Abstract
Hepatitis C virus (HCV) infects over 3% of the world population and is the leading cause of chronic liver disease worldwide. HCV has long been known to associate with circulating lipoproteins, and its interactions with the cholesterol and lipid pathways have been recently described. In this work, we demonstrate that HCV is actively secreted by infected cells through a Golgi-dependent mechanism while bound to very low density lipoprotein (vLDL). Silencing apolipoprotein B (ApoB) messenger RNA in infected cells causes a 70% reduction in the secretion of both ApoB-100 and HCV. More importantly, we demonstrate that the grapefruit flavonoid naringenin, previously shown to inhibit vLDL secretion both in vivo and in vitro, inhibits the microsomal triglyceride transfer protein activity as well as the transcription of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase and acyl-coenzyme A:cholesterol acyltransferase 2 in infected cells. Stimulation with naringenin reduces HCV secretion in infected cells by 80%. Moreover, we find that naringenin is effective at concentrations that are an order of magnitude below the toxic threshold in primary human hepatocytes and in mice.
Conclusion
These results suggest a novel therapeutic approach for the treatment of HCV infection.
Hepatitis C virus (HCV) infection is a global public health problem, affecting over 3% of the world population. HCV infection develops into a chronic condition in over 70% of the patients, ultimately leading to cirrhosis and hepatocellular carcinoma.1 Current standards of care consist of interferon (α2A) and ribavirin, which have been found to be effective in only 50% of the cases.1 However, this treatment is poorly tolerated by patients and is associated with significant side effects. Therefore, there is a pressing need for the development of alternative strategies for the treatment of HCV infection.
HCV has long been known to associate with β-lipoproteins [very low density lipoprotein (vLDL) and low-density lipoprotein (LDL)] circulating in patients’ blood.2 Its E1/E2 receptors have been found to bind to both LDL and high-density lipoprotein,3 whereas HCV core protein has been shown to associate with apolipoprotein AII (ApoAII)4 and lipid droplets in HepG2 cells.5 In addition, HCV replication has been shown to be up-regulated by fatty acids and inhibited by statins; this suggests an interaction between HCV, cholesterol, and lipid metabolism.6 The recent development of an efficient cell culture system in which the full lifecycle of HCV infection is captured has opened new opportunities for the study of the viral secretion.7,8 Using this system, Gastaminza et al.9 demonstrated that intercellular HCV particles have a higher density than their secreted counterparts, suggesting that HCV might bind low-density particles prior to viral egress. Just recently, Huang et al.10 demonstrated that HCV secretion is dependent on both apolipoprotein B (ApoB) expression and vLDL assembly in a chromosomally integrated complementary DNA (cDNA) model of HCV secretion.
These results strongly suggest that HCV might be “hitching a ride” along the lipoprotein lifecycle. Therefore, compounds previously shown to influence lipoprotein assembly and secretion could possibly exert a similar effect on HCV. To test this hypothesis, we used the full-length, RNA-based HCV full lifecycle model (JFH1/Huh7.5.1) previously shown to capture important aspects of viral replication, assembly, and infection. Using this model, we demonstrate that HCV is being actively secreted by infected cells in a Golgi-dependent pathway while bound to vLDL. Silencing ApoB messenger RNA (mRNA) by transfection with short hairpin RNA (shRNA) is shown to induce a 70% reduction in the secretion of ApoB, HCV core protein, and HCV RNA. More importantly, we find that the grapefruit flavonoid naringenin, previously shown to inhibit vLDL secretion both in vivo and in vitro, is able to reduce HCV secretion from infected cells by 80% ± 10%. We demonstrate that naringenin inhibits ApoB secretion by inhibiting the activity of the microsomal triglyceride transfer protein (MTP) as well as the transcription of 3-hydroxy-3-methyl-glutaryl-coenzyme reductase (HMGR) and acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2). Moreover, we find that naringenin is effective at a concentration of 200 µM, which is well below its toxic concentration for primary human hepatocytes and severe combined immunodeficient (SCID) mice.
Materials and Methods
Reagents and Antibodies
Fetal bovine serum (FBS), phosphate-buffered saline (PBS), Dulbecco’s modified Eagle medium (DMEM), penicillin, streptomycin, and trypsin–ethylene diamine tetraacetic acid (EDTA) were obtained from Invitrogen Life Technologies (Carlsbad, CA). Lipoprotein-free FBS was purchased from Biomedical Technologies (Stoughton, MA). Insulin was obtained from Eli-Lilly (Indianapolis, IN). Oleate, naringenin, and brefeldin A were purchased from Sigma-Aldrich Chemicals (St. Louis, MO). Immunofluorescence-grade paraformaldehyde was purchased from Electron Microscope Sciences (Hatfield, PA). OptiMEM basal medium and Lipofectamine 2000 were purchased from Invitrogen Life Technologies. The SureSilencing shRNA plasmid kit for human ApoB [green fluorescent protein (GFP)] was purchased from SuperArray (Frederick, MD). An MTP fluorescent activity kit was purchased from Roar Biomedical (New York, NY). Unless otherwise noted, all other chemicals were purchased from Sigma-Aldrich Chemicals. For immunoprecipitation, Protein A-Sepharose was purchased from Invitrogen, whereas horseradish peroxidase–conjugated goat anti-mouse secondary was purchased from Santa Cruz Biotech (Santa Cruz, CA). For immunofluorescence studies, normal donkey serum and secondary F(ab′)2 antibody fragments (multiple-labeling [ML] grade) were obtained from Jackson Immunore-search (Bar Harbor, ME). Mouse anti-HCV core antigen (5 µg/mL) was purchased from US Biological (Swampscott, MA). Goat anti-ApoB (10 µg/mL) was purchased from R&D Systems, Inc. (Minneapolis, MN).
Cells and Viruses
The Huh7.5.1 human hepatoma cell line and a plasmid containing the JFH-1 genome were kindly provided by Dr. Chisari (Scripps Research Institute, La Jolla, CA) and Dr. Wakita (National Institute of Infectious Diseases, Tokyo, Japan), respectively. Huh7.5.1 cells were cultured in DMEM supplemented with 10% FBS, 200 units/mL penicillin, and 200 mg/mL streptomycin in a 5% CO2–humidified incubator at 37°C. In vitro transcribed genomic JFH-1 RNA was delivered to cells by liposome-mediated transfection as described by Zhong et al.8 Infected Huh7.5.1 cells were passaged every 3 days and used at passage <15. The presence of HCV in these cells and corresponding supernatants were determined by quantitative, reverse-transcription, polymerase chain reaction (qRT-PCR) and immunofluorescence staining. Primary human hepatocytes were purchased from BD Biosciences (San Jose, CA) and cultured on a collagen-coated 12-well plate in a C+H culture medium composed of DMEM supplemented with 10% heat-inactivated FBS, 200 U/mL penicillin/streptomycin, 7.5 µg/mL hydrocortisone, 20 ng/mL epidermal growth factor (EGF), 14 ng/mL glucagons, and 0.5 U/mL insulin. The medium was supplemented with 2% dimethyl sulfoxide for long-term culture of the primary cells.
