Abstract
Sexual reproduction is notoriously complex in fungi with species able to produce sexual progeny by utilizing a variety of different mechanisms. This is even more so for species employing multiple sexual strategies, which is a surprisingly common occurrence. While heterothallism is relatively well understood in terms of its physiological and molecular underpinnings, homothallism remains greatly understudied. This can be attributed to it involving numerous genetically distinct mechanisms that all result in self-fertility; including primary homothallism, pseudohomothallism, mating type switching, and unisexual reproduction. This review highlights the need to classify these homothallic mechanisms based on their molecular determinants and illustrates what is currently known about the multifaceted behaviours associated with homothallism.
Keywords: Mating, homothallism, sexual reproduction, unisexual reproduction
INTRODUCTION
Sexual reproduction is generally accepted as a process that occurs when opposite mating partners interact physically to produce genetically variable and, ideally over time, better adapted offspring. However, the ability to reproduce sexually in the absence of a partner is known to occur in some eukaryotes, where it has been described in species as diverse as the fan worm (Tovar-Hernández et al. 2009), land snail (Chen 1994), and is commonplace in plants (Pannell 2015). Fungi are no exception, and indeed display a fascinating range of sexual strategies found in self-fertile basidiomycetes and ascomycetes, as reviewed by Lin & Heitman (2007). These fungi are known as homothallic, in contrast to heterothallic where outcrossing is obligatory (Blakeslee 1904).
Homothallism in fungi has historically been defined as the ability of a single spore to produce a sexually reproducing colony when propagated in complete isolation. This is the definition by which self-fertility was first described (Blakeslee 1904) and it is the strategy used to define the sexual status of a newly described fungal species. However, “homothallism” is an all-inclusive term that describes diverse sexual strategies. Describing a fungus as homothallic is therefore over-simplified, given that it can utilize one or more strategies to give rise to sexual progeny. The rapidly growing availability of fungal genome data (Scazzocchio 2014) is allowing more intensive genetic inquiry into fungal mating and this will result in a more informed perspective on sexual reproduction, especially with respect to self-fertility. In this regard, it has become possible to define the various mechanisms underpinning homothallism from a genetic perspective and thus improve our understanding of the evolutionary outcomes of sexual reproduction as a whole.
In order to consider homothallism from a genetic standpoint, it is important to understand that sexual reproduction and mating specificity in fungi is controlled largely by genes present at the mating type (MAT1) locus (Kronstad & Staben 1997). The allelic variants, or idiomorphs, of this locus encode one of two classes of proteins; those that give rise to the MAT-1 phenotype- the genes at the MAT1-1 idiomorph- and those that give rise to the MAT-2 phenotype- the genes at the MAT1-2 idiomorph (Turgeon & Yoder 2000). In its most commonly understood form, sexual reproduction requires the expression of genes from both these idiomorphs (Ni et al. 2011). Thus, fungal mating systems can be classified based on the genic content of the MAT1 locus. Using this designation, homothallic individuals possess genes of both idiomorphs within a single cell, enabling combined MAT1-1 and MAT1-2 expression by a single individual. This is in contrast to individuals of heterothallic species that possess genes from only one of the two idiomorphs. In these species, the combined expression of genes from both these idiomorphs requires the presence of two opposite mating type partners.
While heterothallism represents a relatively simple and well-understood opposite mate interaction, homothallism constitutes a variety of distinct strategies that collectively allow for single individuals to sexually reproduce independently of an opposite mating partner. Although the genetic and biochemical mechanisms underlying a few of these homothallic sexual strategies have been elucidated in some (typically model) fungi, the majority remain unstudied. This review seeks to compare and contrast these mechanisms in terms of their causative molecular basis. It further aims to particularly emphasize the most recently described and unique form of homothallism known as unisexual reproduction.
CONVENTIONAL CATEGORIES OF HOMOTHALLISM
Primary homothallism
Primary homothallism is the classic mechanism by which self-fertility is achieved. Here, species adhere to the strict genetic requirement of the combined expression of MAT1-1 and MAT1-2 genes in a single genome (Ni et al. 2011; Fig. 1). This is exactly the opposite of heterothallism and, as such, primary homothallic species possess all the MAT genes typically found in both MAT-1 and MAT-2 isolates of a closely-related heterothallic species (Lin & Heitman 2007). Genes from the alternate idiomorphs can either exist at a single locus (linked or fused) or be present in separate regions (unlinked) within the genome (Pöggeler et al. 1997, Yun et al. 1999, Galagan et al. 2005).
