Abstract
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative approach in patients with multiple myeloma, but its use for consolidation of first remission has not yet been fully explored. Twenty-two myeloma patients with very good partial response (VGPR) or CR received allogeneic peripheral blood grafts as consolidation from HLA-matched donors between 2007 and 2012. Conditioning regimens were fludarabine (30 mg/m2 i.v. if with bortezomib and 40 mg/m2 i.v. when without bortezomib, ×4 days) plus melphalan (70 mg/m2 intravenously ×2 days) with (n = 13) or without (n = 9) bortezomib (1.3 mg/m2). The cumulative incidence of grades 2–4 acute GVHD at day 100 was 45% (95% CI: 24–65%) and moderate-to-severe chronic GVHD at 2 years was 46% (95% CI: 19–69%). With a median follow-up of 18 (range, 2–61) months, the 2-year PFS estimate is 74.8% (95% CI: 45–90%), which compares favorably with the 52% (95% CI: 35–66%) after autologous HCT for similar patients (a median follow-up of 30 (range, 9–55) months). We are conducting a phase 2 study to assess the efficacy of allogeneic HCT as post-remission therapy.
Keywords: allogeneic hematopoietic cell transplantation, multiple myeloma, bortezomib, fludarabine, melphalan, conditioning
INTRODUCTION
Despite recent advances in treatment options and improved survival, multiple myeloma remains incurable with standard chemotherapy and autologous transplant for most patients with a median time to progression in the range of 40 months with lenalidomide post-transplant maintenance.1,2 Until now, the questions regarding the role of allogeneic hematopoietic cell transplantation (HCT) for remission consolidation could not be fully addressed, in part, due to significantly high burden of myeloma after conventional chemotherapy.3–6 However, the time to address such a question has arrived with the incorporation of bortezomib and lenalidomide into the initial systemic therapy resulting in higher rates of very good partial response (VGPR) and CR.7–9
The use of reduced-intensity conditioning preparative regimens early after initial responses in the era of novel agents might provide an avenue to explore the potent graft-versus-myeloma effect while minimizing non-relapse mortality (NRM).10–15 Therefore, we hypothesized that incorporation of allogeneic HCT early in the disease course as a consolidation therapy for the initial response would maximize the potential benefits of allografting. We report initial results of a reduced-intensity conditioning of fludarabine and melphalan with or without bortezomib in patients with multiple myeloma undergoing allogeneic HCT in their first VGPR or CR following systemic therapy containing bortezomib or lenalidomide.
PATIENTS AND METHODS
Eligibility criteria
Eligible patients for this analysis were those with treatment-responsive multiple myeloma in their first VGPR or CR at the time of allogeneic HCT after initial systemic therapy, aged between 18 and 60 years. We analyzed the outcomes of patients treated with fludarabine and melphalan ± bortezomib conditioning for allogeneic HCT during 2007–2012. Bortezomib was given with fludarabine and melphalan under the clinical protocol (MCC 15697), which was registered at Clinical Trials.gov as NCT 00948922 and approved by the Institutional Review Board (IRB) of University of South Florida (USF). Those patients without bortezomib were treated off. For some patients who were PR after initial systemic therapy, they were eligible to be treated first with autologous HCT using melphalan (100 mg/m2 i.v. ×2 days) plus bortezomib (1.3 mg/m2 i.v., given immediately after the second dose of melphalan) conditioning prior to receiving allogeneic HCT under MCC 15697. In the same time period, there were only two additional patients who achieved PR after initial systemic therapy, and who received allogeneic HCT with fludarabine and melphalan conditioning at our institution, and they were not included in the analysis. All patients signed informed consent in accordance with the Declaration of Helsinki for the study of long-term follow-up after HCT approved by the IRB of USF.
Donors were 8/8 HLA-matched (HLA-A, -B, -C and -DRB1) siblings or unrelated adults. Patients were considered eligible for allogeneic HCT if they have Karnofsky performance status of ≥60%, creatinine clearance >30 mL/min, platelet count ≥30 000/μL, ANC ≥1000/μL, left ventricular ejection fraction ≥40%, bilirubin ≤2 mg/dL, transaminases ≤2.5-times the upper limit of normal and carbon monoxide diffusion capacity ≥50%. A high-risk group by cytogenetic changes was defined by the presence of hypodiploidy, t(4;14), t(14;16), or loss of 17p13, by conventional cytogenetics or FISH. Deletion of chromosome 13q was considered high-risk only if detected by conventional cytogenetics.