HCV Secretion
HCV-infected Huh7.5.1 cells were plated on a 6-well plate at a density of 1×105 cells/cm2 and cultured overnight in the standard medium. Prior to the beginning of the experiment, the cells were washed 3 times with PBS and cultured with DMEM containing 5% lipoprotein-free FBS. Oleate, insulin, naringenin, and brefeldin A were added at this time as described in the text. Following 24 hours of incubation, the plate was gently agitated to release mechanically bound particles, and the medium was collected, filtered to remove cellular debris, and stored at −80°C for further analysis. The attached cells were washed 3 times with PBS, harvested, pelleted, and stored at −80°C for further analysis.
Coimmunoprecipitation
The binding of Huh7.5.1-secreted JFH1 particles to ApoB was assessed with coimmunoprecipitation. Anti-human ApoB-100 antibody (5 µg) was bound to 100 µL of Protein A-Sepharose on ice. Three milliliters of the JFH1-infected Huh7.5.1 conditioned medium (1×106 cells/mL) was added to the mixture, which was subsequently rotated for 4 hours at 4°C. The sample was spun down at 10,000g in a microcentrifuge and washed 3 times with 50 mM trishydroxymethylaminomethane (Tris)-HCl (pH 7.5) containing 5 mM EDTA. Finally, the sample was eluted in 100 µL of 10 mM Tris-HCl (pH 8.5) containing sodium dodecyl sulfate. The protein concentration in the eluted buffer was quantified as described later, and 20 µg of protein was loaded onto a 7.5% Tris-HCL resolving gel. Resolved proteins were transferred to a polyvinylidene fluoride membrane and stained against HCV core (0.5 µg/mL).
HCV Infectivity
The infectivity of the secreted HCV particles was measured as previously described.8 Naïve Huh7.5.1 cells were grown to 80% confluence and exposed to cell culture supernatants diluted 10-fold in the culture medium. Following 1 hour of incubation at 37°C, the medium was replaced, and the cells were cultured for 3 additional days. Levels of HCV infection were determined by immunofluorescence staining for HCV core protein. The viral titer is expressed as focus forming units per milliliter of supernatant.
Human ApoB Enzyme-Linked Immunosorbent Assay (ELISA)
Huh7.5.1–secreted and primary human hepatocyte–secreted ApoB was detected in the medium with the ALerCHEK, Inc. (Portland, ME), total human ApoB ELISA kit. The medium was diluted 1:10 with the specimen diluent, and the assay was carried out according to the manufacturer’s directions.
HCV Core Antigen ELISA
Huh7.5.1–secreted HCV core antigen was detected in the medium with the Wako Chemicals (Cambridge, MA) ORTHO HCV antigen ELISA kit. The medium was used as is, and the assay was carried out according to the manufacturer’s directions.
Total Protein Assay
The total protein content of the cells was measured with the Bio-Rad Laboratories (Hercules, CA) protein assay based on the Bardford method. Briefly, a cell pellet was lysed in 350 µL of 0.1% Triton X-100, and 5-µL samples were loaded onto a 96-well plate and incubated for 15 minutes with 250 µL of Coomassie Blue reagent at room temperature. Absorbance was measured at 595 nm and compared to a bovine serum albumin standard.
Quantitative, Real-Time, Reverse-Transcription Polymerase Chain Reaction (PCR)
Virus samples collected in each experiment were filtered with a 0.45-µm filter, and a volume of 100 µL for each sample was heated at 95°C for 45 minutes. The reverse-transcription reaction step was performed on a Mastercycler epgradientS (Eppendorf) instrument using Omniscript and Sensiscript RT kits (Qiagen). Real-time PCR was performed on a Light Cycler LC-24 (Idaho Technology) using Super-Script III Platinum CellsDirect Two-Step qRT-PCR kits (Invitrogen). For the reverse-transcription step, 2 µL of a sample without RNA extraction was used. For real-time PCR, 1 µL of the reverse-transcription reactions was used. All reactions were performed according to the manufacturer’s instructions with the primers detailed in Table 1.
Table 1.
PCR Primers
| Gene | Primer | |
|---|---|---|
| HCV 5′ untranslated region | Forward | 5′-GCAGAAAGCGTCTAGCCATGGCGT-3′ |
| Reverse | 5′-CTCGCAAGCACCCTATCAGGCAGT-3′ | |
| MTP | Forward | 5′-GAGGTTTCTCTATGCCTGTGGATTT-3′ |
| Reverse | 5′-CCCAGGATTAACTTCTTAGCTTCCA-3′ | |
| ACAT1 | Forward | 5′-CAATACAATGGTGGGTGAAGAGAAG-3′ |
| Reverse | 5′-AAAATCTTTCCTTGTTCTGGAGGTG-3′ | |
| HMGR | Forward | 5′-GACCCCTTTGCTTAGATGAAAAAGA-3′ |
| Reverse | 5′-GGACTGGAAACGGATATAAAGGTTG-3′ | |
| Actin | Forward | 5′-GTCGTACCACTGGCATTGTG-3′ |
| Reverse | 5′-CTCTCAGCTGTGGTGGTGAA-3′ | |
| ACAT2 | Forward | 5′-CATGCGGGAGGCTATACAAT-3′ |
| Reverse | 5′-GTAGATGGTGCGGAAATGCT-3′ |
Cellular Viability
The viability of both Huh7.5.1 cells and primary human hepatocytes was studied with Thermo Fisher Scientific (Waltham, MA) Infinity aspartate aminotransferase (AST) liquid reagent. Medium samples (15 µL/well) were loaded onto a 96-well plate in triplicates and mixed with 150 µL of the AST liquid reagent. Absorbance decay was measured at the wavelength of 340 nm with 15-second intervals in a Bio-Rad Benchmark Plus spectrophotometer. Values were normalized to the total amount of AST available per culture, which was determined by total cell lysis induced by 1% Triton X-100 for 20 minutes at room temperature. Cell viability for all conditions reported in the Results section was greater than 90%.
