediately after ablation (13) | RFA & CA | 0.5% |
*The incidence of typical complications is almost identical for both methods. Only the risk of bleeding is slightly higher for cryoablation compared with radiofrequency ablation, as CA has no cauterizing effect. CA, cryoablation; RFA, radiofrequency ablation; h, hours
Follow-up
The aim of the short-term follow-up after 3 months is to identify any residual tumor tissue early on. Long-term follow-up is meant to detect any local recurrences and is recommended after 6 months and subsequently at yearly intervals (13, 26). Follow-up imaging requires the intravenous application of contrast media as any residual tumor tissue or local recurrences are identified as focal contrast-enhancing lesions. The suspicion that an unclear lesion is a recurrence is strengthened when the mass progressively increases in size during follow-up. These changes should be distinguished from a small ribbon of scar tissue (Figure 2) which delimits the outer ablation margin (23).
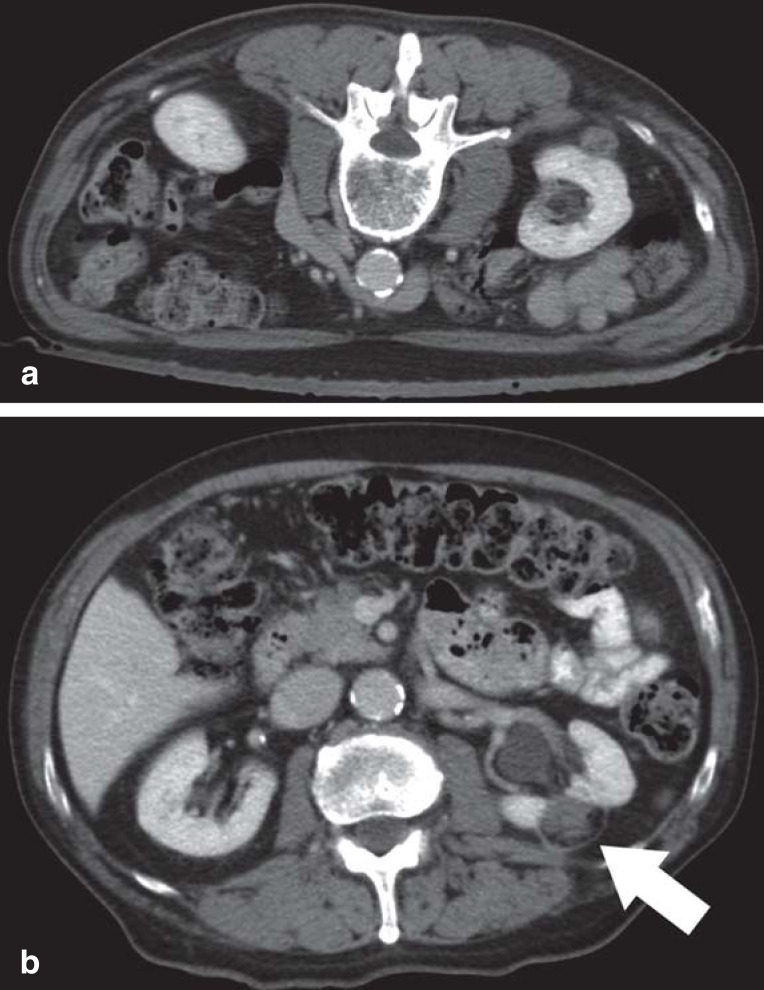
Figure 2.
Computed tomography (CT)–guided radiofrequency ablation and follow-up CT twelve months after ablation.
a) The pre-interventional CT scan in prone position shows the space-occupying exophytic lesion on the dorsal aspect of the kidney.
b) The follow-up CT scan performed 12 months after the successful RFA showed a small ribbon of scar tissue, demarcating the wedge-shaped defect. The ablation area appears as a hypodense defect. No contrast-enhancing lesion suspicious of recurrence is present
Results
Radiofrequency ablation
Over the last decade, numerous authors have reported comparable post-RFA survival data. Table 2 provides a comprehensive overview of current publications and their results. Although the majority of these studies are descriptive case series with heterogeneous study designs, they show several important similarities.