Autologous cohort
To perform an exploratory comparison of efficacy between fludarabine and melphalan ± bortezomib conditioning regimen of allogeneic HCT and conventional autologous HCT in a similar patient population, we retrospectively identified a cohort of myeloma patients. From January 2006 to December 2009, there were 362 myeloma patients who underwent high-dose chemotherapy (melphalan-based) followed by autologous HCT without post-transplant maintenance therapy. Of those, 41 patients were ≤60 years of age and were in their first VGPR or CR at the time of autologous HCT, and were selected for this analysis.
Treatment plan
Fludarabine and melphalan preparative regimen consisted of fludarabine (if creatinine clearance <70 mL/min, then reduced dose) at 30 mg/m2 (with bortezomib) or 40 mg/m2 (without bortezomib) administered i.v. over 30 min on days −6, −5, −4 and −3, and melphalan 70 mg/m2 administered intravenously over 30 min on days −4 and −3. Under MCC 15697 clinical trial, bortezomib was given on day −3 at 1.3 mg/m2 i.v. push over 3–5 s followed by normal saline flush immediately after melphalan (n = 13). For those three patients who received autologous HCT (prior to allogeneic HCT) under MCC protocol 15967, conditioning regimen consisted of melphalan 100 mg/m2 administered intravenously daily over 30 min on days −4 and −3, and bortezomib at 1.3 mg/m2 administered intravenously over 3–5 s immediately after the melphalan. Two patients were enrolled to maintenance lenalidomide trial after allogeneic HCT with fludarabine and melphalan conditioning. None of the patients on the fludarabine, melphalan and bortezomib conditioning regimen protocol (MCC 15697) received post-transplant maintenance regimen nor donor-lymphocyte infusion.
GVHD prophylaxis consisted of tacrolimus/MTX, tacrolimus/mycophenolate mofetil, or tacrolimus/sirolimus, administered as per the institutional guidelines. Immunosuppression was tapered in the absence of GVHD at the discretion of the treating physician. Antibacterial, antifungal and antiviral prophylaxes were provided following the institutional standards. Myeloma disease monitoring with serum and urine protein electrophoresis, and serum-free light chain assays were performed every 3 months after allogeneic HCT until disease progression. Quality of life (QOL) was assessed using the Functional Assessment of Cancer Therapy-Blood and Marrow Transplantation (FACT-BMT) questionnaire at baseline (before allogeneic HCT), and on days +30, +90, +180, +270, +540 and +740 after allogeneic HCT under MCC 15697 (n = 12).16
Statistical endpoints
Response definition was based on International Uniform Response Criteria.17 Neutrophil engraftment was defined as the first day of ANC ≥500/μL for 3 consecutive days and platelet engraftment was defined as the first day of the platelet count ≥20 000/μL for 7 consecutive days independent of transfusions. GVHD was scored according to the published guidelines.18,19 OS and PFS were calculated using the methods of Kaplan and Meier and compared using the log-rank test.20 Cumulative incidence of grade II–IV acute GVHD and moderate to severe chronic GVHD were estimated and compared by the Gray test.21 Death and progression of disease were considered as a competing risk. Pointwise 95% confidence intervals for survival curves and cumulative incidence curves were computed using log–log transformation.
RESULTS
Patient characteristics
Patient characteristics and transplant variables are summarized in Table 1. Details of initial systemic therapy for allogeneic HCT recipients were summarized in Table 2. A total of 22 myeloma patients with first VGPR or CR received allogeneic HCT and 41 patients with VGPR or CR received autologous HCT. Three of 22 patients received autologous HCT for cytoreduction before allogeneic HCT. All patients received G-CSF-mobilized peripheral blood stem cells from 8/8 HLA-A, -B, -C and -DRB1 matched related/unrelated donors or autografts.
Table 1.
