Abstract
Objective
Medication non-adherence is a major cause of uncontrolled hypertension, but clinicians are poor at judging adherence, and the gold standard for measuring adherence, electronic monitoring, is rarely available in clinical settings. Self-report questionnaires (SRQs), by contrast, are inexpensive, easy to administer, and hence, may be useful for “diagnosing” non-adherence. In this study we evaluated the validity of two commonly used medication adherence SRQs among patients with uncontrolled hypertension, using electronic pillbox measurement as the gold standard.
Methods
A total of 149 patients with uncontrolled hypertension had adherence to their antihypertensive medication regimen monitored using a 4-compartment electronic pillbox (MedSignals®) between two primary care visits (median 50 days). Participants completed the 8-item Morisky Medication Adherence Scale© (MMAS-8) and the Visual Analog Scale (VAS) at the second visit. Likelihood ratios (LRs) were calculated using <80% correct dosing adherence by electronic measurement as the gold standard.
Results
SRQ scores indicating low adherence (MMAS-8 <6 and VAS <80%, 23% and 9% of participants, respectively) had LRs of 2.00 (95% confidence interval [CI] 1.10-3.65) and 7.72 (95% CI 1.77-33.6), respectively, for detecting non-adherence compared to electronic measurement. SRQ scores indicating highest adherence (MMAS-8 =8 and VAS =100%, 43% and 61% of participants, respectively) had LRs of 0.55 (95% CI 0.35-0.85) and 0.76 (95% CI 0.57-1.01), respectively, for detecting non-adherence.
Conclusion
The MMAS-8 and VAS are modestly useful in identifying antihypertensive medication non-adherence. Other tools, including electronic measurement, may be needed to guide titration of antihypertensive medications among patients with uncontrolled hypertension.
Keywords: adherence, hypertension, self-report, questionnaire, electronic monitoring
Introduction
Hypertension is one of the most common chronic diseases seen in primary care, affecting nearly one third of U.S. adults, and is a leading risk factor for cardiovascular disease and morality.1-3 Despite improvements in awareness and increased use of antihypertensive medications, approximately 30% of American adults treated for hypertension have uncontrolled blood pressure (BP),2,4 often due to medication non-adherence.5-7 Assessment of adherence is thus an important component of achieving BP control. Unfortunately, providers are often unsure about patients’ adherence levels,8 and clinicians’ predictions of non-adherence are little better than chance.9-12 Measures of adherence to antihypertensive medications that are both reliable and suitable for use in clinical practice are therefore needed.
While electronic monitoring is widely viewed as the gold standard measurement of day-to-day medication adherence,13 it is not readily available to most practitioners. Self-report questionnaires (SRQs), by contrast, are quick, inexpensive, and easy to administer, but may not be accurate due to patients’ tendency to over-report adherence.13 Several antihypertensive medication SRQs have been compared with objective adherence measures, including pharmacy refill data, pill counts, and electronic monitoring.14 Although some SRQs have been positively associated with these objective measures,15-20 studies evaluating the test properties of these SRQs for identifying non-adherence in clinical settings are lacking.17, 20-22 Two of the more commonly used SRQs in patients with hypertension are the 8-item Morisky Medication Adherence Scale© (MMAS-8) and the Visual Analog Scale (VAS).14 The MMAS-8 has been associated with BP control23 and pharmacy refill data,24 but has not been compared with day-to-day electronic adherence monitoring. One small study compared VAS scores with electronic monitoring but did not assess the ability of the instrument to detect non-adherence.15
Before an SRQ is used in clinical settings, an understanding of its ability to identify non-adherence is needed. Thus, the goal of this study was to determine the test properties of the MMAS-8 and VAS in identifying antihypertensive medication non-adherence in patients with uncontrolled hypertension, using electronic measurement as the gold standard comparator.
Methods
Participants
Participants were enrolled from two hospital-based primary care practices in New York City (Internal Medicine Associates, Mount Sinai Medical Center and Associates in Internal Medicine, Columbia University Medical Center) as part of a study investigating barriers to antihypertensive medication adherence among patients with uncontrolled hypertension. Both practices are located in low-income, racially and ethnically diverse neighborhoods. The study was approved by the Institutional Review Board of each medical center, and all participants provided written informed consent.
