Abstract
Nitric oxide (NO) and carbon monoxide (CO) are synthesized at high levels in asthmatic airways. NO can oxidize hemoglobin (Hb) to methemoglobin (MetHb). CO binds to heme to produce carboxyhemoglobin (COHb). We hypothesized that MetHb and COHb may be increased in asthma. COHb, MetHb, and Hb were measured in venous blood of healthy controls (n=32) and asthmatics (n=31). Arterial COHb and oxyhemoglobin were measured by pulse CO-oximeter. Hb, oxyhemoglobin, and deoxyhemoglobin were similar among groups, but arterial COHb was higher in asthmatics than controls (p=0.04). Venous COHb was similar among groups, and thus arteriovenous COHb (a-v COHb) concentration difference was greater in asthma compared with controls. Venous MetHb was lower in asthma compared to controls (p=0.01) and correlated to venous NO (p=0.009). The greater a-v COHb in asthma suggests CO offloading to tissues, but lower than normal MetHb suggests countermeasures to avoid adverse effects of high NO on gas transfer.
Keywords: carboxyhemoglobin, asthma, methemoglobin, nitric oxide, hemoglobin
Introduction
Asthma is a chronic inflammatory disease of the airways characterized by reversible airflow obstruction and inflammation. The fraction of exhaled NO (FENO) is increased in asthmatics due to the induction of NO synthase, an enzyme that catalyzes the production of NO from L-arginine, by proinflammatory cytokines. The majority of NO exists as nitrate and nitrite in the blood, and the measure of these NO metabolites can be used to estimate the NO produced. The NO metabolites will be referred to as venous NO. Venous NO content is increased in asthmatic individuals as compared to healthy controls [1]. Nitrite reaction with the iron (Fe2+) in heme leads to Fe3+ heme, i.e., methemoglobin (MetHb) [2]. MetHb cannot bind oxygen, and consequently, high NO may result in lower oxygen carrying capacity in asthma. Similar to the inducible isoform of nitric oxide synthase, heme oxygenase-1 is upregulated in asthma [3–6]. This enzyme catalyzes free heme to carbon monoxide (CO), free iron, and biliverdin. Exhaled CO is increased in asthmatics patients, which is attributed to greater heme oxygenase-1 levels [7–9]. Recently, CO has been shown to have cytoprotective effects against chronic inflammatory injury to tissue [10–12]. However, given that CO is increased in asthmatics and binds to heme with a much greater affinity than oxygen, asthmatic individuals may have higher than normal carboxyhemoglobin levels (COHb), which are related to asthma severity. A prior study showed that the arteriovenous carboxyhemoglobin (a-v COHb) concentration difference is increased in asthmatics having an acute asthma exacerbation, but the a-v COHb concentration difference is unknown in stable asthma [9]. NO and CO both interact with hemoglobin (Hb) much more avidly than O2 [13]. Because asthmatics have higher than normal CO and NO, the heme group of Hb may be bound differentially to CO, O2, and/or NO. The interaction of Hb with these gases may impact O2 delivery in the capillary beds.
Here we hypothesized that CO and NO, both at higher levels in asthma, may interact with the heme of Hb and produce higher levels of COHb and/or MetHb. To test this, we measured venous levels of oxyhemoglobin, deoxyhemoglobin, COHb, MetHb, Hb, hematocrit, and NO, as well as arterial levels of COHb and oxyhemoglobin by oximetry.
Methods
Study Population
Healthy controls and asthmatics over the age of 18 were enrolled. Asthma diagnosis was based on having either a 12% increase in FEV1 after short-acting bronchodilator or a methacholine dose at which an FEV1 drop greater than 20% from baseline occurred of 16mg/mL or less. All asthmatics were well controlled on medications. Healthy controls did not have a history of lung disease and were nonsmokers, defined as no cigarette smoking currently and within the last year. Because of sample limitations of blood drawing, samples were not available for all tests in all individuals testing; the number of samples assessed is provided with each result. The Cleveland Clinic Institutional Review Board approved the study, and all participants gave written informed consent.
Lung Functions and NO
Spirometry was performed using an automated spirometer (Koko, Longmont, CO) according to American Thoracic Society guidelines with National Health and Nutrition Examination Survey (NHANES III) predicted equations. Subjects withheld short-acting β-agonist treatments for 4 hours, long-acting β-agonist treatments for 12 hours, and other asthma medications for the appropriate length of time to avoid interference with the spirometry. FENO was completed using a NIOX Mino (Aerocrine C&S, Sweden) consistent with American Thoracic Society guidelines.