Patient demographics and transplant variables
| Allogeneic HCT cohort (n = 22) | Autologous HCT cohort (n = 41) | |
|---|---|---|
| Median (range) age at HCT, years | 49 (25–59) | 54 (40–60) |
| Median time (range) from diagnosis to HCT, days | 269 (141–637) | 279 (144–990) |
| Immunoglobulin subtype | ||
| IgG | 12 (55%) | 15 (37%) |
| IgA | 3 (13%) | 11 (27%) |
| IgD | 0 | 1 (2%) |
| Light chain only | 7 (32%) | 12 (29%) |
| Non-secretory | 0 | 2 (5%) |
| Durie–Salmon staging | ||
| 1A | 1 (4%) | 1 (2%) |
| 2A | 2 (9%) | 9 (22%) |
| 3A | 14 (64%) | 24 (59%) |
| 3B | 5 (23%) | 7 (17%) |
| Cytogenetics/FISH | ||
| High-risk | 7 (32%) | 5 (21% = 5/24) |
| Not available | 0 | 17 |
| Disease status at HCT | ||
| sCR | 6 (27%) | 13 (32%) |
| CR | 4 (18%) | 13 (32%) |
| VGPR | 12 (55%) | 15 (36%) |
| Number of prior therapy | 1 (100%) | 1 (100%) |
| Prior therapya | ||
| Lenalidomide-based | 2 (9%) | 8 (19%) |
| Bortezomib-basedb | 9 (41%) | 16 (39%) |
| Both lenalidomide and bortezomib | 11 (50%) | 4 (10%) |
| Other regimens | 0 | 13 (32%) |
| Donor relation | ||
| Related | 9 (41%) | — |
| Unrelatedc | 13 (59%) | — |
| Donor/recipient gender matching | (Male/female = 25/16) | |
| Female/female | 5 (23%) | — |
| Female/male | 4 (18%) | — |
| Male/female | 6 (27%) | — |
| Male/male | 7 (32%) | — |
| Donor/recipient CMV | ||
| Negative/negative | 6 (27%) | — |
| Negative/positive | 7 (32%) | — |
| Positive/negative | 3 (14%) | — |
| Positive/positive | 6 (27%) | — |
| GVHD prophylaxis | ||
| Tacrolimus/MTX | 8 (36%) | — |
| Tacrolimus/mycophenolate mofetil | 6 (28%) | — |
| Tacrolimus/sirolimus | 8 (36%) | — |
| Median CD34 (range) cell dose (× 106/kg)d | 6.38 (1.43–10.65) | 3.53 (2.02–30.06) |
| Median Karnofsky performance status (range) at HCT | 90 (80–100) | 90 (70–100) |
Abbreviations: HCT = hematopoietic cell transplantation; sCR = stringent CR; VGPR, very good partial response
All patients received dexamethasone.
Two patients received liposomal doxorubicin containing triplet therapy and two others received CY-containing triplet therapy.
One donor was mismatched at DQ locus (9/10 matched)
Recipient who received 1.43 × 106 CD34+ cells/kg later received stem cell boost.
Table 2.
Initial systemic therapy for allogeneic HCT recipients
| Regimens | n |
|---|---|
| CVDD | 4 |
| Rev/Dex | 2 |
| Rev/Dex followed by RVD | 3 |
| Vel/Dex followed by RVD | 2 |
| RVD followed by Vel/Dex | 1 |
| RVD | 4 |
| DVD | 2 |
| Vel/Dex | 1 |
| Ve/Dex followed by CyBorD followed by Rev/Dex | 1 |
| CyBorD | 1 |
| Thal/Dex followed by DVD | 1 |
Abbreviations: CVDD = CY, bortezomib (Velcade), liposomal doxorubicin (Doxil) and dexamethasone; CyBorD = CY, bortezomib and dexamethasone; Dex = dexamethasone; DVD = liposomal doxorubicin (Doxil), bortezomib (Velcade) and dexamethasone; Rev = lenalidomide (Revlimid); RVD = lenalidomide (Revlimid), bortezomib (Velcade) and dexamethasone; Thal = thalidomide
Engraftment and chimerism
Neutrophil engraftment was achieved at medians of 15 (range, 11–19) days after transplant in allogeneic HCT recipients and 17 (range, 12–21) days after autologous HCT. Platelet engraftment was achieved at medians of 16 (range, 12–21) days after allogeneic HCT and 12 (range, 10 – 17) days after autologous HCT. At day +30, CD3- and CD33-sorted peripheral blood cells (n = 20) were medians of 100 (range, 80.99–100) % donor type and 100 (range, 98.76–100) % donor type, respectively. There were no graft failures. BM engraftment at 6 (n = 10) and 12 months (n = 8) were medians of 100 (range, 95–100) and 100 (range, 96–100) % donor type, respectively.