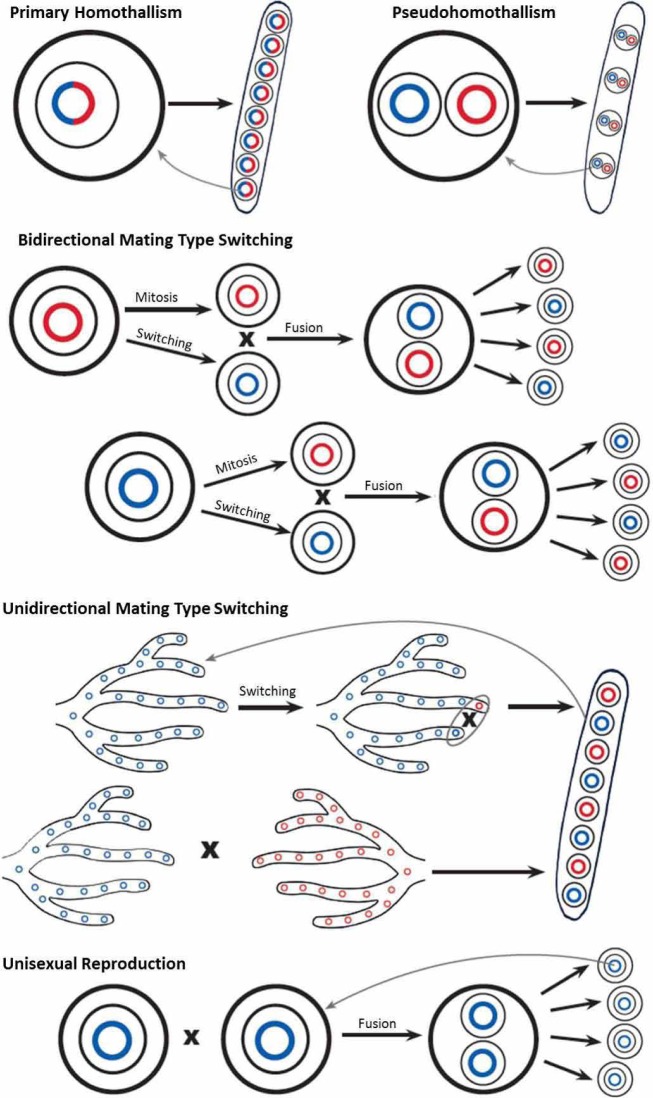
Fig. 1.
A diagrammatic representation of the various genetic mechanisms of homothallism in fungi. Red and blue circles represent haploid genomes of opposite mating type, large black circles represent single fungal cells and small black circles within black ovals represent ascospores within asci. Primary homothallism: The presence of both MAT idiomorphs within a single genome allows the independent production of eight uninucleate ascospores identical to the parental cell as seen in Aspergillus nidulans. Pseudohomothallism: Self-fertility is the result of the packaging of two, opposite mating type nuclei within a single cell. Independent sexual reproduction results in the production of four binucleate ascospores as seen in Neurospora tetrasperma. Bidirectional mating type switching: Cells of either mating type undergo mitosis to form two identical cells, one of which is then able to switch to the opposite mating type. This leads to a mixed colony capable of sexual reproduction via functional heterothallism as seen in Saccharomyces cerevisiae. Unidirectional mating type switching: Cells of the hyphae of one mating type are able to switch mating type, producing a mixed mating type culture capable of sexual reproduction. The other mating type is unable to switch and thus requires the presence of a second individual to undergo sexual reproduction. This mating system is seen in Chromocrea spinulosa. Unisexual reproduction: Cells of the same mating type are able to interact and produce sexual spores regardless of the absence of an opposite mating type partner as in Cryptococcus neoformans.
The mechanism that governs primary homothallism has been relatively well studied in comparison to the other forms of this condition. This is most likely due to the model species Aspergillus nidulans exhibiting a primary homothallic sexual cycle (Pontecorvo et al. 1953). In this species, individuals derived from a single uninucleate and haploid cell are able to undergo sexual reproduction under environmentally conducive conditions (Wang et al. 2010). Genetically, primary homothallism was confirmed in A. nidulans when both the MAT1-1-1 gene (possessing the conserved alpha domain) and the MAT1-2-1 gene (possessing the conserved HMG box domain) were identified and shown to be expressed in a single genome (Paoletti et al. 2007). This is in contrast to heterothallic Aspergillus species, such as A. parasiticus and A. fumigatus, where individuals possess either the MAT1-1-1 or the MAT1-2-1 gene (Horn et al. 2009, O’Gorman et al. 2009).
Pseudohomothallism
Pseudohomothallism adheres to the physiological definition of homothallism and, as such, single spores of species exhibiting this type of self-fertility are fully able to undergo independent sexual reproduction (Whitehouse 1949). However, from a cellular and molecular standpoint, the underlying mechanism is more complex than that of primary homothallism and has consequently been referred to as an example of secondary homothallism (Whitehouse 1949) or functional heterothallism (Dodge 1957, Lin & Heitman 2007).
In species exhibiting pseudohomothallic behaviour, self-fertility is the result of the packaging of two independent and opposite mating type nuclei within a single spore (Fig. 1). This ensures that when the spore germinates, a heterokaryotic and thus self-fertile mycelium is produced (Dodge 1927). In this situation, a single spore actually contains the genetic complement of two opposite mating partners as two discrete nuclei. Functionally, it thus represents a heterothallic system that is able to occur within a single originating cell (Dodge 1957). This behaviour has been described in several species including Neurospora tetrasperma (Dodge 1927), Podospora anserina (Ames 1934), and Gelasinospora tetrasperma (Dowding 1933).