Blood Gas Measurements
Whole blood from a sodium heparin tube was used to measure venous COHb, MetHb, Hb, and hematocrit levels using a spectrophotometer (Radiometer ABL700, Westlake, OH). A Masimo Rad-87 CO-oximeter (Irvine, CA) was used to measure the arterial COHb and oxygen saturation. The sensor was placed on the patient's fingertip, and COHb and oxyhemoglobin levels were determined by the characteristic absorbance of visible and infrared light.
Venous NO Measurement
Nitrogen oxides in plasma samples were measured using chemiluminescence method as previously described [14]. Samples were treated with ZnSO4/NaOH to remove fat/protein. Total nitrogen oxides were converted to NO by a saturated solution of VCl3 in 1 M HCl, and NO gas generated was detected by the Sievers NOA 280i (GE Analytical Instruments, Boulder, CO).
Statistical Analysis
Quantitative measures were reported as mean ±SE and comparisons made using the Student's t test or analysis of variance (ANOVA). Categorical values were evaluated by Pearson's chi-squared tests. All data were analyzed in JMP version 7.0.1. A p value ≤0.05 was defined as statistically significant.
Results
Study Population
The study population included 63 study participants consisting of 31 individuals with asthma and 32 healthy individuals (Table 1). Asthmatics were similar to healthy controls in terms of age, gender, race, and BMI, but asthmatics had lower FEV1%, FEV1/FVC, and higher FENO as compared to healthy controls (Table 1). Of the asthmatics, 16% (n=5) were receiving high dose inhaled corticosteroids, 29% (n=9) low dose inhaled corticosteroids, 6.5% (n=2) oral steroids, and 42% (n=13) on a long acting beta agonist as a part of their daily treatment. All others were on intermittent short acting beta-agonist as needed. Patients had not experienced exacerbations of asthma for at least 6 weeks prior to testing.
Table 1.
Study Population
| Variable | Healthy Control (n=32) | Asthma (n=31) | p |
|---|---|---|---|
| Age | 41 ± 2 | 40 ± 2 | 0.90 |
| Gender (M/F) | 10/22 | 12/19 | 0.5 |
| BMI | 28.7 ± 1.2 | 30.7 ± 1.3 | 0.25 |
| Race | 17 Caucasian/10 AA/2 Asian/1 Latin/ 2 Not declared | 15 Caucasian/15 AA/1 Hispanic | 0.26 |
| FEV1% | 98 ± 2 | 85 ± 2 | 0.0006 |
| FVC% | 100 ± 2 | 94 ± 3 | 0.06 |
| FEV1/FVC | 0.80 ± 0.01 | 0.75 ± 0.02 | 0.04 |
| FENO (ppb) | 22 ± 2 | 36 ± 5 | 0.008 |
| Arterial O2 Saturation (%) | |||
| Male | 97.1 ± 0.4 | 97.2 ± 0.5* | 0.93 |
| Female | 97.9 ± 0.3 | 98.4 ± 0.3* | 0.24 |
| Venous Oxyhemoglobin (%) | |||
| Male | 80.7 ± 8.4 | 63.8 ± 7.4 | 0.15 |
| Female | 72.5 ± 5.6 | 68.0 ± 5.8 | 0.58 |
| Venous Deoxyhemoglobin (%) | |||
| Male | 17.1 ± 8.5 | 34.0 ± 7.5 | 0.16 |
| Female | 24.8 ± 5.7 | 29.7 ± 5.9 | 0.55 |
| pCO2 (mmHg) | |||
| Male | 46.7 ± 3.4 | 52.0 ± 3.0 | 0.26 |
| Female | 50.2 ± 2.0 | 48.8 ± 2.1 | 0.64 |
| pO2 (mmHg) | |||
| Male | 61.7 ± 15.3 | 50.4 ± 13.5 | 0.59 |
| Female | 53.7 ± 8.7 | 51.6 ± 9.0 | 0.87 |
| pH | |||
| Male | 7.38 ± 0.01 | 7.36 ± 0.01 | 0.20 |
| Female | 7.36 ± 0.01 | 7.35 ± 0.01 | 0.64 |
| Hb (g/dL) | |||
| Male | 15.4 ± 0.6* | 14.7 ± 0.6 | 0.37 |
| Female | 13.1 ± 0.4* | 13.5 ± 0.4 | 0.57 |
| Hematocrit (%) | |||
| Male | 46.4 ± 2.9* | 43.1 ± 1.5 | 0.36 |
| Female | 39.2 ± 1.5* | 41.0 ± 1.1 | 0.31 |
BMI, body mass index; FENO, fraction exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; Hb, hemoglobin
Indicates male, female values are different at p<0.05
Hemoglobin in Asthma
Asthmatics had hemoglobin and hematocrit similar to controls, taking gender into account (Table 1). Healthy men had higher hematocrit and hemoglobin than healthy women, but asthmatic men had similar hematocrit and hemoglobin to asthmatic women. Asthmatic men tended to have lower hematocrit and hemoglobin than control men, which did not reach statistical significance. Arterial saturation was similar among controls (n=30) and asthmatics (n=31). Asthmatic women had a higher arterial oxyhemoglobin saturation than asthmatic men, and healthy women similarly tended to have higher arterial oxyhemoglobin saturation than healthy men (Table 1).