Patients were eligible for the study if they had an established relationship with a primary care provider who was enrolled in the study; were age 18 or older; spoke English or Spanish; were prescribed at least one antihypertensive medication; and had uncontrolled hypertension at the baseline study visit and at their previous clinic visit, as defined by criteria from the Seventh Joint National Committee report:25 systolic BP (SBP) ≥140 mmHg or diastolic BP (DBP) ≥90 mmHg in patients without diabetes mellitus (DM) or chronic kidney disease (CKD, estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2), SBP ≥130 mmHg or DBP ≥80 mmHg in patients with DM or CKD). Patients were ineligible for participation if any of the following criteria were met: severe uncontrolled hypertension (SBP ≥200 mmHg or DBP ≥130 mmHg); severe physical, cognitive, or psychiatric impairment that limited ability to self-administer antihypertensive medications; terminal non-cardiovascular illness; unavailability for follow-up; or enrollment in another cardiovascular clinical trial. All recruitment procedures and patient interviews were conducted in participants’ preferred language (English or Spanish), and all questionnaires were professionally translated into Spanish.
Electronic adherence measurement
Each participant was enrolled at a routine clinic visit and was provided a 4-compartment MedSignals® pillbox (LIFETechniques Inc., San Antonio, TX), which can monitor adherence to up to four medications simultaneously. The device records a pill as taken each time the lid of the individual pillbox compartment is opened and closed. Data on adherence are transmitted from the pillbox to the MedSignals server via landline, or, among the newer generation of devices, via a cellphone embedded in the device. Participants were instructed to fill the pillbox with their antihypertensive medications and take them as they normally would between the baseline visit and their next scheduled clinic visit. For each monitored antihypertensive medication, adherence was calculated as the percent of days on which the correct number of doses was taken as prescribed. For medications with more than one daily dose, participants were considered partially adherent if they took at least one daily dose (e.g., for twice daily medications, participants were considered 50% adherent on days they took the medication once). Extra doses did not count for or against the adherence percentage (e.g. for once daily medications, participants were considered 100% adherent on days they took the medication twice). Participants were asked about days on which they were hospitalized or had been instructed to omit or discontinue a monitored medication; such periods were excluded from calculations of adherence rates. The overall regimen adherence percentage was calculated as the mean adherence across all the antihypertensive medications being taken. As has become convention for studies of antihypertensive medication adherence, non-adherence was defined as overall adherence less than 80%.26
Self-report questionnaires (SRQs)
At the second clinic visit, participants returned the electronic pillbox and completed a single MMAS-8 and VAS, querying adherence to all of their antihypertensive medications since their last visit. The MMAS-823,24, 27 consists of eight items that assess extent of non-adherence (e.g. how frequently patients fail to take their antihypertensive medications) and reasons for non-adherence (e.g. whether they feel taking daily antihypertensive medications is a hassle). The questionnaire is scored from 0 to 8, with 8 indicating highest adherence. According to cutpoints recommended by the developers of the MMAS-8, a score less than 6 indicates low adherence, 6 to less than 8 indicates medium adherence, and 8 indicates high adherence.
The VAS consists of a numbered line with intervals of 10% from 0% to 100% for each of the electronically monitored medications. For each medication, participants marked an “X” on the line corresponding to their estimated adherence over the monitoring period. A participant’s overall VAS score was calculated by averaging scores across all antihypertensive medications. VAS scores range from 0% to 100%, with 100% indicating highest adherence. Following conventions used in previously published studies, a score less than 80% indicates low adherence.15
Other measures
Demographic information was obtained by self-report at the baseline visit. Data on participants’ medical comorbidities were obtained by review of their medical records. Acceptability of use of the electronic pillbox was assessed by questionnaire, using a five-category Likert scale, during the second visit.
Cohort assembly
Out of 522 patients screened, 43 (8%) declined to participate and 279 (53%) were ineligible. After these exclusions, 200 patients enrolled in the study. Of enrolled participants, 152 (76%) had data available from the electronic pillbox, and 149 of these participants also completed the SRQs at the second study visit. Reasons for not having valid electronic pillbox data included dropout from study (4%), loss to follow-up (2%), failure to return landline pillboxes (7%), failure to use the pillbox during the monitoring period (7%), and pillbox technical problems (2%).