Response
Stringent CR (sCR) was noted in 6 patients (27%) before the allogeneic HCT, which was increased to 15 recipients (68%) at best response after the transplant. One patient progressed after achieving sCR at +446 days, while all other sCR recipients maintained their responses at the time of analysis. Of those 12 patients who were in VGPR at the time of transplant, 4 converted to CR and 5 converted to sCR at their best responses; 2 recipients remained in VGPR. In the autologous cohort, sCR was achieved in 24 patients (57%) and CR was achieved in 9 patients (21%) after autologous HCT. Cumulative incidence of relapse at 24 months was 8.3% (95% CI: 0.4%–32.4%) for the allogeneic cohort and 45.6% (94% CI: 29.4%–60.5%) for the autologous cohort.
GVGD
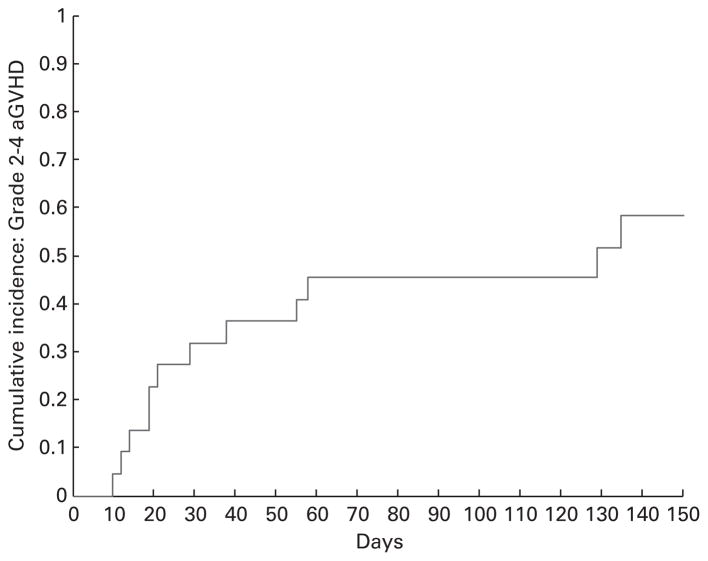
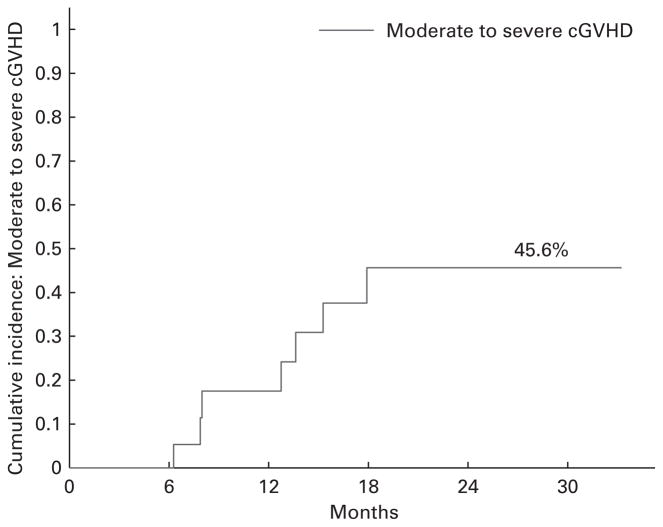
After allogeneic HCT, the cumulative incidence of grades II–IV acute GVHD at day 100 was 45% (95% CI: 24–65%) overall (Figure 1). The cumulative incidence of moderate-to-severe chronic GVHD at 2 years based on NIH consensus criteria was 46% (95% CI: 26–79%) (Figure 2). Of 10 patients who were treated with systemic corticosteroid for GVHD, 3 (30%) were tapered to off at the time of analysis. There were 7 patients who received tacrolimus and MTX GVHD prophylaxis with bortezomib as part of their conditioning and only 1 developed acute GVHD.
Figure 1.