Pseudohomothallism has been intensively studied in N. tetrasperma, where it was first described (Dodge 1927). In this species, a significant majority of the resulting ascospores are larger than those produced by closely-related heterothallic species. Furthermore, while heterothallic Neurospora species typically produce eight ascospores, N. tetrasperma produces only four (Dodge 1927). These large ascospores, representing about 90 % of those produced (Raju 1992), possess two independent nuclei: one of which expresses the MAT-1 phenotype while the other expresses the MAT-2 phenotype (Dodge 1927). This comes about due to a programmed meiotic spindle alignment that allows two opposite mating type nuclei derived from a single diploid nucleus to be packaged in a single ascospore. This might be considered as a form of reproductive “assurance” allowing unhindered sexual reproduction even in the absence of a suitable mating partner (Merino et al. 1996). The remaining ~10 % of the ascospores produced in N. tetrasperma are much smaller, possess only a single nucleus, and thus express only one of the two mating types (Raju 1992). These spores have been suggested to represent a mechanism whereby outcrossing can be maintained in the species, along with the benefits attributed to functional recombination (Raju 1992). Asci comprised of both the large and small spores ensure that both homothallic and heterothallic reproduction can occur, thereby maintaining the benefits associated with both.
MATING TYPE SWITCHING
A second mechanism that allows for homothallic behaviour is referred to as mating type switching and it represents yet another example of secondary homothallism (Lin & Heitman 2007). In this mating strategy, an individual of one mating type can undergo a switch to the opposite mating type, either bidirectionally (reversibly) or unidirectionally (irreversibly). This allows a single cell to produce a colony of mixed mating type that is subsequently able to reproduce sexually (Lin & Heitman 2007; Fig. 1).
Bidirectional Mating Type Switching
The first case of this unusual mating behaviour was described in Saccharomyces cerevisiae, after the species had been described as homothallic by one group of researchers (Winge & Laustsen 1937) but heterothallic by another (Lindegren & Lindegren 1943, 1944). It was later shown that populations of this ascomycetous yeast are comprised of both self-fertile and self-sterile individuals. Once the genetic mechanism underlying this type of homothallism had been elucidated, it became apparent that every individual within the population, regardless of mating behaviour, possessed sequence of the MAT1-1 idiomorph (termed MATα) as well as sequence of the MAT1-2 idiomorph (termed MATa).
The presence of both mating type idiomorphs in a single cell appeared to be congruent with the standard homothallic requirement of the expression of genes from both mating types in a single cell. However, it did not explain the presence of self-sterile individuals in the population that also possessed all the genes typically required for sexual reproduction. Only then was it shown that, in addition to the typical MAT1 locus that is present in this species, there are also two MTL (mating-type like) loci that flank the MAT1 locus (Nasmyth & Tatchell 1980). While the active MAT1 locus harbours sequence of only one of the MAT idiomorphs, the first MTL possesses MAT1-1 sequence and is termed HMLα and the second possesses MAT1-2 sequence and is termed HMRa. These MTL, however, are transcriptionally silent due to the presence of tightly wound heterochromatin. Consequently, the only MAT genes expressed in a single cell are those found at the active MAT1 locus itself (Rusche et al. 2003). This implies that any one cell can either be classified as MAT-1 or MAT-2, despite possessing sequence for both mating types. The consequence is that the species undergoes a traditional heterothallic cycle.
In S. cerevisiae, homothallic behaviour is due to a gene entirely independent of the MAT1 locus and the associated MTL. This type of self-fertility is a result of the HO gene and its ability to initiate mating type switching events. The HO+ gene encodes an active endonuclease that recognizes the active MAT1 locus, makes a double stranded DNA break and excises the locus (Nasmyth 1982). The flanking MTL of the opposite mating type is then used as a template from which a new MAT1 locus can be constructed via gene conversion. This mechanism allows for a switch to take place from one mating type to the other due to the presence of MTL loci for both the MATα and MATa idiomorphs. It is, therefore, possible for a single originating cell to produce a colony of mixed mating types that is consequently able to sexually reproduce in a functionally heterothallic manner. If a cell possesses the inactive copy of the HO gene (HO-), it cannot switch and is thus considered self-sterile and not able to independently produce a sexually competent colony. The genetic mechanisms and cellular processes underlying this mechanism have been comprehensively reviewed (Haber 1998, 2012).
Unidirectional Mating Type Switching
While the mechanisms underlying the bidirectional switching as observed in S. cerevisiae are well-defined, those relating to unidirectional switching remain poorly understood. This is possibly because this form of switching has only been observed in a small number of fungi residing in distantly-related genera including Chromocrea, Ceratocystis, Glomerella, and Sclerotinia (Wheeler 1950, Mathieson 1952, Uhm & Fujii 1983, Perkins 1987, Harrington & McNew 1997, Witthuhn et al. 2000, Wilken et al. 2014). These fungi have not been included amongst the model organisms used in fungal genetics studies and thus their sexual strategies have not been clearly elucidated.
Unidirectional mating type switching has been partially investigated in Chromocrea spinulosa, where spore size is intricately linked to mating type and is most likely a pleiotropic expression of mating type (Mathieson 1952). The asci of this species have eight two-celled ascospores, half of which are small and produce individuals of l mating type while the others are large and produce individuals of the l+ mating type (Mathieson 1952). When single ascospore isolates of C. spinulosa were originally produced in order to identify the species as heterothallic or homothallic, they segregated into both self-fertile and self-sterile individuals. The sexually competent individuals were shown to have arisen from the larger spores. Those remaining barren of sexual structures had been produced from smaller spores and could only undergo sexual reproduction when co-cultured with individuals derived from large spores (Mathieson 1952).