Statistical analyses
Participant characteristics were calculated for the overall study population. The relationship between MMAS-8 and VAS scores and adherence by the electronic pillboxes was calculated using the linear-by-linear association test. Using adherence from the pillboxes as the gold standard, sensitivity, specificity, and positive and negative likelihood ratios (LRs) for detecting non-adherence were calculated for the MMAS-8 and VAS separately. LRs indicate how much the result from an SRQ increases or decreases the pretest probability of non-adherence, and are advocated in the assessment of the usefulness of diagnostic tests.28 LRs greater than 10 or less than 0.1 are considered to generate large and important changes from pretest to posttest probability; LRs of 5 to 10 and 0.1 to 0.2 generate moderate shifts from pretest to posttest probabilities; LRs from 2 to 5 and 0.5 to 0.2 generate small shifts from pretest to posttest probabilities; and LRs from 1 to 2 and 0.5 to 1 generate very small and rarely important changes from pretest to posttest probability. Analyses were performed using two different cutpoints on the MMAS-8 (<6 vs. 6-8 and <8 vs. 8) and on the VAS (<80% vs. 80-100% and <100% vs. 100%). The methods described by Simel and colleagues were used to calculate 95% confidence intervals for each of these estimates.29 Sensitivity analyses were performed restricted to: (1) participants who had adherence monitored for at least 2 weeks, (2) participants taking no more than two antihypertensive medications, and (3) participants whose preferred language was English. All analyses were performed using SPSS (version 21.0, IBM Corp., Armonk, NY).
Results
Participant characteristics
Baseline characteristics of study participants are shown in Table 1. Mean (SD) age was 64 (9) years; 72% of participants were female, 75% were Hispanic, and 83% had Medicaid. The prevalence of medical comorbidities in the study population was high: 57% had DM, 29% had CKD, and 15% had coronary artery disease. Participants were prescribed a mean (SD) of 2.56 (0.98) antihypertensive medications.
Table 1.
Baseline characteristics of participants (n = 149)
| Characteristic | Description |
|---|---|
| Mean (SD) age (years) | 64 (9) |
| Female, n (%) | 108 (72) |
| Black race, n (%) | 60 (40) |
| Hispanic ethnicity, n (%) | 112 (75) |
| Insurance, n (%) | |
| Medicare only | 17 (11) |
| Medicaid only | 46 (31) |
| Medicare and Medicaid | 78 (52) |
| Neither Medicare nor Medicaid | 8 (5) |
| Country of birth, n (%) | |
| United States (not including Puerto Rico) | 37 (25) |
| Puerto Rico | 18 (12) |
| Dominican Republic | 83 (56) |
| Other | 11 (7) |
| Preferred language, n (%) | |
| English | 51 (34) |
| Spanish | 98 (66) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 85 (57) |
| Chronic kidney disease* | 43 (29) |
| Coronary artery disease | 22 (15) |
| Heart failure | 14 (9) |
| Cerebrovascular disease | 15 (10) |
| Peripheral vascular disease | 8 (5) |
| Hyperlipidemia | 109 (73) |
| Number of antihypertensives prescribed, n (%) | |
| 1 | 22 (15) |
| 2 | 50 (34) |
| 3 | 52 (35) |
| 4 | 22 (15) |
| 5 | 3 (2) |
Defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2
Mean (SD) SBP and DBP at the first clinic visit were 159 (19) mmHg and 85 (12) mmHg, respectively. A majority (80%) of participants continued to have uncontrolled BP at the second visit: mean (SD) SBP and DBP were 149 (21) mmHg and 81 (12) mmHg, respectively.
Electronic adherence data
Adherence was monitored for a median of 50 days (range 6-188 days). A majority (91%) of participants reported that the device was very easy or somewhat easy to use. Median adherence by the MedSignals pillbox was 86% (range 0-100%); 42% of participants were categorized as non-adherent by the threshold of less than 80% adherence.
Test properties of SRQs
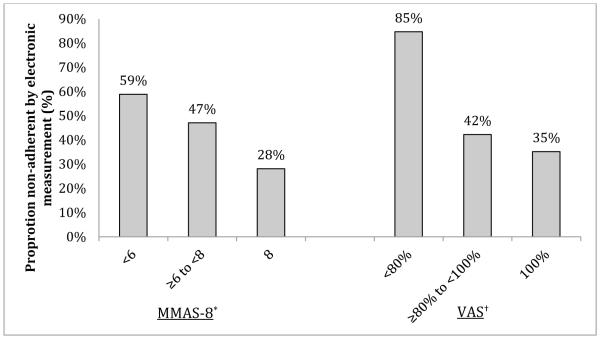
The median MMAS-8 score was 7.00 (range 2.75-8.00), and the median VAS score was 100% (range 0-100%). As shown in the Figure, lower levels of adherence on the MMAS-8 and VAS were each associated with a higher proportion of patients who were non-adherent by electronic measurement (linear-by-linear association p-values = 0.002 and 0.004, respectively).