MTP Activity Assay
MTP activity was analyzed with an MTP assay kit as previously described.11 The assay is based on a transfer of a fluorescent signal between donor and acceptor particles due to MTP activity. Briefly, confluent Huh7.5.1 cells were stimulated with naringenin or a carrier control for 24 hours and were then washed with ice-cold PBS and scraped off the dish with a cell scraper. Samples were homogenized by sonication (3 × 5 seconds) in a buffer containing protease inhibitors. The MTP assay was performed by the incubation of 50 µg of cellular protein with 10 µL of donor and acceptor solutions in 250 µL of total buffer (15 mM Tris, pH 7.4; 40 mM NaCl; 1 mM EDTA). The increase in the fluorescent signal was measured over 12 hours at 37°C at the excitation wavelength of 465 nm and emission wavelength of 538 nm.
Animal Studies
Male SCID mice (8 weeks old, 20–25 g) were obtained from Charles River Laboratories (Wilmington, MA). Animals were treated in accordance with National Institutes of Health guidelines and the Massachusetts General Hospital Subcommittee on Research Animal Care. The mice were allowed free access to laboratory chow and water ad labium. Naringenin was dissolved in 0.5% Tween 20 diluted in saline and given by intraperitoneal injection. Two days following the treatment, animals were sacrificed, and blood was withdrawn by cardiac puncture. AST and alanine aminotransferase (ALT) enzyme levels were assessed as described previously. Total triglycerides were measured with a kit purchased from Sigma-Aldrich Chemicals according to the manufacturer’s instructions.
Silencing ApoB mRNA
HCV-infected Huh7.5.1 cells were plated in T-25 tissue culture flasks at a density of 1 × 105 cells/cm2 and cultured overnight in the standard medium. Prior to silencing, the cells were washed 3 times with PBS, and the medium was replaced with OptiMEM basal medium. SureSilencing shRNA (GFP) plasmids against human ApoB100 as well as shRNA plasmid control (500 ng/mL) were combined with Lipofectamine 2000 in OptiMEM and incubated with the cells overnight. SureSilencing shRNA plasmids code for GFP, which was used to sort the transfected Huh7.5.1 cells with FACSAria (BD Biosciences) located at the Partners AIDS Research Center. Transfected cells (10% of the total population) were sorted directly into a 12-well plate and allowed to adhere overnight. The culture medium was conditioned by the transfected cells for 24 hours and analyzed as described previously.
Immunofluorescence Microscopy
Huh7.5.1 cells were washed 3 times with PBS and fixed in 4% electron microscopy–grade paraformaldehyde for 10 minutes at room temperature. Slides were then washed with PBS and incubated in 100 mmol/L glycine for 15 minutes to saturate reactive groups. Samples were permeabilized for 15 minutes with 0.1% Triton X-100, blocked for 30 minutes with 1% bovine serum albumin and 5% donkey serum at room temperature, and stained with primary antibodies overnight at 4°C. After additional washes with PBS, samples were stained with fluorescently tagged secondary antibodies for 45 minutes at room temperature.
Results
Huh7.5.1-Secreted HCV Is Bound to ApoB
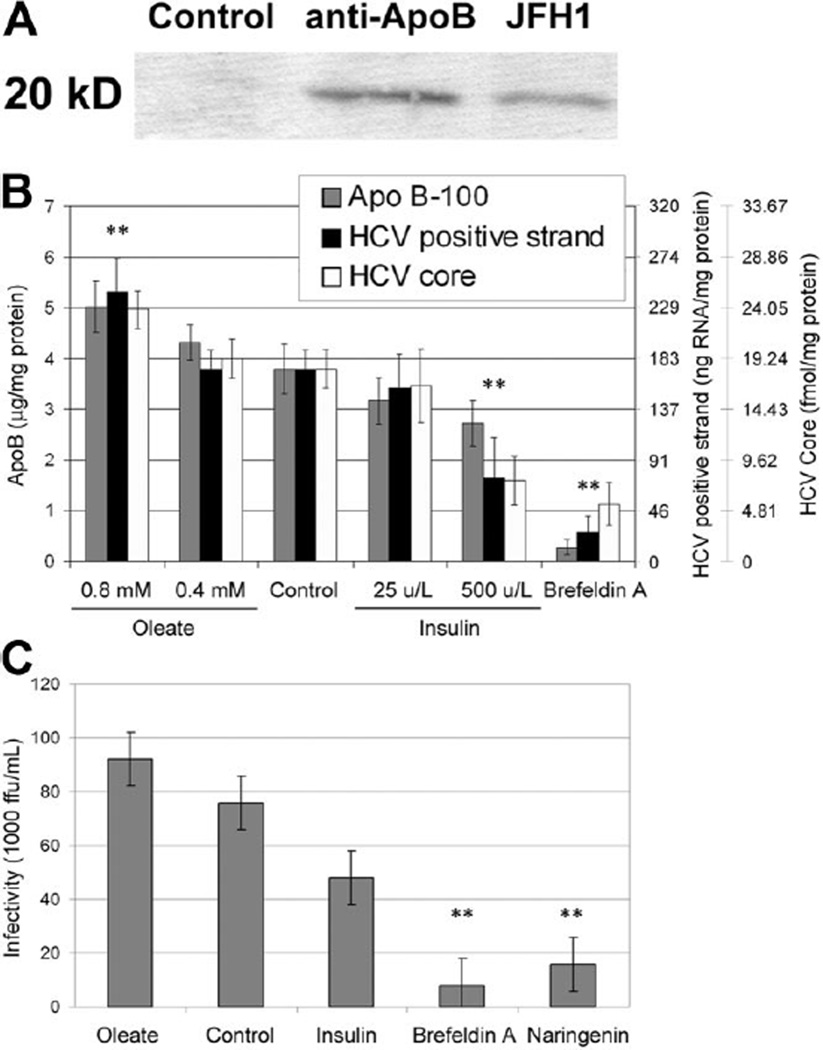
Recent evidence suggests that HCV binds to low-density particles prior to virus egress9 and that viral secretion requires both ApoB expression and vLDL assembly to occur.10 Therefore, HCV secreted by the JFH1/Huh7.5.1 full viral lifecycle model could potentially be secreted while bound to vLDL. To determine if Huh7.5.1-produced HCV is bound to vLDL, we immunoprecipitated the Huh7.5.1-conditioned medium against human ApoB antibodies and detected bound HCV core protein in the eluted sample. The results presented in Fig. 1A demonstrate that HCV core protein is bound to ApoB-100 in our samples. HCV core could not be detected when the sample was precipitated against irrelevant antibody (control) but was easily detected in the cell medium (JFH1).
Fig. 1.