Table 2. Comprehensive selection of published studies with at least 30 patients treated with interstitial therapy and their success and complication rates, based on an extensive search of the PubMed database.
| Author | Year | Patients (n) | Tumor size (cm) | Method | Primary success rate (%) | Follow-up (mDonths) | Freedom of recurrence (%) | Severe complication (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | ||||||||
| Mayo-Smith (e10) | 2003 | 32 | 1–5 | RFA | 81 | 3–36 | 100 | 96 | 0 | |
| Varkarakis (27) | 2005 | 49 | 1–4 | RFA | 87 | 12–48 | 100 | 1.8 | ||
| Gervais (e11) | 2005 | 100 | 1.1–8.9 | RFA | 90 | 3–72 | 100 | 98 | 4 | |
| Levinson (e12) | 2008 | 31 | 1–4 | RFA | 97 | 41–80 | 97 | 94 | 90 | |
| Ferakis (30) | 2010 | 31 | 1.3–7.5 | RFA | 90 | 36–84 | 97 | 90 | 88 | |
| Tracy (32) | 2010 | 208 | 1–5.4 | RFA | 98 | 36–90 | 98 | 97 | 93 | |
| Zagoria (31) | 2011 | 41 | 0.7–8.2 | RFA | 88 | 36–68 | 88 | 83 | 66 | 0 |
| Ji (e13) | 2011 | 106 | 0.9–5.5 | RFA | 99 | 12–48 | 98 | 98 | 0 | |
| Karam (e14) | 2013 | 150 | 0.9–7.1 | RFA | 100 | 6–95 | 100 | 96 | ||
| Atwell (38) | 2013 | 222 | 1–3 | RFA | 98 | 6–102 | 100 | 97 | 93 | 4 |
| Bandi (e15) | 2007 | 78 | Ø 2.6 | CA | 95 | 3–60 | 100 | 98 | 8 | |
| Georgiades (36) | 2008 | 46 | 1–7 | CA | 100 | 4–81 | 97 | 8 | ||
| Spreafico (e16) | 2011 | 36 | 1.2–7 | CA | 96 | 1–36 | 96 | 96 | 6 | |
| Guazzoni (e17) | 2010 | 123 | 0.5–4 | CA | 98 | 12–96 | 100 | 98 | 5 | |
| Rodriguez (24) | 2011 | 117 | 1–7 | CA | 100 | 7–172 | 98 | 92 | 7 | |
| Pirasteh (37) | 2011 | 70 | 1–7 | CA | 99 | 6–60 | 98 | 93 | ||
| Guillotreau (e18) | 2012 | 226 | Ø 2.2 | CA | 100 | 8–66 | 95 | 89 | 3 | |
| Duffey (e19) | 2012 | 116 | 1.1–5.5 | CA | 94 | 1–112 | 91 | 91 | 85 | 2 |
| Atwell (38) | 2013 | 163 | 0.9–3 | CA | 98 | 6–78 | 97 | 95 | 95 | 5 |
CA, cryoablation; RFA, radiofrequency ablation
Stage T1a renal tumors with diameters <4 cm can be treated very effectively and successfully with RFA. In early studies, Varkarakis et al. and Clark et al. reported primary ablation success rates of 89–96% (27, 28). The local recurrence rate in these patient populations was 4–7% after 1–3 years; repeat ablation secured a 100% secondary treatment success. These data were confirmed in a larger sample of 105 patients (29) where a primary ablation success rate of 90% was revealed; the success rate rose to 100% for tumors <3.5 cm and no recurrence was detected within the first 3 years. Another study showed that 3 years after RFA, 92% of patients were free of recurrence (24).
In patients with an initial tumor size ≤ 3 cm at the time of RFA, the primary success rate was 95–98% (30, 31).
Since RFA has only in recent years been used more widely for the curative treatment of renal tumors, initially the promising results achieved with RFA were based on short follow-up periods of 3 years at most. Now, very impressive data covering long-term periods of at least 5 years after percutaneous ablation are starting to become available, defining the role of RFA in the long-term oncology setting. Two independent study groups have reported recurrence-free 5-year survival rates of 88% and 89%, respectively, for tumors ≤4 cm (30, 31). Distant metastasis was not observed in either of the 2 study populations. Tracy et al. confirmed these positive long-term results and reported metastasis-free and disease-specific survival rates of 95% and 99%, respectively (32). In a study with 200 patients, Georgiades et al. achieved a success rate of 96% six years after RFA and concluded that RFA should be considered equivalent to partial nephrectomy in the treatment of T1a renal tumors (23).