Cumulative incidence curve of grade 2–4 acute GVHD.
Figure 2.
Moderate-to-severe chronic GVHD.
OS and PFS
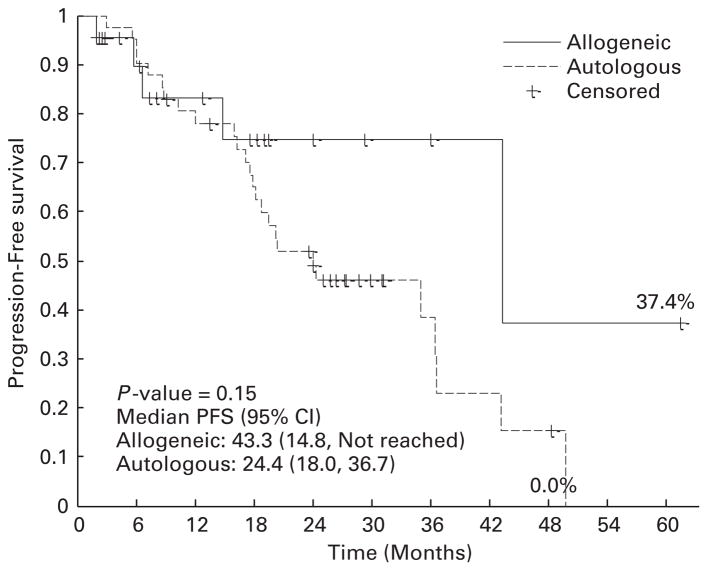
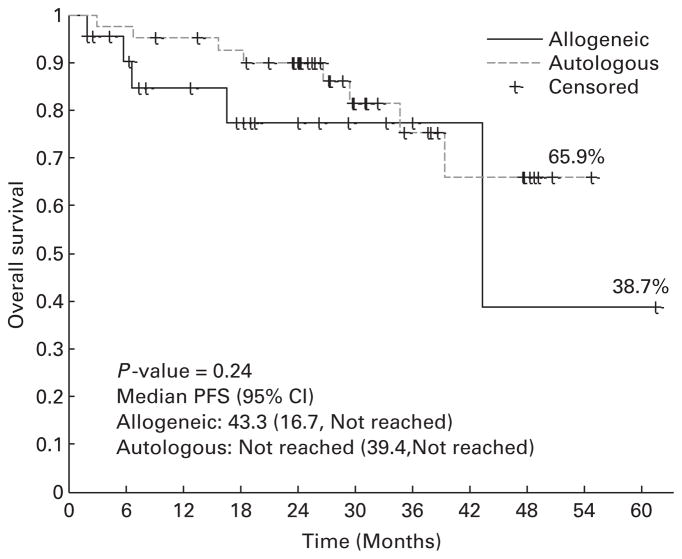
After allogeneic HCT, 4 patients died of GVHD-related complications, 1 died of disease progression and 18 remained alive at the time of analysis. The 2-year PFS estimate is 74.8% (95% CI: 44.6–90.1%) with a median follow-up of 18 (range, 2–61) months for allogeneic HCT cohort, and 51.9% (95% CI: 35.4–66.1%) for the autologous HCT cohort with a median follow-up of 30 months (range, 9–55 months) (Figure 3). The 2-year OS estimate is 77.5% (95% CI: 49.7–91.1%) for allogeneic HCT cohort and 90.0% (95% CI: 75.4–96.1%) for the autologous cohort (Figure 4). There were no differences in PFS or OS when allogeneic HCT patients were stratified based on their cytogenetic risk group (P = 0.914). NRM at 6 24 months are 10.5% (95% CI: 1.6–29%) and 16.9% (95% CI: 3.8 –8%) for the allogeneic cohort, and 2.4% (95% CI: 0.2–11.2%) and 2.4% (95% CI: 0.2–11.2%) for the autologous cohort.
Figure 3.
PFS.
Figure 4.
OS.