(A) Immunoprecipitation of Huh7.5.1-secreted ApoB followed by anti-HCV core staining (coimmunoprecipitation). (B) Cell culture secretion of ApoB, HCV-positive strand RNA, and HCV core protein in JFH-1–infected Huh7.5.1 cells in response to oleate, insulin, and brefeldin A. The secretions of ApoB, HCV RNA, and HCV core protein are significantly up-regulated by oleate and down-regulated by insulin in a dose-dependent manner. Brefeldin A, which blocks Golgi-dependent secretion of proteins, significantly inhibits the secretion of ApoB, HCV RNA, and HCV core. Cell viability for all conditions was greater than 90%. (C) Infectivity of cell culture supernatant assessed by colony formation on naïve Huh7.5.1 cells: oleate (0.8 mM), insulin (500 U/L), brefeldin A (2.5 µg/mL), and naringenin (200 µM). **P < 0.01.
HCV Secretion Mirrors That of vLDL
The interaction between HCV and ApoB suggests that the virus might be actively secreted by the cells while bound to vLDL. However, the interaction between these particles might also occur outside the cell. To determine if HCV is being actively secreted by the cells while bound to vLDL, we studied viral secretion in response to oleate and insulin stimulation, which was previously shown to oppositely modulate ApoB secretion in culture.12 Figure 1B shows ApoB, HCV core, and HCV-positive strand RNA secretion by Huh7.5.1 cells infected with the JFH-1 virus. As expected, ApoB secretion is significantly up-regulated by oleate (P = 0.0023, n = 5) and down-regulated by insulin (P = 0.0073, n = 5) in a dose-dependent manner. Similarly, HCV core protein secretion is significantly up-regulated by oleate (P = 0.0073, n = 3) and down-regulated by insulin (P = 0.0223, n = 3) in a dose-dependent manner. The secretion of HCV-positive strand RNA, measured by qRT-PCR, follows the same path. However, intracellular levels of HCV RNA remained unchanged following both treatments.
Brefeldin A is a commonly used toxin that disrupts communication between the endoplasmic reticulum and the Golgi, inhibiting the active secretion of proteins.12,13 Not surprisingly, the addition of brefeldin A (2.5 µg/mL) blocked ApoB secretion (P = 0.0001, n = 5). Interestingly, brefeldin A significantly inhibits the secretion of HCV core protein (P = 0.0021, n = 4) and HCV-positive strand RNA (P = 0.0006, n = 3). To assess whether the changes in HCV core protein and RNA secretion correlate with changes of viral infectivity in the cell supernatant, we measured the ability of the secreted virus to infect naïve Huh7.5.1 cells. Figure 1C shows that the infectivity of the cell supernatant increased following oleate stimulation, decreased because of insulin, and was strongly inhibited following brefeldin A stimulation by 89% ± 10% (P = 0.001, n = 3). These results suggest that HCV is being actively secreted by the cells, perhaps while bound to vLDL.
HCV Core Antigen Colocalizes with ApoB
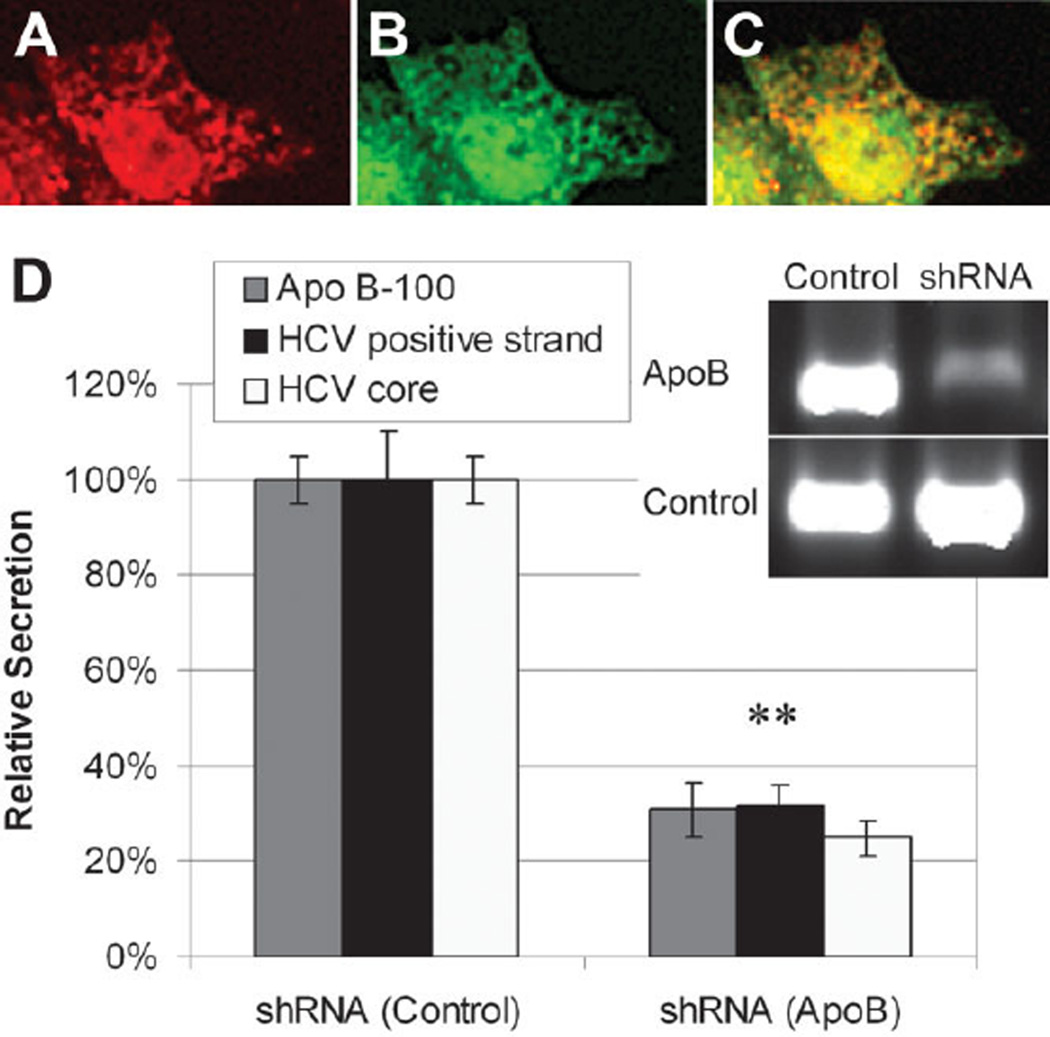
Previously, HCV core protein was shown to associate with ApoAII4 and lipid droplets in HepG2 cells5 overexpressing the core protein. Just recently, Huang et al.10 demonstrated that HCV core protein colocalizes with ApoB in a chromosomally integrated cDNA model of HCV. To ascertain if HCV core protein associates with ApoB in JFH-1 virus–infected Huh7.5.1 cells, we double-stained Huh7.5.1 cells 2 days post infection by immunofluorescence for both viral and native proteins. Figure 2 demonstrates the colocalization of HCV’s core and ApoB100 in infected cells. HCV core protein associates with areas in the cytoplasm that are positive to ApoB100. However, we note that although the proteins appear to be closely associated, we fail to find a one-to-one correspondence between the viral and native proteins in our model of the full viral lifecycle.
