Abstract
In OVA-sensitized and challenged mice, γδ T cells expressing Vγ1 enhance airway hyperresponsiveness (AHR) but the underlying mechanism is unclear. These cells also reduce IL-10 levels in the airways, suggesting that they might function by inhibiting CD4+CD25+ regulatory T cells (Treg) or other CD4+ T cells capable of producing IL-10 and suppressing AHR. Indeed, sensitization and challenge with OVA combined with inactivation of Vγ1+ cells increased CD4+CD25+ cells in the lung, and markedly those capable of producing IL-10. The cellular change was associated with increased IL-10 and TGF-β levels in the airways, and a decrease of IL-13. Treg include naturally occurring Foxp3+ Treg, inducible Foxp3− Treg, and antigen-specific Treg many of which express folate receptor 4 (FR4). Although Foxp3 gene expression in the lung was also increased pulmonary CD4+ T cells, expressing Foxp3-protein or FR4 remained stable. Therefore, the inhibition by Vγ1+ γδ T cells might not be targeting Foxp3+ Treg but rather CD4+ T cells destined to produce IL-10.
Keywords: rodent, T cells, cell differentiation, cytokines, lung
1. Introduction
The mechanisms of airway hyperresponsiveness (AHR) and inflammation in allergic asthma and related diseases of the lung are not yet fully understood. T cells play an important role but different populations have diverse effects that are often in opposition. More recent studies have implicated γδ T cells, but their influence on the allergic response appears to be equally complex [1–4].
γδ T cells fall into discrete subpopulations, which differ in their expression of TCRs [5, 6]. The observation that TCR-Vγ expression alone can be sufficient to define functionally distinct γδ T cell subsets [7, 8] led us to examine the role of such subsets in allergic airway inflammation and AHR. We found that Vγ4+ γδ T cells suppress AHR in a manner independent of αβ T cells and largely bypassing the inflammatory response [9–11]. In contrast, Vγ1+ γδ T cells enhance AHR [12, 13], in different settings, and probably through more than one mechanism. In the absence of conventional, allergen-specific T cells they are capable of mediating AHR, as long as synergistic iNKT cells are present [13]. In the presence of allergen-specific αβ T cells, in normal C57BL/6 and BALB/c mice, Vγ1+ γδ T cells also enhance AHR, although their effect on cytokine levels in the airways differs [12]. In mice of the C57BL/6 genetic background, their regulatory effect involves IL-10, because depletion of the Vγ1+ cells resulted in increased levels of IL-10 in BAL fluid, whereas reconstituting Vγ1+ cells in mice lacking γδ T cells had the opposite consequence [12]. IL-10 is produced by several types of T cells including CD4+CD25+ regulatory T cells (Treg), which produce IL-10 themselves [14, 15], enhance IL-10 production by other cells [16, 17], and are capable of regulating the allergic response in the lung [18–21]. Consistently, recent studies have demonstrated an important role for both IL-10 and Treg in the resolution of airway inflammation and AHR [17, 22]. However, at least in the regulation of the allergic pulmonary response, regulatory activity was also observed when Treg themselves did not produce IL-10 [17, 22]. In view of our earlier findings we reasoned that one mechanism by which Vγ1+ γδ T cells enhance the allergic response in the lung might be through inhibiting the development of IL-10-producing T cells in the lung, including perhaps pulmonary Treg. Indeed, selective inactivation of the Vγ1+ cells by in vivo treatment with anti Vγ1 mAbs resulted in an increase in the lung of inducible CD4+CD25+ T cells capable of producing IL-10, associated with an increase in anti-inflammatory and a decrease in proinflammatory cytokines, but not with an increase in Foxp3-protein+ Treg.
2. Materials and Methods
2.1. Animals, sensitization and airway challenge
Female C57BL/6 mice were obtained from OrientBio (Sungnam, Korea) or from Jackson Laboratories (Bar Harbor, Maine), were maintained on ovalbumin (OVA)-free diets, and were investigated at an age of 8–12 wks, under protocols approved either by the Institutional Animal Care and Use Committees of the Chungbuk National University, or of the National Jewish Medical and Research Center, Denver Colorado. Mice were sensitized by i.p. injection of 20 µg OVA (Grade V; Sigma Chemical Co., St. Louis, MO) emulsified in 2.25 mg aluminum hydroxide (AlumImuject; Pierce Chemical, Rockford, IL) in a total volume of 100 ml on days 0 and 14, and challenged via the airways with OVA (1% in saline) for 20 min on days 28, 29 and 30 by ultrasonic nebulization (particle size 1–5 mm; De Vilbiss, Somerset, PA). Mice were sacrificed for further assay at 48 h after the last allergen challenge.
2.2. Administration of anti-TCR mAbs
Hamster anti-TCR Vγ1 mAb 2.11 [23] and anti Vγ4 mAb UC3 [24] were purified from hybridoma culture supernatants using a Protein G-Sepharose affinity column (Pharmacia, Uppsala, Sweden). T cell functional depletion/inactivation was achieved after injection of 200 µg hamster anti-TCR Vγ4 or Vγ1 mAbs into the tail veins of mice, 3d before each of the two OVA sensitizations, and the effect of the antibody treatment on TCR expression was monitored as previously described [12]. Using this method, we effectively remove TCR-expression (> 80% reduction based on antibody staining) and specifically remove the function of the targeted T cells (based on functional tests and comparison of the antibody treatment and cell transfer experiments). Whether this treatment also eliminates the targeted cells is not clear. Sham antibody treatments were performed with nonspecific hamster IgG (Jackson Laboratories, Bar Harbor, ME). Note: Throughout this paper, we use the nomenclature and numbering system for murine Vγ genes introduced by Heilig et al. [25].