Quality of life
For allogeneic HCT recipients (n = 12), mean FACT-G scores were 82.4 at baseline (n = 11), 68.9 at day +30 (n = 12), 79.1 at day +90 (n = 8), 84.4 at day +180 (n = 11), 78.6 at day +270 (n = 11), 79.3 at day +360 (n = 5), 77.3 at day +540 (n = 6) and 87.1 at day +740 (n = 3). Mean FACT-BMT total scores were 109.4 at baseline (n = 11), 92.5 at day +30 (n = 12), 106.1 at day +90 (n = 8), 115.8 at day +180 (n = 11), 106.1 at day +270 (n = 6), 106.3 at day +360 (n = 5), 102.9 at day +540 (n = 6) and 115.4 at day +740 (n = 3) (Figure 5). QOL data were not collected for autologous HCT cohort.
Figure 5.
FACT-BMT Total Score.
DISCUSSION
Our study illustrates that allogeneic HCT as consolidation of VGPR or CR with fludarabine and melphalan ± bortezomib conditioning can produce excellent disease control with 2-year PFS of 74.8% (95% CI: 44.6–90.1%) and low NRM of 10.5% (95% CI: 1.6–29%) at 6 months. Thirteen patients received bortezomib with fludarabine + melphalan conditioning under the clinical trial and nine patients received fludarabine + melphalan conditioning regimen off study. There is increasing evidence to suggest that the timing of reduced-intensity transplant is crucial for lowering NRM and improving the disease control rate.22 Patients who received allografting with reduced-intensity conditioning after the failure of a previous autograft have a much higher risk of disease progression/relapse and NRM than those patients who remained in remission at the time of allogeneic HCT.23,24
Recent trends in myeloma allogeneic HCT reported by the Center for International Blood and Marrow Transplantation (CIBMTR) showed increasing recipient age (53% were >50 years) and more frequent unrelated donor grafts. OS at 1 year improved over time (63% in years 2001–2005), in part, secondary to a decrease in NRM (22%). However, relapse remains a major obstacle (58% at 5 years), especially as 29% were chemotherapy-resistant at the time of transplant.25 Multiple trials have examined the allogeneic HCT platform where cytoreduction of multiple myeloma was achieved with autologous HCT followed by reduced-intensity conditioning allogeneic HCT (tandem auto-allo HCT approach).6,10,11,26–28 A phase 3 biologic assignment trial by BMT Clinical Trial Network (BMT CTN 0102 protocol) comparing auto-allo HCT approach to tandem autologous HCT showed equivalent outcomes at 3 years in standard-risk patients, but a longer follow-up is likely required to assess the true survival benefits of this strategy.6 Initial cytoreduction remained an issue as only 42% in standard-risk and 6–32% in high-risk group, respectively, were in VGPR + CR at the time of first autologous HCT.
Achieving better response, that is, VGPR or CR, prior to HCT has been associated with at least PFS and some with OS benefits in the context of autologous HCT.29,30 Obtaining molecular response after allogeneic HCT has been demonstrated to translate into longer PFS, which further substantiates the concept of targeting deeper response in myeloma therapy in order to improve treatment outcomes.4,15,31 Another consideration would be the cost of achieving VGPR or CR by performing autologous HCT as a cytoreductive measure.32,33 Similar response may be achieved with novel inductions without autologous HCT, though the universal application of these agents could be problematic due to prohibitive developmental costs and potential toxicities.
Although there are significant interests in exploring the antimyeloma therapy to augment graft-versus-myeloma effects in the post-HCT setting, there has been no consensus on maintenance therapy after allogeneic HCT.34 The HOVON 76 trial investigated the role of lenalidomide maintenance after allogeneic HCT, but the strategy did not appear to be feasible, due to GVHD.35 Another study is currently being conducted by CIBMTR, examining lenalidomide maintenance after allogeneic HCT. Wolschke et al.36 conducted a phase I/II trial of post-allografting lenalidomide and showed significantly increased peripheral γ-IFN-secreting CD4+ and CD8+ T cells during the 1st week of lenalidomide, with subsequent rise in regulatory T cells. In addition, anti-myeloma natural killer cell activity was increased after lenalidomide in responding myeloma patients. Immunomodulatory agents with increased natural killer cell-mediated cytotoxicity warrant further investigation in order to augment graft-versus-myeloma potential after allogeneic HCT.
Dramatic improvement of initial systemic therapy with significant reduction in disease burden may obviate the need for cytoreductive autologous HCT and has set the stage for asking questions regarding the curative potential of allogeneic HCT.7,13,37 International Myeloma Working Group (IMWG) has released the statement that allogeneic HCT should be conducted under clinical trials.34 Our ongoing allogeneic HCT trial as a consolidation therapy of first response targets those myeloma patients who achieved VGPR or CR to circumvent the need for debulking and to avoid the pitfalls of early relapse.