After complete surgical removal of a T1a renal tumor, the expected 5-year survival is 97% (33). Since thermal ablation is associated with the risk that isolated vital cell nests may persist in the periphery of the tumor, nephrectomy still seems to offer the highest safety level, despite the excellent long-term success rates achieved with RFA.
RFA should be seen as an effective treatment alternative, offering the additional advantage of possible repeat application in case of recurrence as it spares the healthy renal parenchyma. With the proven linear correlation between the number of functional nephrons and long-term survival (7, 8), the fact that RFA causes only minimal loss of renal function underscores the significance of this technique. As part of the overall treatment strategy, RFA is of great benefit especially in patients with impaired renal function, kidney transplant, contralateral recurrences or hereditary diseases associated with multiple renal tumors.
At the same time, the minimal invasiveness of RFA is convincing. Additional advantages of RFA over surgery that have helped to establish radiofrequency ablation as a curative treatment for small renal cell carcinomas include the low complication rate and possibility to perform the intervention on an outpatient basis.
Cryoablation
The few authors who reported their primary success rates after CA found somewhat higher rates compared with RFA (34). One argument in favor of CA is that the result of the ablation treatment can already be visualized during the intervention as a hypodense ice ball. Based on this, the success of the procedure can be evaluated. For cryoablation, a close correlation between initial tumor size and probability of successful treatment has also been demonstrated (35). For space-occupying lesions <4 cm, Georgiades et al. achieved a success rate of 100% one year after ablation (36). Over a follow-up period of 3 years, Pirasteh et al. demonstrated lasting treatment success in 93% of patients (37). Atwell et al. reported similarly promising results for the treatment of 145 small renal tumors <3 cm (38). The recurrence-free survival rates after 1, 3 and 5 years were 97.3%, 90.6% and 90.6%, respectively.
The currently available long-term data consistently indicate that in interstitial renal tumor treatment both techniques are equivalent (38– 40).
However, it should be mentioned that so far no randomized controlled trials comparing the two techniques have become available and that the published data are primarily based on retrospective analyses. In addition, no randomized controlled trials directly comparing surgical resection procedures with locally ablative techniques are available. Besides the comparison of percutaneous interstitial and surgical techniques with regard to tumor control, survival and complication rates, prospective randomized studies should especially address patient-reported quality of life after tumor treatment to enable a conclusive evaluation of the significance of interstitial techniques. Given their minimal invasiveness, it is to be expected that percutaneous ablation techniques will help to improve the health-related quality of life of these patients.
Percutaneous vs. laparoscopic ablation
Relevant advantages of percutaneous ablation are its minimally invasive nature and the possibility to perform the intervention on an outpatient basis. This does not only help to reduce patient stress, but also translates into cost savings of 30–50% (e1, e2). Hui et al. showed in their meta-analysis that the rate of severe complications is significantly lower after percutaneous intervention compared with laparoscopic tumor ablation (33). Based on data from MDCT scans performed as part of the follow-up care, efficacy is independent of the access route (19). A prospective study showed that in cases where vital cell nests remained after percutaneous thermal ablation, repeat percutaneous ablation lead to tumor necrosis equivalent to that observed after laparoscopic ablation (33). However, these data are to be confirmed in future randomized controlled trials. In patients where the space-occupying lesion is located very close to the hilum, preference should be given to the laparoscopic approach, as this increases the likelihood that renal blood vessels and ureter are spared. The published data show no advantage of laparoscopic over percutaneous thermal ablation in terms of oncological control of small renal tumors; consequently, preference should be given to the less invasive percutaneous technique (11, 19).
Guideline recommendations for the use of thermal ablation techniques
The American Urological Association (AUA) was the first medical specialty society in the world to include thermal ablation techniques in its recommendations for the treatment of localized renal tumors and to support the use of RFA and CA as an alternative to surgical procedures, especially for patients in poor clinical condition; however, it also warns of their potential for local recurrence (e3).