2.3. Bronchoalveolar lavage and Measurement of cytokines in BAL fluid
Lungs were lavaged with Hank’s balanced salt solution (HBSS, 1 ml) via an intratracheal tube, and total leukocyte numbers were measured using a Coulter Counter (Coulter Corporation, Hialeah, FL). Differential cell counts were performed by counting at least 200 cells on cytocentrifuged preparations (Cytospin 2; Cytospin, Shandon Ltd., Runcorn, Cheshire, UK), stained with Leukostat (Fisher Diagnostics, Fair Lawn, NJ), and differentiated by standard hematological criteria [3]. IL-10, IL-13, and TGF-β in BAL fluid samples were detected by ELISA using commercial kits (R&D Systems, Minneapolis, MN), as previously described [12]. Cytokine levels were determined by comparison with known standards. The limits of detection were 7.8 pg/ml for IL-13 and 15.6 pg/ml for the other cytokines.
2.4. Flow cytometric analysis
Lung tissue cells, prepared as previously described in detail [10], were resuspended in RPMI medium supplemented with 2 mM L-glutamine, 20 µM 2-mercaptoethanol, 100 units of penicillin/ml, 50 µg of streptomycin per ml, and 10% FBS, and stimulated overnight at 37° C in the presence of 50 ng/ml phorbol 12-myristate 13-acetate (PMA), 500 ng/ml of calcium ionomycin, and 10 µg/ml brefeldin A (BFA). Cells were harvested, washed, and resuspended at 2×105/well in staining buffer (BSS containing 1% sodium azide and 2% FBS). Cells were first stained with FITC-conjugated hamster anti-mouse CD4 and CD25 mAbs (Pharmingen, San Diego, CA) for 30 min at 4°C, and then fixed in 2% paraformaldehyde for 20 min.
For intra-cytoplasmic staining, cells were washed and incubated in staining buffer containing 0.1% saponin for 10 minutes. Continuously exposed to saponin, cells were then stained with PE-conjugated mouse IgG1 anti-murine IL-10 mAb (Pharmingen) for 30 min at 4°C. After washing with staining buffer containing saponin, cells were washed again with staining buffer without saponin to allow membrane closure.
In the experiment described in Table 1, freshly isolated cells from lung and spleen were passed over nylon wool columns, and non-adherent cells (NAD) were stained with antibodies specific for CD3ε, CD4, CD25 and either Foxp3 or folate receptor 4 (FR4). Stained cells were analyzed on a FACScalibur flow cytometer (Becton Dickinson) using Lysis II and Cellquest software. All subsequent analyses used a light scatter gate designed to include only small lymphocytes. A minimum of 25,000 events per gated region was counted. Estimated cell numbers were obtained by multiplying relative cell frequencies with total numbers of digested cells per one lung of each mouse, gated for viability and low forward and side scatter. These numbers likely under represent population sizes but are useful in indicating absolute changes in the examined cell populations.
Table 1. T cells expressing Foxp3 and folate receptor 4 in lung and spleen (Mean±SEM).
Expression of Foxp3 and folate receptor 4 proteins in pulmonary and splenic CD4+CD25+ T cells of OVA-sensitized and challenged C57BL/6 mice treated with antibodies against TCR-γδ
| Treatment | Lung | Spleen | ||||||
| Foxp3+ cells among CD3+ cells |
FR4+ cells among CD3+ cells |
Foxp3+ cells among CD3+ cells |
FR4+ cells among CD3+ cells | |||||
| % | # of cells (×103) |
% | # of cells (×103) | % | # of cells (×103) |
% | # of cells (×103) | |
| 2ip3n + hamster IgG | 4.6±0.4 | 5.9±0.6 | 22.6±3.5 | 27.9±1.8 | 0.9±0.1 | 43.3±10.3 | 26.6±0.5 | 1221.0±250.1 |
| 2ip3n + mAb 2.11 | 6.2±1.2 | 7.9±0.9 | 29.1±2.0 | 37.9±6.3 | 0.9±0.2 | 32.9±7.5 | 25.5±0.3 | 917.4±110.5 |
| 2ip3n + mAb UC3 | 5.1±0.3 | 17.8±8.9 | 31.8±2.7 | 100.5±40.6 | 1.1±0.2 | 27.8±4.4 | 24.3±0.4 | 706.8±203.1 |
| Foxp3+ cells among CD3+CD4+ cells |