Fig. 2.
Double immunofluorescence staining of JFH-1–infected Huh7.5.1 cells. (A) Staining for HCV core protein (red). (B) Staining for ApoB100 (green). (C) Superpositioning of the images demonstrates that HCV core protein associates with ApoB100 in the cytoplasm. (D) Relative secretion of ApoB, HCV-positive strand RNA, and HCV core protein in JFH-1–infected Huh7.5.1 cells following silencing of ApoB100 mRNA by SureSilencing shRNA transfection. **P < 0.01.
The association between ApoB100 and HCV core protein as well as previous data suggests that HCV might be “tagging along” ApoB secretion. Therefore, silencing ApoB production in the cell might decrease HCV secretion. Figure 2D demonstrates a 69% ± 6% decrease in ApoB secretion following transfection with SureSilencing shRNA (P = 0.0001, n = 3). Interestingly, HCV core protein secretion was significantly decreased by 75% ± 4% at the same time (P = 0.0002, n = 3). HCV-positive strand RNA secretion was also significantly decreased by 69% ± 4% (P = 0.0015, n = 3).
HCV Secretion Is Inhibited by Naringenin
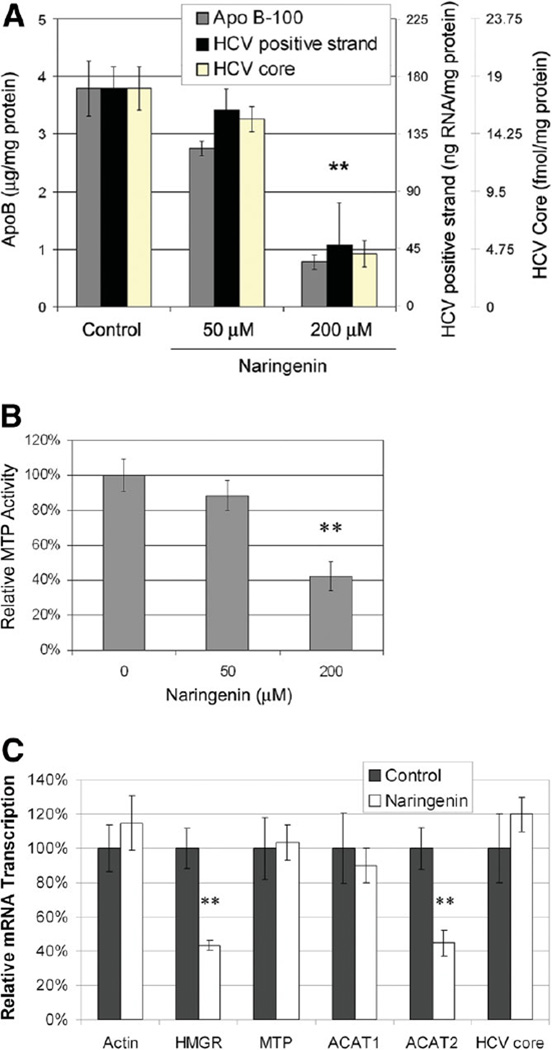
Naringenin is a grapefruit flavonoid previously shown to reduce cholesterol levels both in vivo14 and in vitro.15 It is thought that naringenin inhibits ApoB secretion by reducing the activity and expression of MTP and ACAT.15,16 To assess if naringenin inhibits HCV secretion in a similar manner, we cultured infected Huh7.5.1 cells in the presence of naringenin for 24 hours. Figure 3A demonstrates that naringenin inhibits the secretion of HCV core (P = 0.0001, n = 6) and HCV-positive strand RNA (P = 0.0006, n = 5) in a dose-dependent manner. At the concentration of 200 µM, naringenin inhibited HCV secretion by 80% ± 10%. Interestingly, intracellular levels of HCV-positive strand RNA (Fig. 3C) as well as intracellular HCV core protein expression (Supplementary Fig. 1) remained unchanged. To assess whether the naringenin-induced inhibition of HCV core protein and RNA secretion correlated with changes of viral infectivity in the cell supernatant, we measured the ability of the secreted virus to infect naïve Huh7.5.1 cells. Figure 1C shows that the infectivity of the cell supernatant was strongly inhibited following naringenin stimulation by 79% ± 10% (P = 0.0018, n = 3).
Fig. 3.
(A) Inhibition of ApoB, HCV-positive strand RNA, and HCV core protein secretion by the grapefruit flavonoid naringenin. Naringenin significantly inhibits the secretion of HCV core (P = 0.0001, n = 6) and HCV-positive strand RNA (P = 0.0006, n = 5) in a dose-dependent manner. At the concentration of 200 µM, naringenin inhibited HCV secretion by 80% ± 10%. Cell viability for all conditions was greater than 90%. **P < 0.01. (B) Naringenin inhibits the activity of MTP in a dose-dependent manner. At the concentration of 200 µM, MTP activity was reduced by 58% ± 8% (P = 0.0012, n = 3). (C) Naringenin induces changes in hepatic gene transcription measured by qRT-PCR. HMGR transcription was reduced by 57% ± 3% (P = 0.010, n = 3), whereas the transcription of ACAT2 was reduced by 55% ± 7% (P = 0.016, n = 3). The mRNA levels of actin, MTP, and ACAT1 remained unchanged. Intracellular RNA levels of HCV core also remained unchanged during the 24 hours of treatment. **P < 0.02.
Although the activity of naringenin has been described in uninfected cells,15,17,18 it has yet to be characterized in HCV-infected cells. Figure 3B demonstrates that naringenin inhibits MTP activity in a dose-dependent manner. At the concentration of 200 µM, MTP activity was reduced by 58% ± 8% (P = 0.0012, n = 3). In addition, we demonstrate that naringenin induces significant changes in hepatic gene transcription measured by qRT-PCR (Fig. 3C). HMGR transcription was reduced by 57% ± 3% (P = 0.010, n = 3), whereas ACAT2 was reduced by 55% ± 7% (P = 0.016, n = 3). In contrast, the mRNA levels of actin, MTP, ACAT1, and HCV remained unchanged.