The guidelines of the European Association of Urology recommend the use of thermal ablation to treat small tumors as well as patients with significant concomitant disease (e4). The S3-guideline of the German Cancer Society’s (DKG) guideline program is expected to be published in early 2015. In view of the growing evidence to support their high long-term oncological efficacy, it can be expected that thermal ablation techniques will be included in future guidelines as a curative minimally invasive alternative to surgical resection, adding to the range of treatment options available for patients with localized renal tumors.
Key Messages.
Image-guided thermal ablation is a novel interstitial treatment, particularly suitable for small renal cell carcinomas <4 cm
After assessing all available treatment options, the decision for thermal ablation is to be made by consensus based on an interdisciplinary tumor board agreement.
Radiofrequency ablation (RFA) and cryoablation (CA) are the most commonly used methods, while microwave ablation of renal tumors is still experimental.
The key advantages of interstitial ablation techniques lie in their minimal invasiveness, comparatively low complication rates and the feasibility of repeat application, along with only minor loss of renal function.
Current data on long-term tumor control in patients with small renal cell carcinomas consistently indicate that RFA and CA achieve long-term treatment success in over 90% of patients.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
Prof. Chun has received consultancy fees from Janssen Cilag and UroTech. He has received fees for preparing continuing medical education events from Astellas.
PD Regier declares that no conflict of interests exists.
References
- 1.Robert Koch-Institut. Robert Koch-Institut (eds.) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (eds.) 9th edition. Vol. 27. Berlin: 2013. Krebs in Deutschland 2009/2010; pp. 1–150. [Google Scholar]
- 2.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Fisher HAG. Management of small renal cancers: Choices for the patient and physician. J Urol. 2006;176:1907–1908. doi: 10.1016/j.juro.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 4.Kouba E, Smith A, McRackan D, Wallen EM, Pruthi RS. Watchful waiting for solid renal masses: Insight into the natural history and results of delayed intervention. J Urol. 2007;177:466–470. doi: 10.1016/j.juro.2006.09.064. [DOI] [PubMed] [Google Scholar]
- 5.Hallscheidt P, Noeldge G, Schawo S, et al. [Advances in the staging of renal cell carcinoma with high-resolution imaging] Rofo. 2007;179:1236–1242. doi: 10.1055/s-2007-963574. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RH, Siddiqui S, Lohse CM, Leibovich BC, Russo P, Blute ML. Partial versus radical nephrectomy for 4 to 7 cm renal cortical tumors. J Urol. 2009;182:2601–2606. doi: 10.1016/j.juro.2009.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. JURO. 2000;163:442–445. [PubMed] [Google Scholar]
- 8.Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. JURO. 2010;183:1317–1323. doi: 10.1016/j.juro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Castle SM, Salas N, Leveillee RJ. Renal cancer. UR. 2011;77:792–797. doi: 10.1016/j.urology.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 10.Nabi G, Goodman C, Melzer A. High intensity focused ultrasound treatment of small renal masses: Clinical effectiveness and technological advances. Indian J Urol. 2010;26:331–337. doi: 10.4103/0970-1591.70561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zagoria RJ, Childs DD. Update on thermal ablation of renal cell carcinoma: Oncologic control, technique comparison, renal function preservation, and new modalities. Curr Urol Rep. 2011;13:63–69. doi: 10.1007/s11934-011-0224-y. [DOI] [PubMed] [Google Scholar]