FR4 high cells among CD3+CD4+ cells |
Foxp3+ cells among CD3+CD4+ cells |
FR4 high cells among CD3+CD4+ cells |
|||||
| % | # of cells (×103) |
% | # of cells (×103) | % | # of cells (×103) |
% | # of cells (×103) | |
| 2ip3n + hamster IgG | 6.7±0.5 | 5.6±0.6 | 31.1±3.2 | 25.6±0.6 | 1.6±0.1 | 40.8±9.8 | 38.4±0.7 | 961.0±197.0 |
| 2ip3n + mAb 2.11 | 8.7±0.8 | 7.2±0.8 | 36.1±2.4 | 30.5±4.4 | 1.6±0.3 | 29.2±5.8 | 37.5±0.5 | 693.7±78.3 |
| 2ip3n + mAb UC3 | 7.5±0.5 | 16.4±8.1 | 37.1±0.4 | 77.2±32.6 | 2.0±0.3 | 25.7±4.1 | 35.7±0.6 | 533.1±170.6 |
| Foxp3+ cells among CD4+CD25+ cells |
FR4 high cells among CD4+CD25+ cells |
Foxp3+ cells among CD4+CD25+ cells |
FR4 high cells among CD4+CD25+ cells |
|||||
| % | # of cells (×103) |
% | # of cells (×103) | % | # of cells (×103) |
% | # of cells (×103) | |
| 2ip3n + hamster IgG | 52.1±2.6 | 3.6±0.4 | 56.5±8.8 | 7.6±2.7 | 19.8±1.5 | 28.6±7.3 | 83.6±3.3 | 126.0±33.2 |
| 2ip3n + mAb 2.11 | 55.8±2.0 | 5.0±0.4 | 60.1±2.4 | 6.6±0.8 | 19.0±4.8 | 20.2±3.8 | 75.0±2.3 | 95.6±14.1 |
| 2ip3n + mAb UC3 | 53.5±2.6 | 11.1±5.7 | 60.6±1.1 | 13.6±5.6 | 21.4±3.0 | 17.0±2.5 | 77.3±1.1 | 60.4±16.2 |
Mice were treated as described in Fig. 1. 48 h after the last OVA-challenge, cells were isolated, passed over nylon wool columns and non-adherent fractions stained with antibodies specific for CD3ε, CD4, CD25 and intra-cellular Foxp-3 protein or cell-surface-expressed folate receptor 4. Cell frequencies were determined cytofluorimetrically as described in 2A. Absolute cell numbers (total cells/mouse) were obtained by multiplying the cell frequencies (percentage of total or Foxp3+ or FR4+, CD4+CD25+CD3ε+ T cells) by the number of cells obtained in lung digests. Results for each group are expressed as mean ± SEM (n = 4 in each group).
2.5. Measurement of Foxp3 gene expression by qRT-PCR
RNA of digested lung cells was isolated using the Absolutely RNA Mini Prep kit (Stratagene, La Jolla, CA), according to the manufacturer’s protocol. The eluted RNA was quantified by using a Qubit Fluorometer (Invitrogen, Carlsbad, CA) and Quant-iT RNA assays (Invitrogen) as recommended by the manufacturer. Synthesis of cDNA was performed by using 5× iScript Reaction Mix (containing oligo dT and random hexamers), iScript reverse transcriptase (Bio-Rad, Hercules, CA), quantified RNA, and RNase-free water mixed in a total volume of 20 µl. The quantitative transcription profile of Foxp3 was determined by qRT-PCR using the SYBR green PCR master mix (QIAGEN, Valencia, CA). To normalize the relative concentration values, PCR of the housekeeping gene GAPDH was also performed. A melting curve analysis was always performed for each PCR amplicon to verify specific amplification, and multiple trials of qRT-PCR were performed with each primer set; Foxp3 sense: 5’-CTGCATCGTAGCCACCAGTA-3’ and Foxp3 antisense: 5’-AAGTAGGCGAACATGCGAGT-3’. qRT-PCRs were run in a Corbett Rotor-Gene RG-3000 (Corbett Life Science, Sydney, Australia) under identical amplification conditions. Fold gene expression of Foxp3 was evaluated using the Delta-Delta Ct method, a calculation method using transcription values based on the change in threshold for control (in this case GAPDH) and target cells. The threshold cycle is defined as the cycle number where fluorescent signaling crosses the threshold of logarithmic increases in cDNA concentration.
2.6. Statistical analysis
Data are prepared as means ± standard error of the mean (SEM). The Mann-Whitney test was used for analysis of the effects of mAb treatment on airway inflammation, and ANOVA for analysis of differences in cytokine levels. Pair wise comparisons were performed using the Tukey-Kramer honest significant difference (HSD) test. Statistically significant levels were set at a p value of 0.05.