Venous blood gases and hemoglobin
Venous pO2, pCO2, and pH were similar among asthmatics (n=23) and controls (n=22). Similarly, venous oxyhemoglobin and deoxyhemoglobin levels were similar among asthmatics (n=23) and controls (n=22) (Table 1).
COHb and MetHb
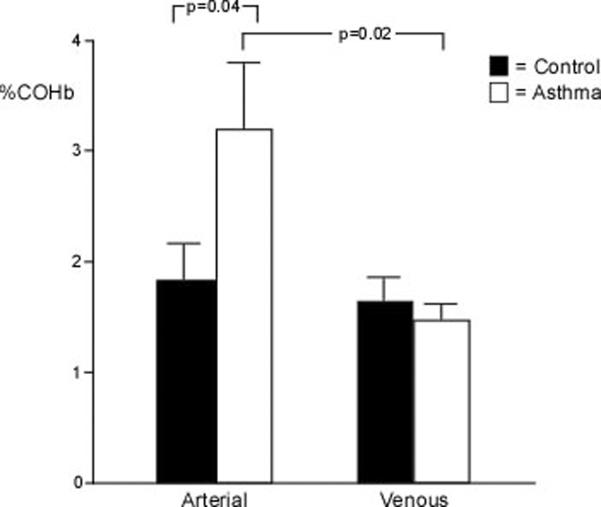
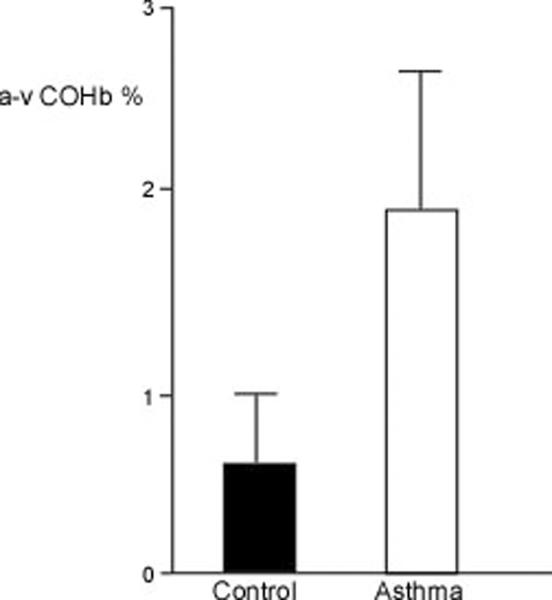
Arterial COHb was higher in asthmatics (n=31) as compared to controls (n=30) (%COHb: controls 1.84±0.32%, asthma 3.24±0.59%; p=0.04), but the venous COHb was similar among asthmatics (n=23) and controls (n=22) (%COHb: controls 1.64±0.20%, asthma 1.48±0.14%; p=0.52) (Figure 1A). This resulted in a significant a-v difference in the asthmatics (n=23) (1.89±0.73; p=0.02) but not the controls (n=20) (0.51±0.55; p=0.37) (Figure 1B). Arterial COHb, venous COHb, and a-v COHb were unrelated to FEV1%, FEV1/FVC ratio, or FENO. Interestingly, the venous MetHb levels were lower in asthmatics (n=23) as compared to controls (n=22) (%MetHb: controls 0.94±0.04%, asthma 0.78±0.04%; p=0.01) (Figure 2A). The MetHb levels were not related to FEV1%, FEV1/FVC ratio, or the arterial or venous COHb in asthmatics and controls.
Figure 1.
[A] Arterial and venous COHb among asthmatics and healthy controls. Asthmatics have higher arterial levels of COHb than controls. [B] a-v COHb differences are greater in asthma (P=0.02). Mean values and standard error are shown.