Interestingly, the asci produced during the self-fertile, l+ sexual cycle of C. spinulosa possessed four large and four small ascospores. This is despite the absence of an interaction with the l mating type partner which would have produced the small ascospores in a typical heterothallic reaction (Mathieson 1952). The most likely explanation for this phenomenon is ‘unidirectional mating type switching’. In this situation, hyphae of the l+ mating type are able to switch via mating type mutation to the l mating type. This results in a mixed mating type culture that is then able to sexually reproduce in a functionally heterothallic process (Mathieson 1952). The mechanism is treated as unidirectional because individuals derived from small ascospores are unable to switch their mating type and subsequently remain sexually inactive unless co-incubated with a suitable partner.
Unidirectional mating type switching is well-known in some species of Ceratocystidaceae (de Beer et al. 2014), a group of important plant pathogens but one that has not received substantial attention from fungal geneticists. This sexual strategy was first recognized in the sweet potato black rot fungus Ceratocystis fimbriata (Webster & Butler 1967). More recent studies of mating type switching in species of Ceratocystidaceae (Harrington & McNew 1997, Witthuhn et al. 2000, Wilken et al. 2014). Wilken et al. (2014) and Lee et al. (2015) have shown that self-fertile isolates of C. fimbriata and C. albifundus have three genes at the MAT1 locus, two of the MAT1-1 idiomorph (MAT1-1-1 and MAT1-1-2) and one of the MAT1-2 idiomorph (MAT1-2-1). Ascospores produced during sexual reproduction segregate into those that produce self-fertile isolates and those that produce self-sterile isolates. Genetically, the switch from a self-fertile to a self-sterile mating strategy is the result of the complete deletion of that MAT1-2-1 gene from the genome. This results in isolates with only MAT1-1 genes (Witthuhn et al. 2000, Wilken et al. 2014). How this change occurs has not been resolved, but it is thought to involve homologous recombination due to identical repeats that flank the MAT1-2-1 gene (Wilken et al. 2014). It remains necessary to determine the significance of the unidirectional mating type switching mechanism in Ceratocystidaceae. This is especially relevant considering that the self-sterile isolates with the MAT1-2-1 deletion are significantly less fit than their self-fertile counterparts (Lee et al. 2015). It would thus be interesting to determine whether the unidirectional mating type switching observed in C. fimbriata could resemble the functionally heterothallic system seen in Chromocrea spinulosa, which would then preserve the self-sterile isolate despite its reduced fitness.
The existence of pseudohomothallism and mating type switching systems challenges our understanding of the concept of homothallism. In populations displaying these systems, fungi are considered functionally heterothallic, despite both mating partners being derived from a single originating cell. It has been speculated that these mating systems allow for the preservation of homothallic mating under circumstances where genetically distinct opposite mating partners are not easily accessible. Additionally, the ability to outcross under conditions where functional recombination is possible is also conserved (Lin & Heitman 2007). Nevertheless, systems such as pseudohomothallism and mating type switching preclude simplistic classification of fungi as either homothallic or heterothallic. While it is convenient to identify fungal species as either homothallic or heterothallic, such designations are often times naïve, especially when the species in question has both types of individuals. Not surprisingly, fungal classification by mating strategy is virtually impossible, not to mention very contentious (Winge & Laustsen 1937, Lindegren 1943).
UNISEXUALITY– AN UNUSUAL FORM OF HOMOTHALLISM
Glass & Smith (1994) discovered a novel form of homothallism in Neurospora africana. The same phenomenon was subsequently described in three other Neurospora species, N. galapagosensis, N. dodgei, and N. lineolata (Beatty et al. 1994). While typically heterothallic Neurospora species occur and have either the mat A (homologous to MAT1-1) or the mat a (homologous to MAT1-2) idiomorphs (Glass et al. 1990a, Staben & Yanofsky 1990), some are self-fertile. These species have both the mat A and mat a idiomorphs in a single genome and in this regard, they exhibit primary homothallism (Beatty et al. 1994). However, only the mat A idiomorph has been found in the homothallic N. africana and related species. They consequently provide an apt example of sexual reproduction in the absence of typically essential MAT genes (Glass & Smith 1994). Interestingly, however, when closely-related heterothallic species are transformed with the N. africana mat A sequence, the self-fertility is not transferred (Glass & Smith 1994). This suggests that other genetic mechanisms are responsible for the homothallic behaviour and that self-fertility is not the result of a unique function of the mat A sequence in the species.
The mating strategy in N. africana and species with the same behaviour has recently been termed unisexual reproduction. Its discovery and recognition represents a paradigm shift in the way we view homothallism. In all previously defined forms of homothallism (primary and secondary), the presence and expression of genes from both the MAT1-1 and MAT1-2 idiomorphs play an essential role in the initiation and full process of sexual reproduction. This is regardless of the origin or physical location of these genes. What is unique about unisexual reproduction is that it describes an atypical system where species are able to complete an entire sexual cycle when only genes from a single MAT idiomorph are expressed (Roach et al. 2014; Fig. 1).