3. Results
3.1. Vγ1+ γδT cells alter airway cytokines but not the leukocytic airway-infiltrate
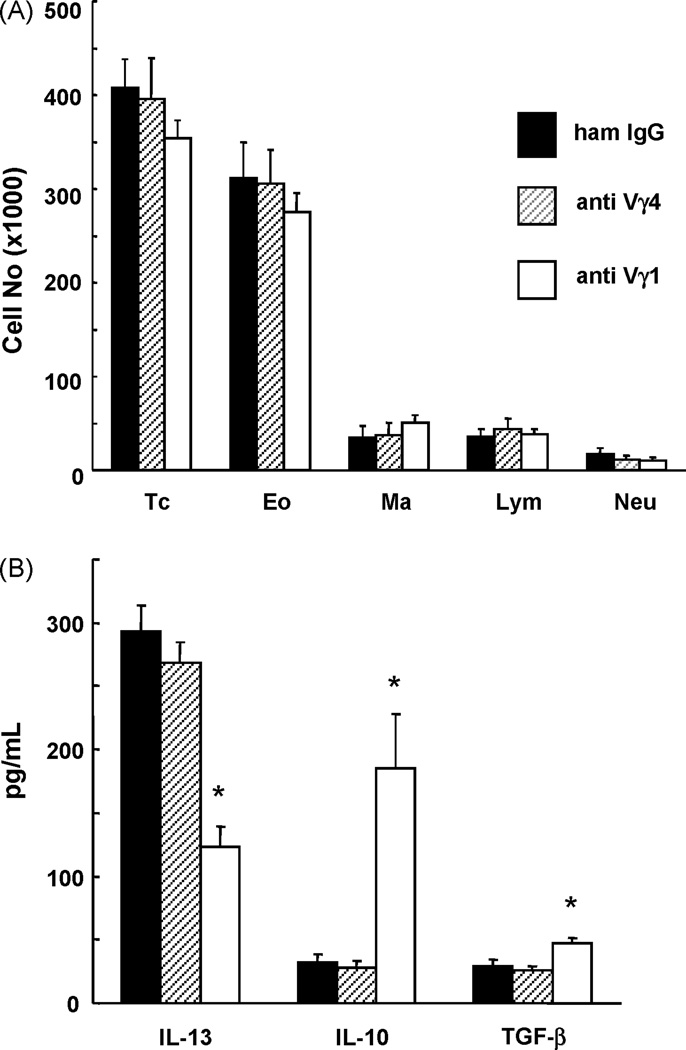
We examined mice sensitized and challenged with OVA, and treated with mAbs to inactivate TCR-Vγ1+ or Vγ4+ γδ T cells or isotype-matched non-specific IgG. The antibodies were injected twice, each 3 days before one of two sensitizing i.p. injections with OVA. By comparison with isotype-matched non-specific antibodies, the treatments with specific γδ T cell-inactivating antibodies did not induce significant changes in total airway infiltrating cells, and relative frequencies of macrophages, eosinophils, neutrophils and lymphocytes remained essentially unchanged (Fig.1A). In marked contrast, one of the antibody-treatments induced substantial changes of cytokine levels in the BAL fluid of these mice. Whereas anti Vγ4 mAb had no effect in comparison with non-specific antibodies, the treatment with anti Vγ1 mAb resulted in a reduced level of IL-13, a slightly increased level of TGF-β, and a greatly increased level of IL-10 (Fig. 1B).
Fig. 1. Inflammatory cell infiltrate and cytokines in the airways of OVA-sensitized and challenged C57BL/6 mice treated with antibodies against TCR-γδ.
A. Mice were sensitized and challenged with OVA (2ip3N protocol). They were further treated twice with anti TCR-Vγ1 mAb, anti TCR-Vγ4 mAb or nonspecific hamster IgG (sham treatment) by i.v. injection 3 days before each of the two sensitizations. Inflammatory cells in BAL fluid were counted 48 h after the last OVA challenge. TC, total cells; Eo, eosinophils; Ma, macrophages; Lym, lymphocytes; Neu, neutrophils. Results for each group are expressed as the mean ± SEM (n = 12 in each group).
B. Mice were treated and BAL fluid collected as described under A. Cytokine assays were performed as indicated, and results for each group expressed as the mean ± SEM (n = 12 in each group). Significant differences (p < 0.05) between the anti TCR Vγ1 mAb-treated group and the other groups are indicated by *.
3.2. Vγ1+ γδ T cells prevent pulmonary accumulation of IL-10-producing CD4+CD25+ T cells
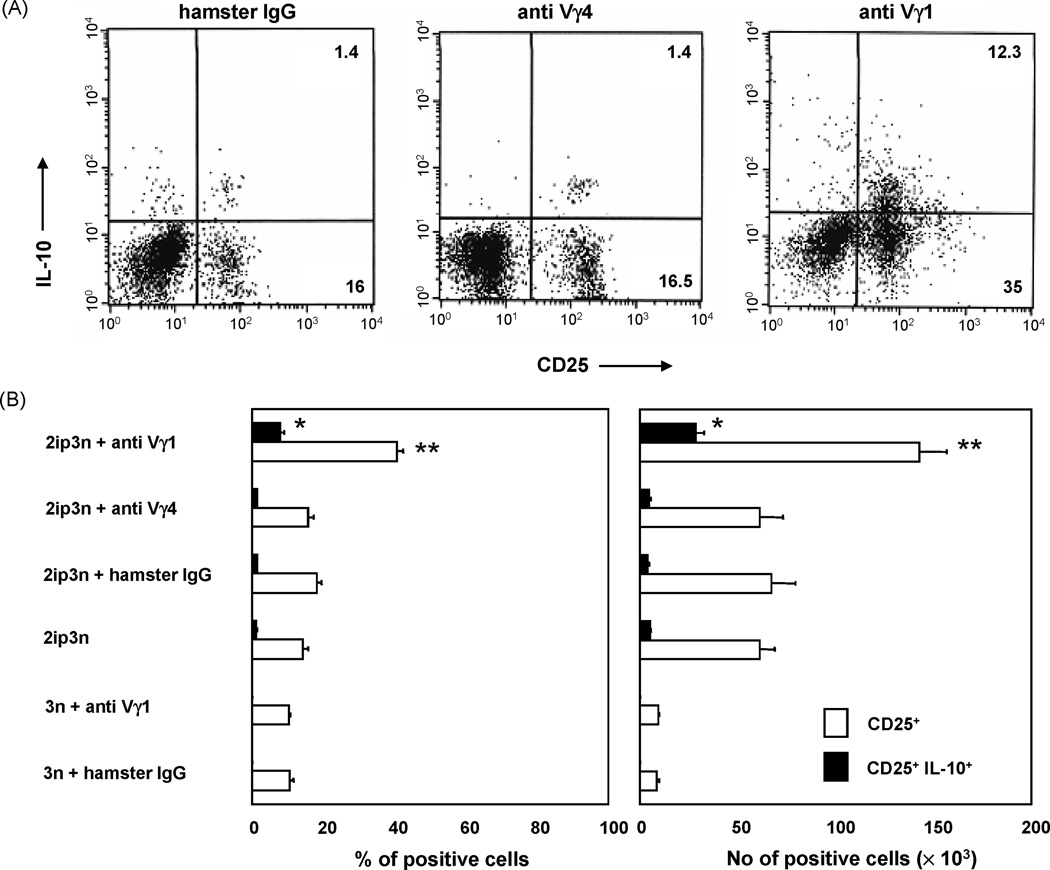
Next, we determined total numbers and relative frequency of CD4+CD25+ T cells in the lung tissue of these mice (Fig. 2). Pulmonary lymphocytes were incubated overnight with PMA/ionomycin and brefeldin A, stained with specific antibodies detecting surface-expressed CD4 and CD25, and intra-cellular IL-10, and enumerated by flow cytometry (Fig. 2A). Compared with controls that were only challenged, OVA-sensitized and challenged mice showed a large increase in the total numbers of pulmonary CD4+CD25+ T cells, accompanied by substantial increase in the relative frequency of these cells within the total CD4+ pulmonary T cell population (Fig. 2B). CD4+CD25+ cells that could be induced to produce IL-10 also appeared in small numbers. However, when the treatment with anti Vγ1 mAb was added to the sensitization and challenge, CD4+CD25+ T cells increased further, and those that could be induced to produce IL-10 increased dramatically, both in absolute numbers and relative frequency (Fig. 2B). Because the treatment with non-specific isotype-matched antibodies or with anti Vγ4 mAb did not have this effect, the data suggest that Vγ1+ γδ T cells in particular selectively suppress the development of inducible, IL-10-producing CD4+CD25+ T cells in the OVA-challenged lung.