Figure 2.
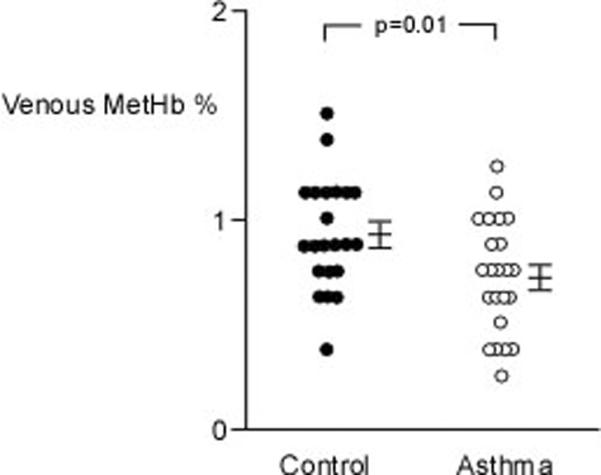
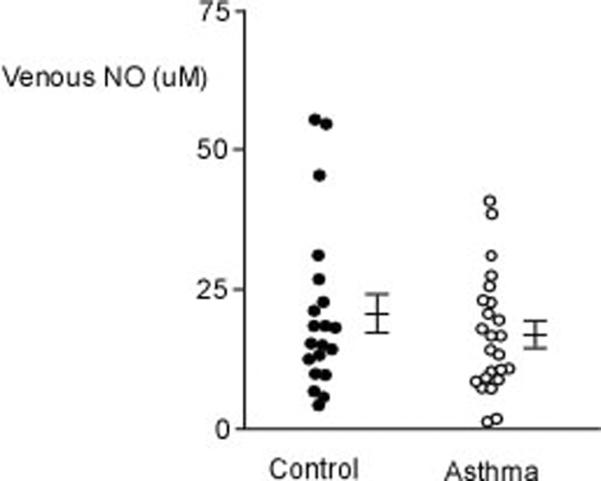
[A] Venous MetHb is lower in asthmatics as compared to healthy controls. [B] Venous NO is similar among asthmatics and controls. Mean values and standard error are shown. Each dot represents an individual measure.
Venous NO and FENO
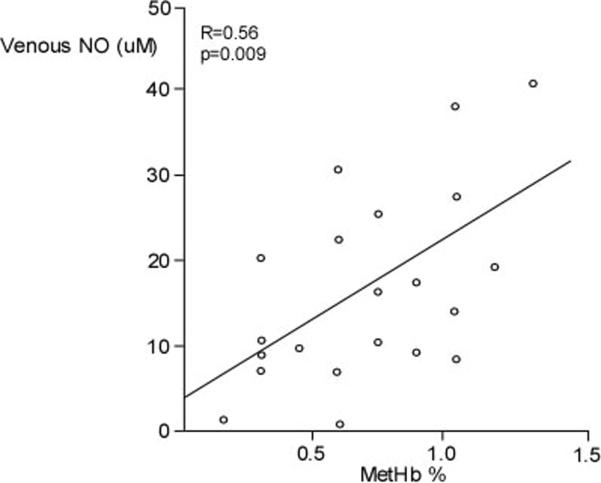
Although FENO was higher in asthmatics as compared to controls (n=30) (Table 1), MetHb was lower in asthmatics than controls (Figure 2A). Furthermore, venous NO was similar among groups (venous NO (μM): controls 21.03±3.34, n=20, asthma 16.53±2.14, n=24; p=0.25) (Figure 2B). MetHb levels were significantly related to venous NO in asthma (R=0.56, p=0.009) (Figure 3), but not controls (R=0.12, p=0.67). However, venous NO or MetHb were unrelated to FENO in asthma or controls.
Figure 3.
Relationship between venous NO and venous MetHb in asthma.
Discussion
This study identifies an increase of arterial COHb levels and a-v COHb difference in stable asthma, suggesting that asthmatics produce more CO in the lung and offload more CO to tissues than healthy controls. To our knowledge, this is the first report that asthmatics have lower levels of MetHb than healthy controls. This indicates that asthmatics have systemic mechanisms to avert adverse consequences of high CO and NO in the airways in order to maintain efficient transport of oxygen to tissues.