A comprehensive description of the genetic and biochemical mechanisms underlying unisexual reproduction has been presented using the basidiomycetous yeast Cryptococcus neoformans as a model (Lin et al. 2005, Feretzaki & Heitman 2013, Ni et al. 2013). This species, which has a well-characterized heterothallic sexual cycle, has also been shown to undergo α-α cell mating, or unisexual reproduction (Lin et al. 2005). Unisexual reproduction has been linked to the pathogenicity of the species and thus has important implications in terms of human health.
Naturally occurring populations of C. neoformans have been shown to be highly clonal, consisting of >99 % α cells (Kwon-Chung & Bennett 1978). Before unisexual reproduction was discovered in the species, this clonality was attributed to high levels of asexual reproduction and an almost non-existent heterothallic mating system, although α and a individuals have been known to mate (Kwon-Chung 1976). It was subsequently observed, however, that α cells were able to undergo a tissue differentiation process similar to that seen during opposite sex mating and that this was the cause of the bias of α cells in C. neoformans populations (Wickes et al. 1996). This system was originally referred to as “monokaryotic fruiting” and was thought to be a strictly mitotic event. It was only more recently recognized as a sexual event that relies on essential meiotic genes, and unisexual reproduction was consequently described in the species (Lin et al. 2005).
The unisexual cycle in C. neoformans can be initiated via one of two pathways. One of these involves endoreplication of the entire genome of a single cell and represents the strictly homothallic version of unisexual reproduction. Alternatively, two cells of the same mating type can undergo cellular and nuclear fusion. The later pathway represents an almost heterothallic system, where outcrossing still takes place despite an identical MAT1 locus. In both cases, the resulting diploid cell undergoes filamentation and subsequent basidium formation. Meiosis then takes place in the basidium and basidiospores are produced (Lin et al. 2005). The unisexual process relies on almost all the genes that are essential in typical bisexual mating. Thus the sexual spores are similar in both types of sexual reproduction, with the exception that unisexual reproduction produces spores of only the α mating type. Consequently, it has been suggested that unisexual reproduction is able to confer the benefits of sexual reproduction. This would, for example, be through allowing genetic recombination while minimizing the cost required to locate a partner, thereby efficiently producing infectious sexual spores in a seemingly clonal population (Phadke et al. 2013).
A unisexual cycle has also been described in the ascomycetous yeast Candida albicans. This species has long been thought of as an asexual, obligate diploid unable to produce sexually recombinant progeny (Noble & Johnson 2007). In addition to unisexual reproduction, C. albicans can also engage in parasexuality under suitable circumstances. The identification of a mating type-like locus (Hull & Johnson 1999) and the discovery of sexually-competent mating partners made it possible to characterize a parasexual cycle in the species (Bennett & Johnson 2003). This cycle involves two diploid cells, a/a and α/α, undergoing cell and nuclear fusion resulting in an unstable tetraploid, which subsequently undergoes concerted chromosome loss until a near-diploid state is reached (Bennett & Johnson 2003).
Unisexual reproduction in C. albicans can be achieved via one of two independent pathways, both of which allow sexual reproduction between cells of the a mating type (Alby et al. 2009). The first involves the mutational inactivation of the Bar1 gene. In this species, the Bar1 gene product is a protease that is produced by a cells in order to degrade the production of endogenously-produced α pheromone (Schaefer et al. 2007). When this protease is defective, a cells are able to respond to this self-made pheromone by the activation of Ste2, the receptor for this pheromone. This leads the cell to initiate sexual reproduction as it would in the presence of a pheromone-producing α cell (Alby et al. 2009). The second pathway by which same-sex matings can be achieved involves ménage à trois matings. Here, a-a mating can occur due to the production of the α pheromone by a limited number of α cells in the population. In this case, a cells are able to recognize the exogenously-produced α pheromone despite the Bar1 protease activity and can initiate sexual reproduction with a second a cell. This form of same-sex mating is also found when a limited number of a cells are co-incubated with α cells which are then able to undergo α-α mating (Alby et al. 2009).
The most recently encountered example of unisexual mating is in the filamentous ascomycete Huntiella moniliformis (Wilson et al. 2015), a saprobic species of Ceratocystidaceae and previously treated in Ceratocystis (de Beer et al. 2014). Unisexual reproduction in this species appears to resemble that in N. africana, where only a single mating type, MAT-2, has been identified despite an extant sexual cycle existing. A closely-related heterothallic species, H. omanensis, possesses individuals of the MAT-1 mating type as well as the MAT-2 mating type and exhibits a typically heterothallic sexual cycle. Surprisingly, unisexual reproduction is not seen in the latter despite the MAT1-2 idiomorph being highly conserved between these two species (Wilson et al. 2015).
Unisexual reproduction is clearly an incompletely described form of homothallism. Although it is currently known in only a small number of species, this does not necessarily mean that it is an uncommon sexual strategy. When it was originally described in N. africana, the evolutionary persistence of the mechanism was questioned due to the negative effects associated with inbreeding. However, the discovery of unisexual reproduction in three related Neurospora species has emphasized the relevance of unisexual mating as an evolutionarily significant reproductive strategy in fungi (Glass et al. 1990b, Glass & Smith 1994, Roach et al. 2014). Furthermore, it has been shown in Cryptococcus neoformans that homothallic unisexual reproduction allows for the production of limited genotypic and subsequent phenotypic diversity de novo. From an evolutionary perspective, this would be beneficial because already well-adapted genotypes are maintained while allowing for a restricted level of genetic admixture to take place within a population (Ni et al. 2013).