Naringenin Does Not Display Hepatic or In Vivo Toxicity
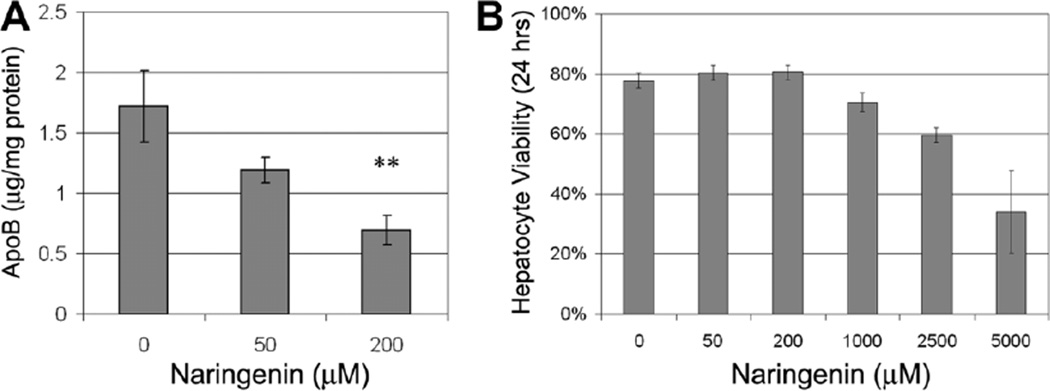
To assess the potential of naringenin-based treatment, we measured ApoB secretion in primary human hepatocytes following 24 hours of stimulation with naringenin. Figure 4A demonstrates a dose-dependent decrease in ApoB secretion following naringenin stimulation. At 200 µM naringenin, ApoB secretion was reduced by 60% ± 7% (P = 0.007, n = 3). The viability of primary human hepatocytes exposed to increasing concentrations of naringenin is shown in Fig. 4B. Human hepatocyte viability was 81% ± 3% at 200 µM naringenin and was not judged to be statistically different than that of the control (78% ± 3%). Human hepatocyte viability dropped significantly only at naringenin concentrations greater than 1000 µM.
Fig. 4.
(A) Naringenin stimulation inhibits ApoB secretion of primary human hepatocytes in a dose-dependent manner. At 200 µM naringenin, ApoB secretion was reduced by 60% ± 7% (P = 0.007, n = 3). (B) Viability of freshly isolated human hepatocytes exposed to increasing concentrations of naringenin for 24 hours. Human hepatocyte viability was 81% ± 3% at 200 µM naringenin and was not judged to be statistically different than the control (77% ± 3%). Human hepatocyte viability dropped significantly only at naringenin concentrations greater than 1000 µM.
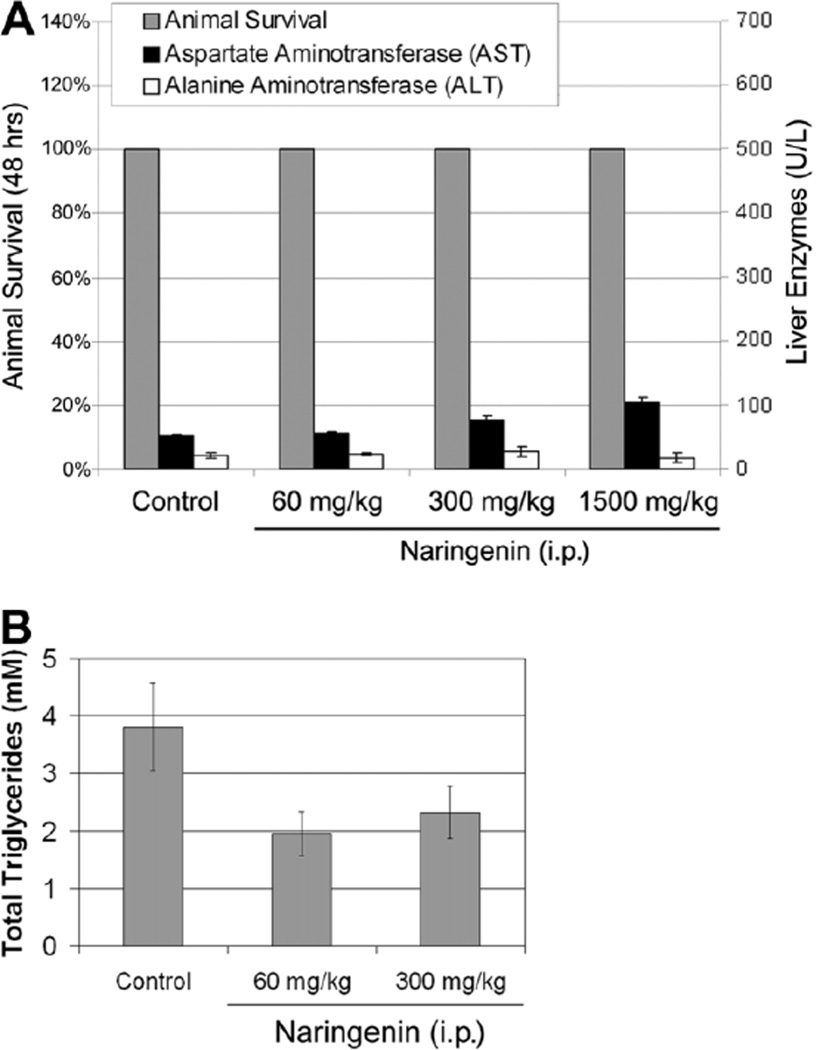
To further assess naringenin potential, we delivered naringenin by intraperitoneal injection to 8-week-old male SCID mice at concentrations of 60, 300, and 1500 mg/kg (approximately 200, 1000, and 5000 µM). Animal survival was not affected by naringenin at these doses. To discern if liver damage occurred, we measured levels of AST and ALT in the animals’ plasma 48 hours following injection. Figure 5 demonstrates that there was no elevation of ALT levels under all conditions. AST levels appeared to increase but remained under 100 U/L even at the highest dose. To assess naringenin’s ability to reduce circulating vLDL levels, we measured total triglyceride levels in animal plasma. Figure 5A demonstrates a decrease in triglycerides following naringenin injection.
Fig. 5.
Animal survival and liver enzyme release following intraperitoneal (i.p.) injection of naringenin into 8-week-old male SCID mice. Animals were injected with naringenin at 60, 300, and 1500 mg/kg of body weight. Animals were sacrificed at 48 hours, at which time liver enzymes (AST and ALT) and total triglycerides were analyzed in the animals’ plasma. (A) Animal survival was monitored for several days following injection and was not affected even at the highest dose (1500 mg/kg). The ALT level appeared unchanged over all conditions, whereas AST was found to be slightly elevated at the highest dose. (B) Total triglycerides analyzed in animal plasma 24 hours following injection decreased in response to naringenin.