Fig. 2. Pulmonary CD4+CD25+ cells of OVA-sensitized and challenged C57BL/6 mice treated with antibodies against TCR-γδ.
A. Mice were treated as described in Fig.1. Pulmonary cells recovered 48 h after the last OVA challenge were stimulated with PMA/ionomycin in the presence of brefeldin A, and then stained with antibodies specific for CD4, CD25 and intracellular IL-10, as described in Materials and Methods. For three-color fluorescent analysis, cells were gated based on their scatter profile (lymphocyte gate) and on CD4-expression. 2.5 × 104 gated cells were counted in each histogram.
B. Cell frequencies were determined cytofluorimetrically as described in 2A. Absolute cell numbers were obtained by multiplying the cell frequencies (percentage of CD4+CD25+ or CD4+CD25+ IL-10+ cells) by the number of cells obtained in lung digests. Results for each group are expressed as mean ± SEM (n = 12 in each group).
3.3. Effect of Vγ1+ γδT cells on Foxp3 expression in the lung
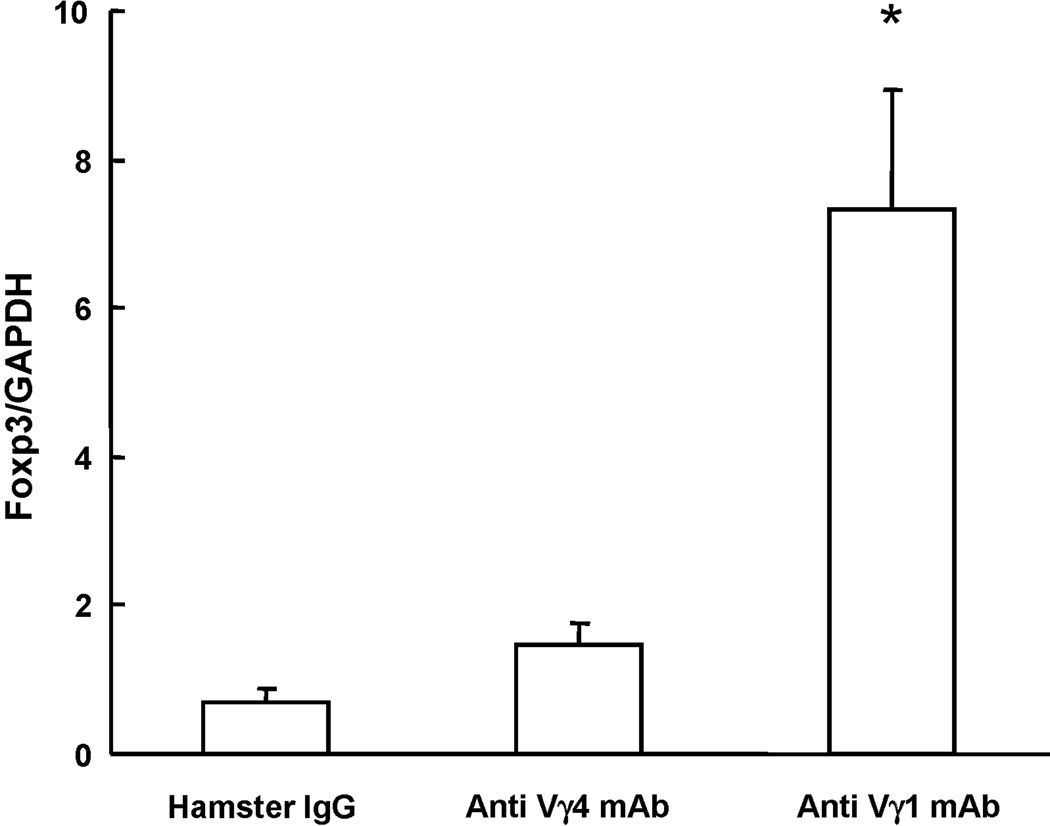
CD4+CD25+ Treg have been divided into inducible and naturally occurring subsets. Naturally occurring Treg are characterized by their expression of the transcription factor Foxp3. Examining the lung of OVA-sensitized and challenged mice, which were also treated with the γδ T cell-inactivating mAbs for overall Foxp3 gene-expression using quantitative RT-PCR, we found increased Foxp3 gene-expression in those mice that had been treated with the anti Vγ1 mAbs, indicating that Vγ1+ γδ T cells in particular exert a regulatory effect on pulmonary cells with the potential of expressing this gene (Fig. 3). However, when we examined freshly isolated pulmonary CD3ε+ T cells or CD4+ or CD4+CD25+ T cell subsets that expressed Foxp3 protein in the anti Vγ1-treated mice, there were no significant changes in relative cellular frequencies or absolute numbers, nor did we find such changes in the spleen (Table 1). We also examined T cell populations expressing folate receptor 4, a recently described cell surface marker associated with the differentiation of antigen-specific Treg [26], with similar results. It thus appears that Vγ1+ γδ T cells target an inducible population among CD4+CD25+ T cells and prevent their development into IL-10-producing cells, without changing the size of cell populations within the same T cell pool that are capable of expressing Foxp3 or FR4-proteins.
Fig. 3. Foxp3 gene expression in the lung of OVA-sensitized and challenged C57BL/6 mice treated with antibodies against TCR-γδ.