CONCLUSIONS
Self-fertility allows for reproductive assurance in species across all major groups of eukaryotes. In fungi, this condition is known as homothallism and ensures that a single individual is able to undergo sexual reproduction, even when a suitable mating partner is not present in the environment. Sexual spores that are typically more environmentally resistant can thus be produced, allowing for growth and persistence in unfavourable environments (Aanen & Hoekstra 2007). Sexual reproduction also allows for recombination, which in the case of homothallism could provide fungi that reproduce predominantly clonally with a means of escaping the accumulation of deleterious mutations (Nielsen 2006).
Homothallism in fungi clearly encompasses a wide variety of genetically distinct mechanisms that all result in sexually reproducing cultures from single originating cells. This review highlights the need for a classification system that will make it possible to fully describe the sexual strategies employed by self-fertile fungi. It also provides a number of molecular and genetic determinants that can aid in the delineation of homothallism into its discrete subcategories. Clearly, homothallism is far more complex than was originally believed, and much work still needs to be done to fully understand the various mechanisms underlying its behaviour. The availability of genome sequences for non-model species is already aiding in this research, allowing for the characterization of the MAT1 locus in addition to classic mycological techniques used to identify the sexual status of a fungus.
Unisexual reproduction has emerged as an intriguingly unique reproductive strategy in fungi. Its unexpected discovery in H. moniliformis leads us to question whether it might not be much more common in fungi than has previously been imagined. That fungi with already well-described homothallic or heterothallic sexual cycles have now also been described as unisexual suggests that many other species could also exhibit this dual mating behaviour. For example, the tree pathogen Cryphonectria parasitica is described as homothallic but it preferentially outcrosses in nature, a situation relatively common in species of Cryphonectriaceae and Dothideomycetes (Milgroom et al. 1993). Could it be that these fungi also have the capacity to undergo unisexual mating?
The numerous and varied mechanisms via which self-fertility has been maintained in fungi suggests that homothallism represents an evolutionarily significant mating strategy. Self-fertility provides these species with the numerous benefits associated with sexual reproduction while minimizing its costs, particularly the costs associated with locating a mating partner. The ability of many species to reproduce sexually both via a heterothallic and homothallic cycle illustrates a fascinating level of reproductive plasticity in fungal mating systems. It allows species to outcross or inbreed depending on the particular environment which they face (Roach et al. 2014).
That most fungal species do not sporulate actively in culture has posed a major challenge relating to the understanding of sexual reproduction in fungi. It is for this reason that most genetic studies have been undertaken in so-called model species, which are typically easy to maintain and mate in culture. However, the increasing availability of fungal genome sequences will allow a much more comprehensive understanding of the reproductive strategies in the fungi. This will be for both model and non-model species and makes for exciting prospects in the near future.
ACKNOWLEDGEMENTS
This project was financed by the University of Pretoria, the Department of Science and Technology (DST)/National Research Foundation (NRF) Centre of Excellence in Tree Health Biotechnology and the Genomics Research Institute (University of Pretoria Institutional Research Theme). This work is based on research supported in part by a number of grants from the National Research Foundation of South Africa (including Grant specific unique reference number (UID) 83924). The Grant holders acknowledge that opinions, findings and conclusions or recommendations expressed in any publication generated by NRF supported research are that of the author(s), and that the NRF accepts no liability whatsoever in this regard. We would also like to acknowledge Glenda Brits of Education Innovation at the University of Pretoria for producing the illustrations used in this review.
REFERENCES
- Aanen DK, Hoekstra RF. (2007) Why sex is good: on fungi and beyond. In: Sex in Fungi: molecular determination and evolutionary implication. (Heitman J, Kronstad JW, Taylor JW, Casselton LA, eds): 527–534. Washington DC: American Society for Microbiology Press. [Google Scholar]
- Alby K, Schaefer D, Bennett R. (2009) Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460: 890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames LM. (1934) Hermaphroditism involving self-sterility and cross-fertility in the ascomycete Pleurage anserina. Mycologia 26: 392–414. [Google Scholar]
- Beatty NP, Smith ML, Glass NL. (1994) Molecular characterization of mating-type loci in selected homothallic species of Neurospora, Gelasinospora and Anixiella. Mycological Research 98: 1309–1316. [Google Scholar]
