Abstract
The receptor for advanced glycation end products (RAGE) is expressed in the heart in cardiomyocytes, vascular cells, fibroblasts, and in infiltrating inflammatory cells. Experiments in murine, rat, and swine models of injury suggest that RAGE and the ligands of RAGE are upregulated in key injuries to the heart, including ischemia/reperfusion injury, diabetes, and inflammation. Pharmacological antagonism of RAGE or genetic deletion of the receptor in mice is strikingly protective in models of these stresses. Data emerging from human studies suggest that measurement of levels of RAGE ligands or soluble RAGEs in plasma or serum may correlate with the degree of heart failure. Taken together, the ligand-RAGE axis is implicated in heart failure and we predict that therapeutic antagonism of RAGE might be a unique target for therapeutic intervention in this disorder.
Keywords: Receptor, Diabetes, RAGE, Myocarditis, Inflammation, Ischemia, Reperfusion, Glycation, S100/calgranulin, HMGB1, Soluble RAGE, Heart failure, Cardiomyocyte, Fibroblast, Endothelium, Pathophysiology
Introduction
The receptor for advanced glycation end products (RAGE) is a member of the immunoglobulin superfamily of cell surface molecules, discovered for its ability to bind advanced glycation end products (AGEs), the products of nonenzymatic glycation and oxidation of proteins and lipids [1]. RAGE binds multiple ligands; our findings that members of the S100/calgranulin family of proinflammatory polypeptides, high-mobility group box 1 (HMGB1), and amyloid-β peptide and β-sheet fibrils signal via RAGE set the stage for understanding that RAGE was implicated in chronic cellular stress responses that lead to tissue damage in lieu of resolution and repair [2–4]. Other molecules that potentially bind RAGE have been reported, such as mac-1, phosphatidyl serine (PS), C3a and CpGA, heparin, and, most recently, PR3, a serine protease molecule identified in the bone marrow of humans with chronic myelogenous leukemia and metastatic prostate cancer [5–9]. The affirmed ligands for RAGE recognize common “patterns” within the RAGE extracellular domain, and ligand binding is driven by electrostatic interactions between the positively charged surface of the RAGE ectodomain and negatively charged ligands [10].
Consequent to ligand-RAGE binding, signal transduction through the receptor mediates a gene expression changes in a cell- and stress-driven manner. Experimental evidence implicates the cytoplasmic domain of RAGE in such signaling events. In vitro and in vivo deletion of the cytoplasmic domain of RAGE suppresses ligand-driven signaling through the receptor [2, 11]. We recently reported that the cytoplasmic domain of RAGE binds to a member of the formin family, mDia1, and that mDia1 is essential for RAGE-mediated signaling in transformed cells and in macrophages [12, 13].
Both RAGE and members of the formin family are expressed in the heart [14, 15]. An aging-associated increase in RAGE expression in the heart has been reported in humans in a manner associated with reduced heart function [16]. In this review, we will discuss the evidence linking RAGE to the pathophysiology of heart failure. Because heart failure may be induced by multiple stimuli, we will focus on those solidly linked to the biology of RAGE, such as ischemia/reperfusion (I/R) injury, diabetes, and inflammation.
RAGE and Ischemia/Reperfusion Injury in the Heart
Isolated Perfused Heart and In Vivo Models of Transient Ligation/Reperfusion of the Left Anterior Descending Coronary Artery
Diabetes is a leading risk factor for the acceleration of atherosclerosis and the increased incidence and severity of heart attacks and strokes [17]. RAGE and its ligands are highly expressed in atherosclerotic plaques in human and murine models of atherosclerosis, particularly in lesional macrophages and smooth muscle cells [18, 19]. Increased accumulation of AGEs and expression of S100A12 have been identified in atherosclerotic plaques, especially in hyperglycemia [18, 19]. These findings led us to probe the effects of RAGE blockade or deletion in murine models of atherosclerosis accelerated by diabetes. In apolipoprotein E (apoE)–deficient mice rendered type 1 diabetic with streptozotocin or by breeding into the db/db background (type 2 insulin-resistant diabetes), administration of the extracellular ligand decoy of RAGE, soluble RAGE (sRAGE), suppressed diabetes-accelerated atherosclerosis. No impact on levels of blood glucose or on total levels of cholesterol or triglyceride has been found with sRAGE [19, 20]. Key to the beneficial impact of sRAGE was its ability to suppress the enhanced vascular inflammation that accompanied diabetes. Even in nondiabetic atherosclerosis-vulnerable mice, sRAGE imparted benefit in reduction of atherosclerosis, albeit to lower degrees than that observed in diabetes. Distinct studies tested the effect of genetic deletion of RAGE in the apoE null or low density lipoprotein (LDL) receptor null background. Deletion of RAGE resulted in reduced atherosclerosis and vascular inflammation compared to wild-type apoE null mice in the diabetic and/or nondiabetic states [11, 21, 22, 23•]. Genetic deletion of RAGE had no effect on glycemia or hyperlipidemia in nondiabetic or diabetic mice; however, mechanisms linked to vascular stress and even further enhanced by diabetes, particularly activation of the ROCK1 branch of the transforming growth factor-β2 family, were significantly attenuated in the aortas of mice devoid of RAGE [23•].