Discussion
HCV is a leading cause of chronic liver disease worldwide. Although the disease develops to cirrhosis in only 20% of the cases, the sheer scope of infection and lack of effective treatment make it a severe global health problem. A simulation of the US population for the years 2010–2019 predicts nearly 200,000 deaths associated with HCV infection and direct medical expenditures in excess of $10 billion. It is for these reasons that there is a pressing need for the development of alternative strategies for the treatment of HCV infection.
The interaction between HCV infection, cholesterol, and fatty acid metabolism has received significant attention, mainly because of the development of liver steatosis in chronically infected patients.1 However, the lack of an efficient cell culture model of HCV replication and infection has significantly limited research in the field. Despite these limitations, several groups have demonstrated that HCV core protein associates with ApoAII4 and lipid droplets in HepG2 cells5 overexpressing the protein. The data suggest that HCV in infected patients might circulate as lipoviral particles.19 The development of HCV replicon systems20 has allowed for the efficient study of viral replication in culture. Using this system, Kapadia and Chisari6 demonstrated that HCV replication is regulated by geranylgeranylation and fatty acid metabolism. Others have demonstrated that HCV nonstructural proteins, such as nonstructural protein 5A, inhibit ApoB secretion.21
The recent development of the JFH-1 virus7 in combination with the Huh7.5.1 cell line8 has allowed for the efficient infection of cells and the generation of large virus titers in culture. This model allows for the identification of intercellular infectious HCV particles with a higher density than that of their secreted counterparts,9 suggesting the binding of HCV to low-density particles in the endoplasmic reticulum. Just recently, Huang et al.10 demonstrated that HCV assembled in ApoB and MTP enriched vesicles and that the viral secretion was dependent on both ApoB expression and vLDL assembly in a chromosomally integrated cDNA model of HCV secretion. As the association between HCV and serum β-lipoproteins (vLDL and LDL) is well known,2 these results strongly suggest that HCV might “hitch a ride” on the lipoprotein-cholesterol lifecycle. This hypothesis is intriguing as it might explain the presence of HCV in intestinal cells, a second site of lipoprotein production.22 In addition, it might explain HCV uptake by LDL receptor,23,24 scavenger receptor class B type I,25 and heparin sulfate.26
Our results strongly support this hypothesis. We demonstrate that HCV produced by the Huh7.5.1 cell line is bound to ApoB and that its secretion is inhibited by brefeldin A, a metabolite of the fungus Eupenicillium brefeldianum, which blocks the communication between the endoplasmic reticulum and the Golgi, effectively inhibiting protein secretion.12,13 We also demonstrate that HCV secretion is up-regulated by the fatty acid oleate and down-regulated by insulin, precisely mirroring ApoB secretion by the cells.12 Moreover, silencing ApoB100 mRNA caused a significant and parallel decrease in HCV core protein secretion. This ApoB-dependent HCV secretion pathway suggests a novel therapeutic approach for the treatment of HCV infection.
Naringin, one of the most abundant flavonoids in citrus fruits, is hydrolyzed by enterobacteria to naringenin prior to being absorbed. Naringenin has been reported to be an antioxidant,27 MTP and ACAT inhibitor,16 and regulator of cytochrome P4503A and 4A activity.28,29 The ability of naringenin, or its glycosylated form, to significantly reduce plasma cholesterol levels has been demonstrated both in vivo and in vitro.14,15 It is thought that naringenin inhibits the expression and activity of MTP, which catalyzes the transfer of lipids to the nascent ApoB molecule as it buds into the endoplasmic reticulum as a vLDL particle.16–18 Our results demonstrate that short-term (24-hour) stimulation of infected hepatocytes with 200 µM naringenin significantly inhibits HCV secretion by 80% ± 10% and the infectivity of the titer by 79% ± 10%. At the same time, transcription of the viral RNA remains unchanged. We suggest that this is due in part to the inhibition of MTP activity by 58% ± 8% as well as the inhibition of HMGR and ACAT2 transcription. To further demonstrate naringenin as a potential therapy, we show that the compound is nontoxic to freshly isolated human hepatocytes up to concentrations greater than 1000 µM. In addition, we demonstrate that naringenin induced a 60% ± 7% decrease in ApoB secretion by primary human hepatocytes.
The concept of supplementing HCV patients’ diets with naringenin is appealing. A recent clinical trial in hypercholesterolemic patients demonstrated that a low dose of naringin (400 mg/day) lowered LDL levels by 17%.30 A similar cholesterol-lowering effect of naringenin was demonstrated in rabbits14,31 and rats.32 However, it is worth noting that the absorbance of naringenin through the intestinal wall is limited (less than 8%), and this suggests that short-term therapeutic doses would need to be delivered intravenously. Prior studies have suggested that the median lethal dose (50% kill) for naringenin is 2000 mg/kg for both rats and guinea pigs by intraperitoneal injection.33 Our results show that doses up to 1500 mg/kg naringenin given by intraperitoneal injection to mice did not cause death or a marked elevation of liver enzymes, suggesting that intravenous administration of naringenin is in the realm of possibility.
The ability of the liver to regenerate in the context of the RNA-based lifecycle of HCV allows for the potential clearance of the viral infection. It is thought that clearance occurs in about 30% of HCV-infected patients. The possible reduction of HCV viral load by inhibiting viral secretion could allow uninfected cells to regenerate, potentially increasing the overall rate of viral clearance. Future studies would focus on the long-term ability of naringenin and perhaps other citrus flavonoids to reduce viral load in animal models, such as the KMT Mouse model,34 and long-term cultures of primary human hepatocytes.
Supplementary Material
Acknowledgment
We thank Chris Pohun Chen for his help with both fluorescence-activated cell sorting and western blotting. We also thank Dr. Francis Chisari (Scripps Research Institute) for the Huh7.5.1 cell line. Microscopic imaging studies were made possible by the Core Morphology Facility of the Boston Shriners Hospital.
Abbreviations
- ACAT
acyl-coenzyme A:cholesterol acyltransferase
- ALT
alanine aminotransferase
- ApoAII
apolipoprotein AII
- ApoB
apolipoprotein B
- AST
aspartate aminotransferase
- cDNA
complementary DNA
- DMEM
Dulbecco’s modified Eagle medium
- EDTA
ethylene diamine tetraacetic acid
- EGF
epidermal growth factor
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- HCV
hepatitis C virus
- HMGR
3-hydroxy-3-methyl-glutaryl-coenzyme reductase
- i.p.
intraperitoneal
- LDL
low-density lipoprotein
- mRNA
messenger RNA
- MTP
microsomal triglyceride transfer protein
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- qPCR
quantitative polymerase chain reaction
- SCID
severe combined immunodeficient
- shRNA
short hairpin RNA
- Tris
trishydroxymethylaminomethane
- vLDL
very low density lipoprotein
Footnotes
Potential conflict of interest: Nothing to report.