- 12.van Poppel H, Becker F, Cadeddu JA, et al. Treatment of localised renal cell carcinoma. Eur Urol. 2011;60:662–672. doi: 10.1016/j.eururo.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 13.Venkatesan AM, Wood BJ, Gervais DA. Percutaneous ablation in the kidney. Radiology. 2011;261:375–391. doi: 10.1148/radiol.11091207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gideon L, Michael G, Mehul D, Merce J, Gaston MB, Raymond L. Long-term oncologic outcomes following radiofrequency ablation with real-time temperature monitoring for T1a renal cell cancer. Urologic Oncology. Seminars and Original Investigations. 2014;1:1–7. doi: 10.1016/j.urolonc.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Georgiades C, Rodriguez R, Azene E, et al. Determination of the nonlethal margin inside the visible “ice-ball” during percutaneous cryoablation of renal tissue. Cardiovasc Intervent Radiol. 2013;36:783–790. doi: 10.1007/s00270-012-0470-5. [DOI] [PubMed] [Google Scholar]
- 16.Floridi C, De Bernardi I, Fontana F, et al. Microwave ablation of renal tumors: state of the art and development trends. Radiol Med. 2014;119:533–540. doi: 10.1007/s11547-014-0426-8. [DOI] [PubMed] [Google Scholar]
- 17.Takaki H, Soga N, Kanda H, et al. Radiofrequency ablation versus radical nephrectomy: Clinical outcomes for stage t1b renal cell carcinoma. Radiology. 2014;270:292–299. doi: 10.1148/radiol.13130221. [DOI] [PubMed] [Google Scholar]
- 18.Al-Alem I, Pillai K, Akhter J, Chua TC, Morris DL. Heat sink phenomenon of bipolar and monopolar radiofrequency ablation observed using polypropylene tubes for vessel simulation. Surgical Innovation. 2014;21:269–276. doi: 10.1177/1553350613505713. [DOI] [PubMed] [Google Scholar]
- 19.Young EE, Castle SM, Gorbatiy V, Leveillee RJ. Comparison of safety, renal function outcomes and efficacy of laparoscopic and percutaneous radio frequency ablation of renal masses. J Urol. 2012;187:1177–1182. doi: 10.1016/j.juro.2011.11.099. [DOI] [PubMed] [Google Scholar]
- 20.Boone J, Bex A, Prevoo W. Percutaneous radiofrequency ablation of a small renal mass complicated by appendiceal perforation. Cardiovasc Intervent Radiol. 2011;35:695–699. doi: 10.1007/s00270-011-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tailly T, Goeman L, Gontero P, Joniau S. The safety and oncologic efficacy of radio-frequency ablation for the treatment of small renal masses: comprehensive review of the current literature. Panminerva Med. 2010;52:319–329. [PubMed] [Google Scholar]
- 22.Joniau S, Tailly T, Goeman L, Blyweert W, Gontero P, Joyce A. Kidney radiofrequency ablation for small renal tumors: oncologic efficacy. J Endourol. 2010;24:721–728. doi: 10.1089/end.2009.0677. [DOI] [PubMed] [Google Scholar]
- 23.Georgiades C, Rodriguez R. Renal tumor ablation. Techniques in Vascular and Interventional Radiology. 2013;16:230–238. doi: 10.1053/j.tvir.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez R, Cizman Z, Hong K, Koliatsos A, Georgiades C. Prospective analysis of the safety and efficacy of percutaneous cryoablation for pT1NxMx biopsy-proven renal cell carcinoma. Cardiovasc Intervent Radiol. 2011;34:573–578. doi: 10.1007/s00270-010-9934-7. [DOI] [PubMed] [Google Scholar]
- 25.Iannuccilli JD, Dupuy DE, Mayo-Smith WW. Solid renal masses: effectiveness and safety of image-guided percutaneous radiofrequency ablation. Abdom Imaging. 2011;37:647–658. doi: 10.1007/s00261-011-9807-9. [DOI] [PubMed] [Google Scholar]
- 26.Feng B, Liang P. Local thermal ablation of renal cell carcinoma. Eur J Radiol. 2012;81:437–440. doi: 10.1016/j.ejrad.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 27.Varkarakis IM, Allaf ME, Inagaki T, et al. Percutaneous radio frequency ablation of renal masses: results at a 2-year mean followup. JURO. 2005;174:456–460. doi: 10.1097/01.ju.0000165655.91152.c5. discussion 460. [DOI] [PubMed] [Google Scholar]