Twenty-three percent of participants had an MMAS-8 score less than 6, and 57% had an MMAS-8 score less than 8. With electronic measurement as the gold standard, an MMAS-8 score less than 6 had a sensitivity of 32% (95% confidence interval [CI] 21-44%) and specificity of 84% (95% CI 76-92%) for detecting non-adherence (Table 2). Positive LR for identifying non-adherence was 2.00 (95% CI 1.10-3.65) and negative LR was 0.81 (95% CI 0.66-0.98). Using an MMAS-8 score less than 8, sensitivity and specificity for identifying non-adherence on electronic monitoring were 71% (95% CI 60-82%) and 53% (95% CI 42-63%), respectively. The positive LR was 1.51 (95% CI 1.15-1.98) and negative LR was 0.55 (95% CI 0.35-0.85).
Table 2.
Test properties of the 8-Item Morisky Medication Adherence Scale and the Visual Analog Scale compared with electronically measured adherence
| Questionnaire, score | Sensitivity, % (95% CI) |
Specificity, % (95% CI) |
LR+ (95% CI) |
LR− (95% CI) |
|---|---|---|---|---|
| MMAS-8, <6 vs. 6-8 | 32 (21-44) | 84 (76-92) | 2.00 (1.10-3.65) | 0.81 (0.66-0.98) |
| MMAS-8, <8 vs. 8 | 71 (60-82) | 53 (42-63) | 1.51 (1.15-1.98) | 0.55 (0.35-0.85) |
| VAS, <80% vs. 80-100% | 18 (8-27) | 98 (95-100) | 7.72 (1.77-33.6) | 0.84 (0.75-0.95) |
| VAS, <100% vs. 100% | 48 (36-62) | 68 (58-78) | 1.50 (1.01-2.24) | 0.76 (0.57-1.01) |
Abbreviations: MMAS-8, 8-Item Morisky Medication Adherence Scale; VAS, Visual Analog Scale; LR+, positive likelihood ratio; LR−, negative likelihood ratio; CI, confidence interval.
Nine percent of participants had a VAS score less than 80%, and 39% had a VAS score less than 100%. VAS scores less than 80% had a sensitivity of 18% (95% CI 8-27%) and specificity of 98% (95% CI 95-100%) for non-adherence by electronic monitoring, with a positive LR of 7.72 (95% CI 1.77-33.6) and negative LR of 0.84 (95% CI 0.75-0.95). A VAS score less than 100% had a sensitivity of 48% (95% CI 36-62%), specificity of 68% (95% CI 58-78%), positive LR of 1.50 (95% CI 1.01-2.24), and negative LR of 0.76 (95% CI 0.57-1.01) for non-adherence by electronic monitoring.
Results were similar in a sensitivity analysis restricted to participants monitored for at least 2 weeks (n = 139, 93%; data not shown). Restricting to participants taking no more than two antihypertensive medications (n = 72, 48%) or those whose preferred language was English (n = 51, 34%) likewise did not change the pattern of results.
Discussion
In this study of 149 primary care patients with uncontrolled hypertension prescribed at least one antihypertensive medication, the MMAS-8 and VAS were modestly helpful for distinguishing between adherent and non-adherent patients when compared with the gold standard of electronic measurement. As expected, participants tended to over-report their adherence. While more than 40% of participants were non-adherent by electronic measurement, fewer than 25% reported low adherence on the MMAS-8, and fewer than 10% indicated low adherence on the VAS. Over-reporting can be mitigated by adjusting the thresholds for non-adherence in the questionnaires, but in our sample neither SRQ achieved both high sensitivity and specificity, regardless of the cutpoints used. Applying low thresholds for non-adherence (MMAS-8 <6 and VAS <80%) to maximize specificity, the MMAS-8 and VAS had moderate (84%) and high (98%) specificity for non-adherence, respectively. However, relatively few participants had scores in this range (fewer than 25% and 10% for the MMAS-8 and VAS, respectively). Even after applying the highest possible thresholds for non-adherence (MMAS-8 <8 and VAS <100%) to maximize sensitivity, the MMAS-8 and VAS had only modest sensitivity (71% and 48%, respectively).