Mice were treated as described in Fig. 1. Pulmonary cells were recovered 48 h after the last OVA-challenge, total RNA was isolated and RT-PCR performed with primers specific for Foxp3. Gene expression levels were determined after normalization to GAPDH. Results for each group are expressed as the mean ± SEM (n = 8 in each group).
4. Discussion
The finding that Vγ1+ γδ T cells enhance AHR in OVA-sensitized and challenged mice [12, 27] raised questions about the underlying mechanism. These cells are effective in very small numbers, are able to decrease levels of IL-10 in the airways, and require the presence of αβ T cells for their regulatory function [12]. Others have shown that IL-10 can regulate the allergic response, and that transferred CD4+CD25+ αβ T cells can increase IL-10 in the lung [17, 22]. The possibility of a connection between the two T cell-types in the regulation of the allergic response let us to examine the effect of Vγ1+ γδ T cells on pulmonary CD4+CD25+ T cells in OVA-sensitized and challenged mice.
Along with an expected Th2 response [28], the OVA-sensitized and challenged mice developed substantially higher numbers of pulmonary CD4+CD25+ T cells than did mice which were only challenged with OVA, an increase likely due to recruitment of these cells into the lung [29–31]. Inactivating Vγ1+ but not Vγ4+ γδ T cells using anti TCR mAb further increased pulmonary CD4+CD25+ cells, both in absolute and relative terms. However, the two-fold increase of CD4+CD25+ cells was small by comparison with the six-fold increase of those CD4+CD25+ cells that were capable of producing IL-10. These cellular changes were accompanied by increases in the airways (BAL) of IL-10 and TGF-β, and a decrease in IL-13, suggesting that the cellular changes in the lung of the antibody-treated mice actually reached sufficient proportions to diminish the allergic response and the pivotal AHR-inducing cytokine IL-13. Thus, our findings suggest that at least one mechanism by which Vγ1+ γδ T cells support the allergic response leading to AHR is indirect, through the inhibition of inducible IL-10-producing CD4+CD25+ T cells in the lung.
The exact role of IL-10, and the mechanism by which CD4+CD25+ T cells suppress the allergic response and AHR, are still unclear. It has been questioned that IL-10 production by CD4+CD25+ T cells is prerequisite for their suppression of Th2 responses because, following adoptive transfer, OVA-specific CD4+CD25+ T cells isolated from IL-10-deficient mice suppressed the allergic response as well as CD4+CD25+ T cells from wildtype mice [17]. However, a study with naturally occurring CD4+CD25+ T cells found that these cells had to be incubated with IL-10 in order to become suppressors when isolated from IL-10-deficient mice, thus emphasizing the importance of IL-10 [22]. In addition, the same report showed that CD4+CD25+ T cells were the major source of IL-10, suggesting that IL-10 is required for the functional differentiation of suppressive CD4+CD25+ T cells, and that the source population also provides the cytokine [22]. Our findings suggest that Vγ1+ γδ T cells might regulate this developmental process.