- 28.Clark TWI, Malkowicz B, Stavropoulos SW, et al. Radiofrequency ablation of small renal cell carcinomas using multitined expandable electrodes: preliminary experience. J Vasc Interv Radiol. 2006;17:513–519. doi: 10.1097/01.RVI.0000204853.75376.2C. [DOI] [PubMed] [Google Scholar]
- 29.Breen DJ, Rutherford EE, Stedman B, et al. Management of renal tumors by image-guided radiofrequency ablation: Experience in 105 tumors. Cardiovasc Intervent Radiol. 2007;30:936–942. doi: 10.1007/s00270-007-9090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferakis N, Bouropoulos C, Granitsas T, Mylona S, Poulias I. Long-term results after computed-tomography-guided percutaneous radiofrequency ablation for small renal tumors. J Endourol. 2010;24:1909–1913. doi: 10.1089/end.2009.0639. [DOI] [PubMed] [Google Scholar]
- 31.Zagoria RJ, Pettus JA, Rogers M, Werle DM, Childs D, Leyendecker JR. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. URL. 2011;77:1393–1397. doi: 10.1016/j.urology.2010.12.077. [DOI] [PubMed] [Google Scholar]
- 32.Tracy CR, Raman JD, Donnally C, Trimmer CK, Cadeddu JA. Durable oncologic outcomes after radiofrequency ablation. Cancer. 2010;116:3135–3142. doi: 10.1002/cncr.25002. [DOI] [PubMed] [Google Scholar]
- 33.Hui GC, Tuncali K, Tatli S, Morrison PR, Silverman SG. Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol. 2008;19:1311–1320. doi: 10.1016/j.jvir.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Tsimafeyeu I, Zart JS, Chung B. Cytoreductive radiofrequency ablation in patients with metastatic renal cell carcinoma (RCC) with small primary tumours treated with sunitinib or interferon- α. BJU Int. 2013;112:32–38. doi: 10.1111/bju.12107. [DOI] [PubMed] [Google Scholar]
- 35.Atwell TD, Farrell MA, Leibovich BC, et al. Percutaneous renal cryoablation: experience treating 115 tumors. J Urol. 2008;179:2136–2140. doi: 10.1016/j.juro.2008.01.144. discussion 2140-1. [DOI] [PubMed] [Google Scholar]
- 36.Georgiades CS, Hong K, Bizzell C, Geschwind JF, Rodriguez R. Safety and efficacy of CT-guided percutaneous cryoablation for renal cell carcinoma. J Vasc Interv Radiol. 2008;19:1302–1310. doi: 10.1016/j.jvir.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Pirasteh A, Snyder L, Boncher N, Passalacqua M, Rosenblum D, Prologo JD. Cryoablation vs. radiofrequency ablation for small renal masses. Academic Radiology. 2011;18:97–100. doi: 10.1016/j.acra.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Atwell TD, Schmit GD, Boorjian SA, et al. Percutaneous ablation of renal masses measuring 30. cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. AJR. 2013;200:461–466. doi: 10.2214/AJR.12.8618. [DOI] [PubMed] [Google Scholar]
- 39.Chalasani V, Martinez CH, Lim D, Abdelhady M, Chin JL. Surgical cryoablation as an option for small renal masses in patients who are not ideal partial nephrectomy candidates: intermediate-term outcomes. Can Urol Assoc J. 2010;4:399–402. doi: 10.5489/cuaj.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cadeddu JA. Laparoscopy/new technology. JURO. 2013;189 [Google Scholar]
- e1.Badwan K, Maxwell K, Venkatesh R, et al. Comparison of laparoscopic and percutaneous cryoablation of renal tumors: a cost analysis. J Endourol. 2008;22:1275–1277. doi: 10.1089/end.2008.0102. [DOI] [PubMed] [Google Scholar]
- e2.Hinshaw JL, Shadid AM, Nakada SY, Hedican SP, Winter TC, Lee FT. Comparison of percutaneous and laparoscopic cryoablation for the treatment of solid renal masses. AJR. 2008;191:1159–1168. doi: 10.2214/AJR.07.3706. [DOI] [PubMed] [Google Scholar]