Moreover, it is unclear whether the likelihood ratios for these SRQs are sufficient to improve clinicians’ confidence in patients’ adherence status in a meaningful way. For example, given a pretest probability of non-adherence of 50%—consistent with studies suggesting that clinicians’ predictions of non-adherence are little better than chance9-12—an MMAS-8 score less than 6 would yield a post-test probability of non-adherence of 67%, and a score of 8 would yield a post-test probability of non-adherence of 35%. Although a VAS score less than 80% had a moderately high positive LR (7.72), there was a wide confidence interval around this estimate owing to the small proportion of participants who reported adherence at this level.
While prior studies have assessed the association of the MMAS with BP control23 and pharmacy refill data,24 ours is the first to compare it with electronic monitoring. One small study of patients with hypertension, diabetes, or dyslipidemia found that VAS scores correlated poorly with electronic adherence measurement.15 In that analysis, however, adherence to a single cardiovascular or diabetic medication was measured as a surrogate for the entire regimen, even though adherence may vary between such medications based upon differences in medication beliefs, side effect profiles, and frequency of dosing.30,31 Furthermore, the VAS was compared with a subsequent electronic monitoring period, and hence, the study did not test whether the VAS could be used to retrospectively determine patients’ adherence status, as would be needed for it to be useful to clinicians titrating antihypertensive medications.
This study has several strengths. First, the MedSignals pillbox enabled us to measure adherence to all or most (up to four) of participants’ antihypertensive medications, rather than monitoring one medication as a surrogate for the entire regimen. Second, we enrolled a cohort of patients from a diverse, low-income background (indicated by the high proportion of participants on Medicaid), a population that is often excluded from research participation. Third, we assessed adherence in a clinical setting. Fourth, in contrast with prior studies, participants were asked about adherence during the period in which it was electronically monitored. There were also some limitations. With electronic monitoring, one cannot confirm that pillbox openings indicate that a medication dose was taken. The Hawthorne effect—the phenomenon by which individuals change their behavior as a result of participating in a study—may have led participants to take their medications more regularly than usual. Moreover, non-adherers may have been less likely to volunteer for the study. Thus, the prevalence of non-adherence in our sample may represent a conservative estimate of the true prevalence of non-adherence in the study population. Innumeracy may have made completing the VAS difficult for some participants. Finally, the results in our study population may not be generalizable to all patients with uncontrolled hypertension.
Our results indicate that objective measures of adherence may be needed to guide clinicians’ management of uncontrolled hypertension.32 Advances in mobile health technology that allow for wireless transmission of adherence data from electronic monitoring devices may make it feasible to integrate electronic monitoring into clinical practice in the near future.33 Pharmacy refill patterns may also be useful for identifying non-adherent patients.34,35 Yet prescription refill records do not provide information on the day-to-day dosing immediately prior to a BP measurement, and therefore, do not maximally reduce uncertainty as to whether uncontrolled BP is due to insufficient medication or insufficient adherence. Bioassays that detect antihypertensive medications in the urine and serum represent another objective approach to identifying patients who are non-adherent.36-40 These tests, however, only measure whether a medication was taken at all during a short period of time preceding the test, and hence, may fail to identify intermittent non-adherers.
In summary, we have shown that two commonly used SRQs, the MMAS-8 and VAS, have modest utility in identifying patients with uncontrolled hypertension with and without non-adherence to antihypertensive medications. Further work is needed to validate other promising SRQs with electronic adherence measurement and to develop new SRQs with better test properties.
Figure.
Association of the 8-Item Morisky Medication Adherence Scale and the Visual Analog Scale with electronically measured adherence
Abbreviations: MMAS-8, 8-Item Morisky Medication Adherence Scale; VAS, Visual Analog Scale
*Linear-by-linear association p = 0.002
†Linear-by-linear association p = 0.004
Use of the ©MMAS is protected by US copyright laws. Permission for use is required. A license agreement is available from: Donald E. Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095-1772.