The health-related QOL is a vital component of comprehensive peri-transplant care.38 In this small cohort of patients, the observed trajectory of reported QOL may be consistent with previously reported results.39 Following an initial decline in some patients, most returned to or exceeded pre-HCT-QOL by 6 months. These data complement the reported survival outcomes and facilitate the understanding of the patients’ experience.
CONCLUSIONS
These initial data indicate that allogeneic HCT for multiple myeloma in first VGPR or CR can be well tolerated and argues for the investigation of allogeneic HCT strategy as a consolidation therapy early in the disease course. A larger number of patients is required to assess the true efficacy of allogeneic HCT used for consolidation of remission in preventing relapse or progression of multiple myeloma.
Acknowledgments
We thank the patients, physicians, nurses and research staffs at MCC. We also thank Ms Pamela Reiersen for data collection on QOL measures.
Footnotes
CONFLICT OF INTEREST
MA is a consultant to and receives research support from Millennium Pharmaceuticals Inc.; RB, receives research support from Millennium Pharmaceuticals Inc. The remaining authors declare no conflict of interest.
References
- 1.Kumar S, Rajkumar S, Dispenzieri A, Lacy M, Hayman S, Buadi F, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 4.Corradini P, Cavo M, Lokhorst H, Martinelli G, Terragna C, Majolino I, et al. Molecular remission after myeloablative allogeneic stem cell transplantation predicts a better relapse-free survival in patients with multiple myeloma. Blood. 2003;102:1927–1929. doi: 10.1182/blood-2003-01-0189. [DOI] [PubMed] [Google Scholar]
- 5.Crawley C, Iacobelli S, Björkstrand B, Apperley J, Niederwieser D, Gahrton G. Reduced-intensity conditioning for myeloma: lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood. 2007;109:3588–3594. doi: 10.1182/blood-2006-07-036848. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12:1195–1203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson P, Weller E, Lonial S, Jakubowiak A, Jagannath S, Raje N, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakubowiak AJ, Kendall T, Al-Zoubi A, Khaled Y, Mineishi S, Ahmed A, et al. Phase II trial of combination therapy with bortezomib, pegylated liposomal doxorubicin, and dexamethasone in patients with newly diagnosed myeloma. J Clin Oncol. 2009;27:5015–5022. doi: 10.1200/JCO.2008.19.5370. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar S, Jacobus S, Callander N, Fonseca R, Vesole D, Williams M, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 11.Rosiñol L, Pérez-Simón JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112:3591–3593. doi: 10.1182/blood-2008-02-141598. [DOI] [PubMed] [Google Scholar]
- 12.Vesole DH, Zhang L, Flomenberg N, Greipp PR, Lazarus HM, Huff CA, et al. A Phase II trial of autologous stem cell transplantation followed by mini-allogeneic stem cell transplantation for the treatment of multiple myeloma: an analysis of Eastern Cooperative Oncology Group ECOG E4A98 and E1A97. Biol Blood Marrow Transplant. 2009;15:83–91. doi: 10.1016/j.bbmt.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tricot G, Vesole D, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87:1196–1198. [PubMed] [Google Scholar]
- 14.Martinelli G, Terragna C, Zamagni E, Ronconi S, Tosi P, Lemoli RM, et al. Molecular remission after allogeneic or autologous transplantation of hematopoietic stem cells for multiple myeloma. J Clin Oncol. 2000;18:2273–2281. doi: 10.1200/JCO.2000.18.11.2273. [DOI] [PubMed] [Google Scholar]
- 15.Cavo M, Terragna C, Martinelli G, Ronconi S, Zamagni E, Tosi P, et al. Molecular monitoring of minimal residual disease in patients in long-term complete remission after allogeneic stem cell transplantation for multiple myeloma. Blood. 2000;96:355–357. [PubMed] [Google Scholar]
- 16.McQuellon RP, Russell GB, Cella DF, Craven BL, Brady M, Bonomi A, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19:357–368. doi: 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 17.Durie B, Harousseau J, Miguel J, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 22.Gahrton G, Svensson H, Cavo M, Apperly J, Bacigalupo A, Björkstrand B, et al. Progress in allogenic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983–93 and 1994–98 at European Group for Blood and Marrow Transplantation centres. Br J Haematol. 2001;113:209–216. doi: 10.1046/j.1365-2141.2001.02726.x. [DOI] [PubMed] [Google Scholar]