The finding of an increase in arterial COHb in asthma is consistent with those of previous studies of unstable asthma [5, 9]. Yasuda et al reported that a-v COHb was high in a study population of 18 patients during an acute asthma exacerbation and that the a-v COHb was related to the degree of airflow obstruction as determined by FEV1 [9]. This led to the concept that the a-v COHb concentration difference was associated with asthma severity and was a reflection of the amount of inflammation in the airway. Here, in stable asthmatics, the COHb and a-v COHb levels were not related to any spirometric value, specifically the FEV1% or the FEV1/FVC ratio.
On the other hand, the finding of a lower than normal MetHb level in asthmatics was unexpected. A higher than normal MetHb level was anticipated in asthma on the basis that NO can oxidize the Fe2+ in heme to Fe3+ to produce MetHb. Nadeem et al showed that venous NO was higher in in asthmatics as compared to controls in a study population of 28 nonsmoking asthmatics who were at least 4 weeks removed from an asthma exacerbation. Here, venous NO was similar among asthmatics and controls, despite high FENO. The FENO and NO in the circulatory system are not related in these stable asthmatics. However, the positive relationship of MetHb to venous NO confirms that NO in the circulatory system contributes to the formation of MetHb. The lower MetHb in asthma may be due to higher levels of methemoglobin reductase (also known as cytochrome b5 reductase), which is an enzyme that converts methemoglobin (Fe3+) to hemoglobin (Fe2+) in order to maintain heme-oxygen binding. More studies are needed to investigate mechanisms for the lower levels of MetHb, and the importance to gas transport in asthma.
One limitation of this study is that we did not collect arterial blood for COHb, nitric oxide, oxyhemoglobin, deoxyhemoglobin, or MetHb levels. This would have provided greater quantitative assessment of the arterial levels, and a-v differences among the various heme binding states. Second, exhaled CO levels were unavailable for subjects, which limits the mechanistic insights of origin of the greater arterial COHb content. Third, methemoglobin reductase was not assessed, as we had anticipated higher than normal MetHb in asthma.
In conclusion, this is the first study to identify variations in the heme state in stable asthma. The gases CO, NO, CO2, and O2 all bind to and influence hemoglobin through the interactions with the heme group. Further work is needed to explain the interactions of these gases with heme in asthma and the relevance to gas transfer.
Acknowledgements
We would like to thank Masimo for providing us with the loan of their equipment. This work is supported by RC1 HL099303, HL081064, HL103453, and UL1 RR024989 from the National Center for Research Resources
Footnotes
Conflicts of Interests: None
References
- 1.Nadeem A, Chhabra SK, Masood A, et al. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–78. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 2.Brooks J. The action of nitrite on haemoglobin in the absence of oxygen. Proc Royal Soc London B Biol Sci. 1937;123:368–382. [Google Scholar]
- 3.Slebos Dirk-Jan, Ryter S, Choi AM. Heme oxygenase-1 and carbon monoxide in pulmonary medicine. Respiratory Research. 2003;4:7. doi: 10.1186/1465-9921-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryter S, Otterbein LE, Morse D, et al. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234–235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuda H, Yamaya M, Yanai M, et al. Increased blood carboxyhaemoglobin concentrations in inflammatory pulmonary diseases. Thorax. 2002;57:779–783. doi: 10.1136/thorax.57.9.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maines MD. The heme oxygenase system: a regulator of second messenger gases. AnnuRev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 7.Zayasu K, Sekizawa K, Okinaga S, et al. Increased carbon monoxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1997;156:1140–1143. doi: 10.1164/ajrccm.156.4.96-08056. [DOI] [PubMed] [Google Scholar]
- 8.Horvath I, Loukides S, Wodehouse T, et al. Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: a new marker of oxidative stress. Thorax. 1998;53:668–672. doi: 10.1136/thx.53.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasuda H, Sasaki T, Yamaya M, et al. Increased arteriovenous carboxyhemoglobin differences in patients with inflammatory pulmonary diseases. Chest. 2004;125:2160–2168. doi: 10.1378/chest.125.6.2160. [DOI] [PubMed] [Google Scholar]
- 10.Otterbein LE, Choi AM. Heme oxygenase: colors of defence against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 11.Morse D, Lin L, Choi AM, et al. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radical Biology and Medicine. 2009;47:1–12. doi: 10.1016/j.freeradbiomed.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryter S, Alam J, Choi AM. Heme oxygenase/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 13.Antonini E, Brunori M. Hemoglobin and myoglobin in their reactions with ligands. North-Holland; Amsterdam: 1971. [Google Scholar]
- 14.Kaneko FT, Arroliga AC, Dweik RA, et al. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med. 1998;158:917–23. doi: 10.1164/ajrccm.158.3.9802066. [DOI] [PubMed] [Google Scholar]