Acknowledgements
None
Sources of Support: This work was supported by funds from the National Heart, Lung, and Blood Institute (NHLBI) (K23 HL-098359 and P01-HL047540) and from the American Heart Association (SDG 10SDG2600321). Mr. Gallagher received support from the NHLBI (T35 HL-007616). Dr. Lin received support from the National Cancer Institute (NCI) (K07 CA-166462).
Footnotes
Prior Presentations: American Heart Association EPI/NPAM Conference, New Orleans, LA, March 2013 (poster presentation).
Conflicts of Interest: The authors declare that they have no conflicts of interest.
References
- [1].Chobanian AV. Shattuck Lecture. The hypertension paradox--more uncontrolled disease despite improved therapy. N. Engl. J. Med. 2009;361(9):878–887. doi: 10.1056/NEJMsa0903829. [DOI] [PubMed] [Google Scholar]
- [2].Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- [3].Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- [5].Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J. Manag. Care Pharm. 2006;12(3):239–245. doi: 10.18553/jmcp.2006.12.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ho PM, Magid DJ, Shetterly SM, et al. Importance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary disease. Arch. Intern. Med. 2008;168(3):271–276. doi: 10.1001/archinternmed.2007.72. [DOI] [PubMed] [Google Scholar]
- [7].DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med. Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- [8].Kerr EA, Zikmund-Fisher BJ, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann. Intern. Med. 2008;148(10):717–727. doi: 10.7326/0003-4819-148-10-200805200-00004. [DOI] [PubMed] [Google Scholar]
- [9].Turner BJ, Hecht FM. Improving on a coin toss to predict patient adherence to medications. Ann. Intern. Med. 2001;134(10):1004–1006. doi: 10.7326/0003-4819-134-10-200105150-00015. [DOI] [PubMed] [Google Scholar]
- [10].Miller LG, Liu H, Hays RD, et al. How well do clinicians estimate patients' adherence to combination antiretroviral therapy? J. Gen. Intern. Med. 2002;17(1):1–11. doi: 10.1046/j.1525-1497.2002.09004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mushlin AI, Appel FA. Diagnosing potential noncompliance. Physicians' ability in a behavioral dimension of medical care. Arch. Intern. Med. 1977;137(3):318–321. doi: 10.1001/archinte.137.3.318. [DOI] [PubMed] [Google Scholar]
- [12].Zeller A, Taegtmeyer A, Martina B, Battegay E, Tschudi P. Physicians' ability to predict patients' adherence to antihypertensive medication in primary care. Hypertens. Res. 2008;31(9):1765–1771. doi: 10.1291/hypres.31.1765. [DOI] [PubMed] [Google Scholar]
- [13].Osterberg L, Blaschke T. Adherence to Medication. N. Engl. J. Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- [14].Garfield S, Clifford S, Eliasson L, Barber N, Willson A. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med. Res. Methodol. 2011;11:149. doi: 10.1186/1471-2288-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zeller A, Ramseier E, Teagtmeyer A, Battegay E. Patients' self-reported adherence to cardiovascular medication using electronic monitors as comparators. Hypertens. Res. 2008;31(11):2037–2043. doi: 10.1291/hypres.31.2037. [DOI] [PubMed] [Google Scholar]
- [16].Cook CL, Wade WE, Martin BC, Perri M., 3rd Concordance among three self-reported measures of medication adherence and pharmacy refill records. J. Am. Pharm. Assoc. (2003) 2005;45(2):151–159. doi: 10.1331/1544345053623573. [DOI] [PubMed] [Google Scholar]
- [17].Prado JC, Jr., Kupek E, Mion D., Jr. Validity of four indirect methods to measure adherence in primary care hypertensives. J. Hum. Hypertens. 2007;21(7):579–584. doi: 10.1038/sj.jhh.1002196. [DOI] [PubMed] [Google Scholar]
- [18].Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med. Care. 1999;37(9):846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- [19].Schroeder K, Fahey T, Hay AD, Montgomery A, Peters TJ. Adherence to antihypertensive medication assessed by self-report was associated with electronic monitoring compliance. J. Clin. Epidemiol. 2006;59(6):650–651. doi: 10.1016/j.jclinepi.2005.10.013. [DOI] [PubMed] [Google Scholar]
- [20].Shalansky SJ, Levy AR, Ignaszewski AP. Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Ann. Pharmacother. 2004;38(9):1363–1368. doi: 10.1345/aph.1E071. [DOI] [PubMed] [Google Scholar]