Inactivating Vγ1+ γδ T cells also increased levels of soluble TGF-β in the airways. CD4+CD25+ T cells can be induced to produce TGF-β, both the secreted and surface-bound forms [14, 32, 33]. Like IL-10, TGF-β might be needed primarily for the further differentiation of CD4+CD25+ T cells, rather than AHR-suppression, because the suppressive action of CD4+CD25+ T cells has been shown to be independent of this cytokine [34, 35]. Again, this suggests a role for the γδ T cells in the development of the AHR-inhibitory CD4+CD25+ T cells. Th2 suppression mediated by CD4+CD25+ T cells is, at least in part, dependent on TCR-ligation, and direct cell-contact or close range mediators, and it is not APC-dependent [36–38]. Therefore, the substantially increased pulmonary CD4+CD25+ T cells observed here might be also directly mediating suppression of the allergic response, in addition to an effect due to the altered cytokine levels.
Although inactivating Vγ1+ γδ T cells increased levels of Foxp3 gene expression in the lung tissue of the OVA-sensitized and challenged mice (based on PCR detection), we did not find significant changes in the relative frequency of Foxp3-protein expressing T cells in the lung or spleen, nor did we find Foxp3-protein expressed at higher levels in the T cells of these organs. It remains possible, however, that the changes in mRNA expression do not result in increased protein levels, or that cells other than CD3+ T cells express Foxp3 at variable regulated levels [39]. Naturally occurring regulatory CD4+CD25+ T cells (Treg) are known to express Foxp3 at high levels, and Foxp3-expression correlates with their regulatory activity [40, 41]. However, inducible CD4+CD25+ T cells with regulatory functions have been described that are Foxp3-negative [42], and both types of Treg as well as CD4+ T cells not classified as Treg are capable of producing IL-10 [15]. Finally, Vγ1+ γδ T cells do not appear to change pulmonary (or splenic) T cell populations expressing folate receptor 4 (FR4) either, a recently described marker of antigen-specific Treg [26].
In conclusion, our study indicates that Vγ1+ γδ T cells regulate certain steps in the development and functional activity of IL-10-producing pulmonary CD4+CD25+ T cells in allergen-sensitized and challenged mice of the C57BL/6 genetic background. This regulation provides a mechanistic explanation for their documented capability to increase the allergic response in normal mice where conventional αβ T cells are present. This indirect mechanism is different and comes into play in addition to another AHR-enhancing mechanism also involving C57BL/6-derived Vγ1+ γδ T cells, which was found to be operational in the absence of conventional αβ T cells and only required iNKT cells in synergy with the αβ T cells [13].
Acknowledgements
The authors would like to thank Drs. Jena French, Christina Roark and Yafei Huang for advice and helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by Korea Research Foundation Grant KRF-2004-202-E00113, and by NIH grants AI40611 and HL65410 to W.K.B..
References
- 1.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265:1869. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 2.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 3.Lahn M, Kanehiro A, Takeda K, Joetham A, Schwarze J, Koehler G, O'Brien R, Gelfand EW, Born W. Negative regulation of airway responsiveness that is dependent on γδ T cells and independent of αβ T cells. Nature Medicine. 1999;5:1150. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 4.Isogai S, Rubin A, Maghni K, Ramos-Barbon D, Taha R, Yoshizawa Y, Hamid Q, Martin JG. The effects of CD8+ gammadelta T cells on late allergic airway responses and airway inflammation in rats. J. Allergy Clin. Immunol. 2003;112:547. doi: 10.1016/s0091-6749(03)01720-2. [DOI] [PubMed] [Google Scholar]
- 5.Havran W, Allison JP. Developmentally ordered appearance of thymocytes expressing different T cell antigen receptors. Nature. 1988;335:443. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 6.Bonneville M, Janeway J, Ito CAK, Haser W, Ishida I, Nakanishi N, Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of γδ T cells. Nature. 1988;336:479. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- 7.Huber SA, Graveline D, Newell MK, Born WK, O'Brien RL. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J. Immunology. 2000;165:4174. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien RL, Xiang Y, Huber S, Ikuta K, Born WK. Depletion of a γδ T cell subset can increase host resistance to a bacterial infection. J. Immunol. 2000;165:6472. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- 9.Lahn M, Kanehiro A, Takeda K, Terry J, Hahn Y-S, Aydintug MK, Konowal A, Ikuta K, O'Brien RL, Gelfand EW, Born WK. MHC class I-dependent Vγ4+ pulmonary T cells regulate αβ T cell-independent airway responsiveness. Proc. Natl. Acad. Sci (USA) 2002;99:8850. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn Y-S, Taube C, Jin N, Takeda K, Park J-W, Wands JM, Aydintug MK, Roark CL, Lahn M, O'Brien RL, Gelfand EW, Born WK. Vγ4+ T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J. Immunol. 2003;171:3170. doi: 10.4049/jimmunol.171.6.3170. [DOI] [PubMed] [Google Scholar]
- 11.Cui Z-H, Joetham A, Aydintug MK, Born WK, Gelfand EW. Reversal of established allergic airway hyperreactivity by long-term allergen challenge depends on γ/δ T cells. Am. J. Respir. Crit. Care Med. 2003;168:1324. doi: 10.1164/rccm.200305-634OC. [DOI] [PubMed] [Google Scholar]