- 23.Giralt S, Aleman A, Anagnostopoulos A, Weber D, Khouri I, Anderlini P, et al. Fludarabine/melphalan conditioning for allogeneic transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2002;30:367–373. doi: 10.1038/sj.bmt.1703652. [DOI] [PubMed] [Google Scholar]
- 24.Kröger N, Perez-Simon J, Myint H, Klingemann H, Shimoni A, Nagler A, et al. Relapse to prior autograft and chronic graft-versus-host disease are the strongest prognostic factors for outcome of melphalan/fludarabine-based dose-reduced allogeneic stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2004;10:698–708. doi: 10.1016/j.bbmt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Zhang MJ, Li P, Dispenzieri A, Milone GA, Lonial S, et al. Trends in allogeneic stem cell transplantation for multiple myeloma: a CIBMTR analysis. Blood. 2011;118:1979–1988. doi: 10.1182/blood-2011-02-337329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kröger N, Schwerdtfeger R, Kiehl M, Sayer HG, Renges H, Zabelina T, et al. Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood. 2002;100:755–760. doi: 10.1182/blood-2002-01-0131. [DOI] [PubMed] [Google Scholar]
- 27.Maloney D, Molina A, Sahebi F, Stockerl-Goldstein K, Sandmaier B, Bensinger W, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102:3447–3454. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 28.Björkstrand B, Iacobelli S, Hegenbart U, Gruber A, Greinix H, Volin L, et al. Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: long-term follow-up. J Clin Oncol. 2011;29:3016–3022. doi: 10.1200/JCO.2010.32.7312. [DOI] [PubMed] [Google Scholar]
- 29.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 30.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 31.Harousseau J, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114:3139–3146. doi: 10.1182/blood-2009-03-201053. [DOI] [PubMed] [Google Scholar]
- 32.Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1620–1628. doi: 10.1016/j.bbmt.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majhail NS, Mau LW, Denzen EM, Arneson TJ. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: a study using a large National Private Claims Database. Bone Marrow Transplant. 2012;48:294–300. doi: 10.1038/bmt.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lokhorst H, Einsele H, Vesole D, Bruno B, San Miguel J, Pérez-Simon JA, et al. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2010;28:4521–4530. doi: 10.1200/JCO.2010.29.7929. [DOI] [PubMed] [Google Scholar]
- 35.Kneppers E, van der Holt B, Kersten MJ, Zweegman S, Meijer E, Huls G, et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 Trial. Blood. 2011;118:2413–2419. doi: 10.1182/blood-2011-04-348292. [DOI] [PubMed] [Google Scholar]
- 36.Wolschke C, Stübig T, Hegenbart U, Schönland S, Heinzelmann M, Hildebrandt Y, et al. Postallograft lenalidomide induces strong NK cell-mediated antimyeloma activity and risk for T cell-mediated GvHD: results from a phase I/II dose-finding study. Exp Hematol. 2012;41:134–142. doi: 10.1016/j.exphem.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Corradini P, Voena C, Tarella C, Astolfi M, Ladetto M, Palumbo A, et al. Molecular and clinical remissions in multiple myeloma: role of autologous and allogeneic transplantation of hematopoietic cells. J Clin Oncol. 1999;17:208–215. doi: 10.1200/JCO.1999.17.1.208. [DOI] [PubMed] [Google Scholar]
- 38.Pidala J, Anasetti C, Jim H. Health-related quality of life following haematopoietic cell transplantation: patient education, evaluation and intervention. Br J Haematol. 2010;148:373–385. doi: 10.1111/j.1365-2141.2009.07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherman AC, Simonton S, Latif U, Plante TG, Anaissie EJ. Changes in quality-of-life and psychosocial adjustment among multiple myeloma patients treated with high-dose melphalan and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:12–20. doi: 10.1016/j.bbmt.2008.09.023. [DOI] [PubMed] [Google Scholar]