- [21].Inui TS, Carter WB, Pecoraro RE. Screening for noncompliance among patients with hypertension: is self-report the best available measure? Med. Care. 1981;19(10):1061–1064. doi: 10.1097/00005650-198110000-00008. [DOI] [PubMed] [Google Scholar]
- [22].Zeller A, Schroeder K, Peters TJ. An adherence self-report questionnaire facilitated the differentiation between nonadherence and nonresponse to antihypertensive treatment. J. Clin. Epidemiol. 2008;61(3):282–288. doi: 10.1016/j.jclinepi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- [23].Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive Validity of a Medication Adherence Measure in an Outpatient Setting. J. Clin. Hypertens. 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [24].Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am. J. Manag. Care. 2009;15(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- [25].Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- [26].Sackett DL, Haynes RB, Gibson ES, et al. Randomised clinical trial of strategies for improving medication compliance in primary hypertension. Lancet. 1975;1(7918):1205–1207. doi: 10.1016/s0140-6736(75)92192-3. [DOI] [PubMed] [Google Scholar]
- [27].Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: final response. J. Clin. Epidemiol. 2011;64(3):262–263. doi: 10.1016/j.jclinepi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jaeschke R, Guyatt GH, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271(9):703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- [29].Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J. Clin. Epidemiol. 1991;44(8):763–770. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- [30].Kronish IM, Woodward M, Sergie Z, Ogedegbe G, Falzon L, Mann DM. Meta-analysis: impact of drug class on adherence to antihypertensives. Circulation. 2011;123(15):1611–1621. doi: 10.1161/CIRCULATIONAHA.110.983874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jung O, Gechter JL, Wunder C, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J. Hypertens. 2013;31(4):766–774. doi: 10.1097/HJH.0b013e32835e2286. [DOI] [PubMed] [Google Scholar]
- [32].Christensen A, Osterberg LG, Hansen EH. Electronic monitoring of patient adherence to oral antihypertensive medical treatment: a systematic review. J. Hypertens. 2009;27(8):1540–1551. doi: 10.1097/HJH.0b013e32832d50ef. [DOI] [PubMed] [Google Scholar]
- [33].Asch DA, Muller RW, Volpp KG. Automated hovering in health care--watching over the 5000 hours. N. Engl. J. Med. 2012;367(1):1–3. doi: 10.1056/NEJMp1203869. [DOI] [PubMed] [Google Scholar]
- [34].Heisler M, Hofer TP, Schmittdiel JA, et al. Improving blood pressure control through a clinical pharmacist outreach program in patients with diabetes mellitus in 2 high-performing health systems: the adherence and intensification of medications cluster randomized, controlled pragmatic trial. Circulation. 2012;125(23):2863–2872. doi: 10.1161/CIRCULATIONAHA.111.089169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schectman JM, Schorling JB, Nadkarni MM, Voss JD. Can prescription refill feedback to physicians improve patient adherence? Am J Med Sci. 2004;327(1):19–24. doi: 10.1097/00000441-200401000-00005. [DOI] [PubMed] [Google Scholar]
- [36].Ceral J, Habrdova V, Vorisek V, Bima M, Pelouch R, Solar M. Difficult-to-control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non-responsiveness from non-adherence to recommended therapy. Hypertension Res. 2011;34(1):87–90. doi: 10.1038/hr.2010.183. [DOI] [PubMed] [Google Scholar]
- [37].Jung O, Gechter JL, Wunder C, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31(4):766–774. doi: 10.1097/HJH.0b013e32835e2286. [DOI] [PubMed] [Google Scholar]
- [38].Strauch B, Petrak O, Zelinka T, et al. Precise assessment of noncompliance with the antihypertensive therapy in patients with resistant hypertension using toxicological serum analysis. J Hypertens. 2013;31(12):2455–2461. doi: 10.1097/HJH.0b013e3283652c61. [DOI] [PubMed] [Google Scholar]
- [39].Brinker S, Pandey A, Ayers C, et al. Therapeutic drug monitoring facilitates blood pressure control in resistant hypertension. J Am Coll Cardiol. 2014;63(8):834–835. doi: 10.1016/j.jacc.2013.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tomaszewski M, White C, Patel P, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100(11):855–861. doi: 10.1136/heartjnl-2013-305063. [DOI] [PMC free article] [PubMed] [Google Scholar]