- Bennett RJ, Johnson AD. (2003) Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO Journal 22: 2505–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee AF. (1904) Sexual reproduction in the Mucorineae. Proceedings of the American Academy of Arts and Sciences 40: 205–319. [Google Scholar]
- Chen X. (1994) Self-fertilization and cross-fertilization in the land snail Arianta arbustorum. Journal of Zoology 232: 465–471. [Google Scholar]
- de Beer ZW, Duong TA, Barnes I, Wingfield BD, Wingfield MJ. (2014) Redefining Ceratocystis and allied genera. Studies in Mycology 79: 187–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge BO. (1927) Nuclear phenomena associated with heterothallism and homothallism in the ascomycete Neurospora. Journal of Agricultural Research 35: 289–305. [Google Scholar]
- Dodge BO. (1957) Rib formation in ascospores of Neurospora and questions of terminology. Bulletin of the Torrey Botanical Club 84: 182–188. [Google Scholar]
- Dowding ES. (1933) Gelasinospora, a new genus of pyrenomycetes with pitted spores. Canadian Journal of Research 9: 294–305. [Google Scholar]
- Feretzaki M, Heitman J. (2013) Genetic circuits that govern bisexual and unisexual reproduction in Cryptococcus neoformans. PLoS Genetics 9: e1003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Cuomo C, Ma LJ, Wortman JR, et al. (2005) Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438: 1105–1115. [DOI] [PubMed] [Google Scholar]
- Glass NL, Grotelueschen J, Metzenberg RL. (1990a) Neurospora crassa A mating-type region. Proceedings of the National Academy of Sciences, USA 87: 4912–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass NL, Metzenberg RL, Raju NB. (1990b) Homothallic Sordariaceae from nature: the absence of strains containing only the a mating type sequence. Experimental Mycology 14: 274–289. [Google Scholar]
- Glass NL, Smith ML. (1994) Structure and function of a mating-type gene from the homothallic species Neurospora africana. Molecular and General Genetics 244: 401–409. [DOI] [PubMed] [Google Scholar]
- Haber JE. (1998) Mating-type gene switching in Saccharomyces cerevisiae. Annual Review of Genetics 32: 561–599. [DOI] [PubMed] [Google Scholar]
- Haber JE. (2012) Mating-type Genes and MAT switching in Saccharomyces cerevisiae. Genetics 191: 33–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington TC, McNew DL. (1997) Self-fertility and Uni-directional mating-type switching in Ceratocystis coerulescens, a filamentous ascomycete. Current Genetics 32: 52–59. [DOI] [PubMed] [Google Scholar]
- Horn BW, Ramirez-Prado JH, Carbone I. (2009) Sexual reproduction and recombination in the aflatoxin-producing fungus Aspergillus parasiticus. Fungal Genetics and Biology 46: 169–175. [DOI] [PubMed] [Google Scholar]
- Hull CM, Johnson AD. (1999) Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 285: 1271–1275. [DOI] [PubMed] [Google Scholar]
- Kronstad JW, Staben C. (1997) Mating type in filamentous fungi. Annual Review of Genetics 31: 245–276. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung KJ. (1976) Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68: 821–833. [PubMed] [Google Scholar]
- Kwon-Chung KJ, Bennett JE. (1978) Distribution of α and a mating types of Cryptococcus neoformans among natural and clinical isolates. American Journal of Epidemiology 108: 337–340. [DOI] [PubMed] [Google Scholar]
- Lee D, Roux J, Wingfield BD, Wingfield MJ. (2015) Variation in growth rates and aggressiveness of naturally occurring self-fertile and self-sterile isolates of the wilt pathogen Ceratocystis albifundus. Plant Pathology: in press. doi: 10.1111/ppa.12349. [Google Scholar]
- Lin X, Heitman J. (2007) Mechanisms of homothallism in fungi and transitions between heterothallism and homothallism. In: Sex in Fungi: molecular determination and evolutionary implication. (Heitman J, Kronstad JW, Taylor JW, Casselton LA, eds): 35–57. Washington DC: American Society for Microbiology Press. [Google Scholar]
- Lin X, Hull CM, Heitman J. (2005) Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434: 1017–1021. [DOI] [PubMed] [Google Scholar]
- Lindegren CC, Lindegren G. (1943) Segregation, mutation, and copulation in Saccharomyces cerevisiae. Annals of the Missouri Botanical Garden 30: 453–468. [Google Scholar]
- Lindegren CC, Lindegren G. (1944) Instability of the mating type alleles in Saccharomyces. Annals of the Missouri Botanical Garden 31: 203–216. [Google Scholar]
- Mathieson M. (1952) Ascospore dimorphism and mating type in Chromocrea spinulosa (Fuckel) Petch n. comb. Annals of Botany 16: 449–468. [Google Scholar]
- Merino ST, Nelson MA, Jacobson DJ, Natvig DO. (1996) Pseudohomothallism and evolution of the mating-type chromosome in Neurospora tetrasperma. Genetics 143: 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgroom MG, Lipari SE, Ennos RA, Liu YC. (1993) Estimation of the outcrossing rate in the chestnut blight fungus, Cryphonectria parasitica. Heredity 70: 385–392. [Google Scholar]
- Nasmyth KA. (1982) Molecular genetics of yeast mating type. Annual Review of Genetics 16: 439–500. [DOI] [PubMed] [Google Scholar]
- Nasmyth KA, Tatchell K. (1980) The structure of transposable yeast mating type loci. Cell 19: 753–764. [DOI] [PubMed] [Google Scholar]
- Nielsen R. (2006) Why sex? Science 311: 960–961. [DOI] [PubMed] [Google Scholar]