- e3.AUA. Guideline for management of the clinical stage 1 renal mass. Kansas City. American Urological Association. 2009;22:1–81. [Google Scholar]
- e4.Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. European Urology. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- e5.Wah TM, Arellano RS, Gervais DA, Saltalamacchia CA, Martino J, Halpern EF, et al. Image-guided percutaneous radiofrequency ablation and incidence of post-radiofrequency ablation syndrome: prospective survey. Radiology. 2005;237:1097–1102. doi: 10.1148/radiol.2373042008. [DOI] [PubMed] [Google Scholar]
- e6.Rhim H, Dodd GD III, Chintapalli KN, et al. Radiofrequency thermal ablation of abdominal tumors: Lessons learned from complications. Radiographics. 2004;24:41–52. doi: 10.1148/rg.241025144. [DOI] [PubMed] [Google Scholar]
- e7.Zagoria RJ. Imaging-guided radiofrequency ablation of renal masses. Radiographics. 2004;24:59–71. doi: 10.1148/rg.24si045512. [DOI] [PubMed] [Google Scholar]
- e8.Maybody M, Solomon SB. Image-guided percutaneous cryoablation of renal tumors. Techniques in vascular and interventional Radiology. 2007;10:140–148. doi: 10.1053/j.tvir.2007.09.009. [DOI] [PubMed] [Google Scholar]
- e9.Permpongkosol S, Nicol TL, Bagga HS, Kohanim S, Kavoussi L, Solomon SB. Prophylactic gelatin sponge tract injection to prevent bleeding after percutaneous renal cryoablation in a swine model. J Vasc Interv Radiol. 2006;17:1505–1509. doi: 10.1097/01.RVI.0000235773.23394.51. [DOI] [PubMed] [Google Scholar]
- e10.Mayo-Smith WW, Dupuy DE, Parikh PM, Pezzullo JA, Cronan JJ. Imaging-guided percutaneous radiofrequency ablation of solid renal masses: techniques and outcomes of 38 treatment sessions in 32 consecutive patients. AJR. 2003;180:1503–1508. doi: 10.2214/ajr.180.6.1801503. [DOI] [PubMed] [Google Scholar]
- e11.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR. 2005;185:64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- e12.Levinson AW, Su L-M, Agarwal D, et al. Long-term oncological and overall outcomes of percutaneous radio frequency ablation in high risk surgical patients with a solitary small renal mass. J Urol. 2008;180:499–504. doi: 10.1016/j.juro.2008.04.031. discussion 504. [DOI] [PubMed] [Google Scholar]
- e13.Ji C, Li X, Zhang S, Gan W, et al. Laparoscopic radiofrequency ablation of renal tumors: 32-month mean follow-up results of 106 patients. Urology. 2011;77:798–802. doi: 10.1016/j.urology.2010.10.014. [DOI] [PubMed] [Google Scholar]
- e14.Karam JA, Ahrar K, Vikram R, et al. Radiofrequency ablation of renal tumours with clinical, radiographical and pathological results. BJU Int. 2013;111:997–1005. doi: 10.1111/j.1464-410X.2012.11608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e15.Bandi G, Christian MW, Hedican SP, Moon TD, Nakada SY. Cryoablation of small renal masses: assessment of the outcome at one institution. BJU Int. 2007;100:798–801. doi: 10.1111/j.1464-410X.2007.07158.x. [DOI] [PubMed] [Google Scholar]
- e16.Spreafico C, Nicolai N, Lanocita R, et al. Crioablazione percutanea TC-guidata delle masse renali in pazienti selezionati. Radiol Med. 2011;117:593–605. [Google Scholar]
- e17.Guazzoni G, Cestari A, Buffi N, et al. Renal cancer. URL. 2010;76:624–629. doi: 10.1016/j.urology.2010.03.078. [DOI] [PubMed] [Google Scholar]
- e18.Guillotreau J, Haber G-P, Autorino R, et al. Robotic partial nephrectomy versus laparoscopic cryoablation for the small renal mass. Eur Urol. 2012;61:899–904. doi: 10.1016/j.eururo.2012.01.007. [DOI] [PubMed] [Google Scholar]
- e19.Duffey B, Nguyen V, Lund E, Koopmeiners JS, Hulbert J, Anderson JK. Third prize: Intermediate-term outcomes after renal cryoablation: Results of a multi-institutional study. J Endourol. 2012;26:15–20. doi: 10.1089/end.2011.0179. [DOI] [PubMed] [Google Scholar]