Supplementary material for this article can be found on the HEPATOLOGY Web site (http://interscience.wiley.com/jpages/0270-9139/suppmat/index.html).
References
- 1.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol Mech Dis. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 2.Thomssen R, Bonk S, Propfe C, Heermann KH, Kochel HG, Uy A. Association of hepatitis C virus in human sera with beta-lipoprotein. Med Microbiol Immunol. 1992;181:293–300. doi: 10.1007/BF00198849. [DOI] [PubMed] [Google Scholar]
- 3.Monazahian M, Kippenberger S, Muller A, Seitz H, Bohme I, Grethe S, et al. Binding of human lipoproteins (low, very low, high density lipoproteins) to recombinant envelope proteins of hepatitis C virus. Med Microbiol Immunol. 2000;188:177–184. doi: 10.1007/s004300000032. [DOI] [PubMed] [Google Scholar]
- 4.Sabile A, Perlemuter G, Bono F, Kohara K, Demaugre F, Kohara M, et al. Hepatitis C virus core protein binds to apolipoprotein AII and its secretion is modulated by fibrates. Hepatology. 1999;30:1064–1076. doi: 10.1002/hep.510300429. [DOI] [PubMed] [Google Scholar]
- 5.Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, et al. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci U S A. 1997;94:1200–1205. doi: 10.1073/pnas.94.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci U S A. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gastaminza P, Kapadia SB, Chisari FV. Differential biophysical properties of infectious intracellular and secreted hepatitis C virus particles. J Virol. 2006;80:11074–11081. doi: 10.1128/JVI.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M, Jr, et al. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci U S A. 2007;104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chretien Y, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185–194. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- 12.Dixon JL, Ginsberg HN. Regulation of hepatic secretion of apolipoprotein B-containing lipoproteins: information obtained from cultured liver cells. J Lipid Res. 1993;34:167–179. [PubMed] [Google Scholar]
- 13.Misumi Y, Misumi Y, Miki K, Takatsuki A, Tamura G, Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986;261:11398–11403. [PubMed] [Google Scholar]
- 14.Kurowska E, Borradaile N, Spence JD, Carroll KK. Hypocholesterolemic effects of dietary citrus juices in rabbits. Nutr Res. 2000;20:121–129. [Google Scholar]
- 15.Allister EM, Borradaile NM, Edwards JY, Huff MW. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes. 2005;54:1676–1683. doi: 10.2337/diabetes.54.6.1676. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox LJ, Borradaile NM, Dreu LED, Huff MW. Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. J Lipid Res. 2001;42:725–734. [PubMed] [Google Scholar]
- 17.Borradaile NM, Dreu LED, Barrett PHR, Huff MW. Inhibition of hepatocyte apoB secretion by naringenin: enhanced rapid intracellular degradation independent of reduced microsomal cholesteryl esters. J Lipid Res. 2002;43:1544–1554. doi: 10.1194/jlr.m200115-jlr200. [DOI] [PubMed] [Google Scholar]
- 18.Borradaile NM, Dreu LED, Barrett PHR, Behrsin CD, Huff MW. Hepatocyte ApoB-containing lipoprotein secretion is decreased by the grapefruit flavonoid, naringenin, via inhibition of MTP-mediated microsomal triglyceride accumulation. Biochemistry. 2003;42:1283–1291. doi: 10.1021/bi026731o. [DOI] [PubMed] [Google Scholar]
- 19.Andre P, Perlemuter G, Budkowska A, Bre’chot C, Lotteau V. Hepatitis C virus particles and lipoprotein metabolism. Semin Liver Dis. 2005;25:93–104. doi: 10.1055/s-2005-864785. [DOI] [PubMed] [Google Scholar]
- 20.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 21.Domitrovich AM, Felmlee DJ, Siddiqui A. Hepatitis C virus nonstructural proteins inhibit apolipoprotein B100 secretion. J Biol Chem. 2005;280:39802–39808. doi: 10.1074/jbc.M510391200. [DOI] [PubMed] [Google Scholar]
- 22.Deforges S, Evlashev A, Perret M, Sodoyer M, Pouzol S, Scoazec JY, et al. Expression of hepatitis C virus proteins in epithelial intestinal cells in vivo. J Gen Virol. 2004;85(pt 9):2515–2523. doi: 10.1099/vir.0.80071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahmias Y, Casali M, Barbe L, Berthiaume F, Yarmush ML. Liver endothelial cells promote LDL-R expression and the uptake of HCV-like particles in primary rat and human hepatocytes. Hepatology. 2006;43:257–265. doi: 10.1002/hep.21016. [DOI] [PubMed] [Google Scholar]
- 24.Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1999;96:12766–12771. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maillard P, Huby T, Andréo U, Moreau M, Chapman J, Budkowska A. The interaction of natural hepatitis C virus with human scavenger receptor SR-BI/Cla1 is mediated by ApoB containing lipoproteins. FASEB J. 2006;20:735–737. doi: 10.1096/fj.05-4728fje. [DOI] [PubMed] [Google Scholar]
- 26.Barth H, Schnober EK, Zhang F, Linhardt RJ, Depla E, Boson B, et al. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J Virol. 2006;80:10579–10590. doi: 10.1128/JVI.00941-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanno S-I, Tomizawa A, Hiura T, Osanai Y, Shouji A, Ujibe M, et al. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice. Biol Pharm Bull. 2005;28:527–530. doi: 10.1248/bpb.28.527. [DOI] [PubMed] [Google Scholar]
- 28.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 29.Huong DT, Takahashi Y, Ide T. Activity and mRNA levels of enzymes involved in hepatic fatty acid oxidation in mice fed citrus flavonoids. Nutrition. 2006;22:546–552. doi: 10.1016/j.nut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO, Park EJ, et al. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr. 2003;22:561–568. doi: 10.1016/s0261-5614(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 31.Lee C-H, Jeong T-S, Choi Y-K, Hyun B-H, Oh G-T, Kim E-H, et al. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem Biophys Res Commun. 2001;284:681–688. doi: 10.1006/bbrc.2001.5001. [DOI] [PubMed] [Google Scholar]
- 32.Kim S-Y, Kim H-J, Lee M-K, Jeon S-M, Do G-M, Kwon E-Y, et al. Naringin time-dependently lowers hepatic cholesterol biosynthesis and plasma cholesterol in rats fed high-fat and high-cholesterol diet. J Med Food. 2006;9:582–586. doi: 10.1089/jmf.2006.9.582. [DOI] [PubMed] [Google Scholar]
- 33.EKMMA8 Eksperimentalna Meditsina i Morfologiya. Vol. 19. Sofia, Bulgaria: Hemus: 1980. p. 207. [Google Scholar]
- 34.Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.