- Ni M, Feretzaki M, Li W, Floyd-Averette A, Mieczkowski P, et al. (2013) Unisexual and heterosexual meiotic reproduction generate aneuploidy and phenotypic diversity de novo in the yeast Cryptococcus neoformans. PLoS Biology 11: e1001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Feretzaki M, Sun S, Wang X, Heitman J. (2011) Sex in fungi. Annual Review of Genetics 45: 405–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM, Johnson AD. (2007) Genetics of Candida albicans, a diploid human fungal pathogen. Annual Review of Genetics 41: 193–211. [DOI] [PubMed] [Google Scholar]
- O’Gorman CM, Fuller HT, Dyer PS. (2009) Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457: 471–474. [DOI] [PubMed] [Google Scholar]
- Pannell JR. (2015) Evolution of the mating system in colonizing plants. Molecular Ecology 24: 2018–2037. [DOI] [PubMed] [Google Scholar]
- Paoletti M, Seymour FA, Alcocer MJ, Kaur N, Calvo AM, et al. (2007) Mating type and the genetic basis of self-fertility in the model fungus Aspergillus nidulans. Current Biology 17: 1384–1389. [DOI] [PubMed] [Google Scholar]
- Perkins DD. (1987) Mating-type switching in filamentous ascomycetes. Genetics 115: 215–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke SS, Feretzaki M, Heitman J. (2013) Unisexual reproduction enhances fungal competitiveness by promoting habitat exploration via hyphal growth and sporulation. Eukaryotic Cell 12: 1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöggeler S, Risch S, Kück U, Osiewacz HD. (1997) Mating-type genes from the homothallic fungus Sordaria macrospora are functionally expressed in a heterothallic ascomycete. Genetics 147: 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontecorvo G, Roper JA, Hemmons LA, MacDonald DK, Bufton WJ. (1953) The genetics of Aspergillus nidulans. Advances in Genetics 5: 141–238. [DOI] [PubMed] [Google Scholar]
- Raju NB. (1992) Functional heterothallism resulting from homokaryotic conidia and ascospores in Neurospora tetrasperma. Mycological Research 96: 103–116. [Google Scholar]
- Roach K, Feretzaki M, Sun S, Heitman J. (2014) Unisexual reproduction. Advances in Genetics 85: 255–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche LN, Kirchmaier AL, Rine J. (2003) The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annual Review of Biochemistry 72: 481–516. [DOI] [PubMed] [Google Scholar]
- Scazzocchio C. (2014) Fungal biology in the post-genomic era. Fungal Biology and Biotechnology 1: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer D, Côte P, Whiteway M, Bennett RJ. (2007) Barrier activity in Candida albicans mediates pheromone degradation and promotes mating. Eukaryotic Cell 6: 907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staben C, Yanofsky C. (1990) Neurospora crassa a mating-type region. Proceedings of the National Academy of Sciences, USA 87: 4917–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Hernández MA, Méndez N, Salgado-Barragán J. (2009) Branchiomma bairdi: a caribbean hermaphrodite fan worm in the south-eastern Gulf of California (Polychaeta: Sabellidae). Marine Biodiversity Records 2: e43. [Google Scholar]
- Turgeon BG, Yoder O. (2000) Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genetics and Biology 31: 1–5. [DOI] [PubMed] [Google Scholar]
- Uhm J, Fujii H. (1983) Heterothallism and mating type mutation in Sclerotinia trifoliorum. Phytopathology 73: 569–572. [Google Scholar]
- Wang C-L, Shim W-B, Shaw BD. (2010) Aspergillus nidulans Striatin (StrA) mediates sexual development and localizes to the endoplasmic reticulum. Fungal Genetics and Biology 47: 789–799. [DOI] [PubMed] [Google Scholar]
- Webster R, Butler E. (1967) The origin of self-sterile, cross-fertile strains and culture sterility in Ceratocystis fimbriata. Mycologia 59: 212–221. [Google Scholar]
- Wheeler H. (1950) Genetics of Glomerella. VIII. A genetic basis for the occurrence of minus mutants. American Journal of Botany 37: 304–312. [Google Scholar]
- Whitehouse H. (1949) Heterothallism and sex in the fungi. Biological Reviews 24: 411–447. [DOI] [PubMed] [Google Scholar]
- Wickes BL, Mayorga ME, Edman U, Edman JC. (1996) Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the alpha mating type. Proceedings of the National Academy of Sciences, USA 93: 7327–7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken PM, Steenkamp ET, Wingfield MJ, De Beer ZW, Wingfield BD. (2014) DNA loss at the Ceratocystis fimbriata mating locus results in self-sterility. PloS One 9: e92180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Godlonton T, van der Nest MA, Wilken PM, Wingfield MJ, et al. (2015) Unisexual reproduction in Huntiella moniliformis. Fungal Genetics and Biology 80: 1–9. [DOI] [PubMed] [Google Scholar]
- Winge O, Laustsen O. (1937) On two types of spore germination and on genetic segregations in Saccharomyces, demonstrated through single-spore cultures. Comptes Rendus des Travaux du Laboratoire Carlsberg 22: 99–117. [Google Scholar]
- Witthuhn RC, Harrington TC, Wingfield BD, Steimel JP, Wingfield MJ. (2000) Deletion of the MAT-2 mating-type gene during uni-directional mating-type switching in Ceratocystis. Current Genetics 38: 48–52. [DOI] [PubMed] [Google Scholar]
- Yun S-H, Berbee ML, Yoder O, Turgeon BG. (1999) Evolution of the fungal self-fertile reproductive life style from self-sterile ancestors. Proceedings of the National Academy of Sciences, USA 96: 5592–5597. [DOI] [PMC free article] [PubMed] [Google Scholar]