- 12.Hahn Y-S, Taube C, Jin N, Sharp L, Wands JM, Kemal Aydintug M, Lahn M, Huber SA, O'Brien RL, Gelfand EW, Born WK. Different potentials of γδ T cell subsets in regulating airway responsiveness: Vγ1+ cells, but not Vγ4+ cells, promote airway hyperreactivity, TH2 cytokines, and airway inflammation. J. Immunol. 2004;172:2894. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 13.Jin N, Miyahara N, Roark CL, French JD, Aydintug MK, JL M, Gapin L, O'Brien RL, Gelfand EW, Born WK. Airway hyperresponsiveness through synergy of gd T cells and NKT cells. J. Immunol. 2007;179:2961. doi: 10.4049/jimmunol.179.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annacker OR, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 2001;166:3008. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 15.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nature Medicine. 2004;10:801. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 16.Jonuleit HE, Schmitt H, Kakirman M, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J. Exp. Med. 2002;196:255. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 2005;202:1539. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman PM, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T cell suppression of allergen-driven T cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 19.Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J. Immunol. 2004;172:3842. doi: 10.4049/jimmunol.172.6.3842. [DOI] [PubMed] [Google Scholar]
- 20.Hadeiba H, Locksley RM. Lung CD25 CD4 regulatory T cells suppress type 2 immune responses but not bronchial hyperreactivity. J. Immunol. 2003;170:5502. doi: 10.4049/jimmunol.170.11.5502. [DOI] [PubMed] [Google Scholar]
- 21.Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, el Haddad B, Schmitt E, Bopp T, Kallen KJ, Herz U, Schmitt S, Luft C, Hecht O, Hohlfeld JM, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Rose-John S, Renz H, Neurath MF, Galle PR, Finotto S. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J. Clin. Invest. 2005;115:313. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joetham A, Takada K, taube C, Miyahara N, Matsubara S, Koya T, Rha Y-H, Dakhama A, Gelfand EW. Naturally occurring lung CD4+CD25+ T cell regulation of airway allergic responses depends on IL-10 induction of TGF-b. J. Immunol. 2007;178:1433. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 23.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of Vγ1-expressing γ/δ T lymphocytes in normal mice. J. Exp. Med. 1995;182:1921. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dent AL, Matis LA, Hooshmand F, Widacki SM, Bluestone JA, Hedrick SM. Self-reactive γδ T cells are eliminated in the thymus. Nature. 1990;343:714. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 25.Heilig JS, Tonegawa S. T-cell γ gene is allelically but not isotypically excluded and is not required in known functional T-cell subsets. Proc Natl Acad Sci USA. 1987;84:8070. doi: 10.1073/pnas.84.22.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Hirota K, Nagahama K, Ohkawa K, Takahashi T, Nomura T, Sakaguchi S. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27:145. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Lahn M, Kanehiro A, Hahn Y-S, Wands JM, Aydintug MK, O'Brien RL, Gelfand EW, Born WK. Aerosolized anti-T-cell-receptor antibodies are effective against airway inflammation and hyperreactivity. Int. Arch. Allergy and Immunology. 2004;134:49. doi: 10.1159/000077533. [DOI] [PubMed] [Google Scholar]
- 28.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 29.Huehn J, Siegmund K, Lehmann JC, Sievert U, Haubold M, Feuerer GF, Debes J, Lauber J, Frey O, Przybylski GK, Niesner U, de la Rosa M, Schmidt CA, Brauer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 2004;199:303. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siewert C, Menning A, Dudda J, Siegmund K, Lauer U, Floess S, Campbell DJ, Hamann A, Huehn J. Induction of organ-selective CD4+ regulatory T cell homing. Eur. J. Immunol. 2007;37:978. doi: 10.1002/eji.200636575. [DOI] [PubMed] [Google Scholar]
- 31.Siegund K, Feuerer GF, Siewert C, Ghani S, Haubold U, Dankof A, Krenn V, Schon MP, Scheffold A, Lowe JB, Hamann A, Syrbe U, Huehn J. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface bound transforming growth factor beta. J. Exp. Med. 2001;194:629. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+CD25+ T cells. J. Autoimmun. 2001;16:115. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- 34.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takanashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self tolerance maintained by CD4+CD25+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 36.Umetsu DT, Akbari O, DeKruyff RH. Regulatory T cells control the development of allergic disease and asthma. J. Allergy Clin. Immunol. 2003;112:480. [PubMed] [Google Scholar]
- 37.Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. Control of T-cell activation by CD4+CD25+ suppressor T cells. Immunol. Rev. 2001;182:58. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 38.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 2005;6:338. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M, Tsuji-Takayama K, Suzuki M, Harashima A, Sugimoto A, Motoda R, Yamasaki F, Nakamura S, Kibata M. Comprehensive analysis of FOXP3 mRNA expression in leukemia and transformed cell lines. Leuk Res. 2008;32:651. doi: 10.1016/j.leukres.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 41.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O'Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol. 2004;172:5986. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]