Based on these findings that RAGE contributed to striking upregulation of atherosclerotic disease in the coronary arteries of atherosclerosis-prone mice, experiments were performed to establish the potential role of underlying RAGE action in I/R injury, a fundamental consequence of advanced atherosclerotic disease. Two distinct experimental strategies for induction of I/R injury were tested to address these concepts.
First, in the isolated perfused heart subjected to ischemia followed by reperfusion in mice or rats, administration of sRAGE or genetic deletion of RAGE resulted in decreased damage to the diabetic and nondiabetic heart as measured by lower levels of lactic dehydrogenase (LDH) release, higher adenosine triphosphate (ATP) levels in the heart, and lower left ventricular developed pressure (LVDP), the latter a marker of cardiac dysfunction. Of note, diabetic hearts were more vulnerable to I/R injury compared to nondiabetic hearts; in both settings, RAGE blockade was beneficial in mitigating the adverse impact of I/R [24, 25]. Evidence of oxidative stress induced by I/R as measured by increased inducible nitric oxide synthase (iNOS) expression and increased nitrotyrosine epitopes in the heart was reduced by sRAGE or deletion of RAGE [25]. In the diabetic myocardium, evidence of apoptosis after I/R, including caspase-3 activity and cytochrome c release and levels of the AGE carboxymethyl lysine (CML), were reduced by modulation of RAGE [24].
Second, rodent models of frank myocardial infarction have been tested in the context of RAGE. Wild-type and RAGE-null mice were subjected to 30-min ligation of the left anterior descending (LAD) coronary artery followed by reperfusion. Mice were sacrificed and measurement of infarction of the left ventricle was undertaken 48 h after the onset of reperfusion. Multiple experimental results indicated that deficiency of RAGE was highly protective; hematoxylin and eosin staining revealed much smaller infarcts in the RAGE-null versus wild-type hearts; cardiac function, as assessed by echocardiography, revealed significantly higher fractional shortening in the RAGE-null versus wild-type heart; terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, cytochrome c release, and caspase-3 activity revealed significantly less apoptosis in the RAGE-null heart compared to the wild-type heart; and phosphorylation of c-Jun N-terminal kinase (JNK) and STAT5 was markedly reduced in the RAGE-null versus the wild-type heart [26••].
Tsoporis and colleagues [27] demonstrated that the RAGE ligand S100B contributed to I/R heart damage. Using rats subjected to coronary artery ligation, they showed that in peri-infarct myocytes, both RAGE and S100B were increased and that the latter was released into plasma. In parallel, in rat neonatal cardiomyocyte cultures, exposure to S100B (at least 50 nmol/L) resulted in myocyte apoptosis and pro-damage signaling that was attenuated by introduction of dominant negative (DN) RAGE [27].
Lu and colleagues treated male minipigs with intracoronary sRAGE or vehicle in a swine model of in vivo myocardial infarction. Although increases in left ventricular end-diastolic volume and end-systolic volume and decreased ejection fraction were not impacted by sRAGE, at least in the experimental conditions employed, the expression of transforming growth factor-β1, particularly in peri-infarct areas, was lower in the sRAGE-treated group compared to the vehicle [28].
Taken together, these experiments revealed that RAGE and its ligands were upregulated by I/R in the heart, even in the absence of diabetes, and, in parallel, evidence of oxidative stress was enhanced. Significant benefit of antagonizing RAGE was observed with reduced infarct volume and less loss of cardiac function compared to controls patients. These data provided strong evidence that RAGE was a key mediator of myocardial I/R injury and a potential target for therapeutic intervention.
Induction of Hypoxia in Cardiomyocytes: Testing the Role of RAGE
In the isolated perfused heart and in vivo model of myocardial infarction, the specific impact of RAGE signaling in cardiomyocytes is difficult to discern because RAGE is also expressed in vascular, inflammatory cells and fibroblasts. To address the role of RAGE in cardiomyocytes, primary adult ventricular cardiomyocytes were retrieved from the hearts of wild-type and RAGE-null mice and subjected to in vitro applied hypoxia (H) for 30 min followed by reoxygenation (R) for 1 h. Compared to normoxia, H/R resulted in a marked upregulation of RAGE in the wild-type myocyte, along with an increase in AGEs; this finding paralleled that observed in the in vivo heart:, that cardiomyocytes express RAGE, and upregulation of RAGE and RAGE ligands in these cells occurs in I/R and H/R stress [29].
Signaling mechanisms were examined in isolated adult cardiomyocytes and revealed that H/R resulted in increased phosphorylation of JNK and suppression of glycogen synthase kinase (GSK)-3β serine 9 phosphorylation; these adverse signaling mechanisms were prevented in cardiomyocytes devoid of RAGE or by pretreatment of mice with sRAGE before retrieval of cardiomyocytes. H/R-induced apoptosis was significantly suppressed in RAGE-deficient or sRAGE-treated wild-type cardiomyocytes [29]. Hence, in primary cardiomyocytes, the adverse effects of H/R were prevented by blockade/deletion of RAGE.
In other studies, rat neonatal cardiomyocytes were stimulated with S100B. In RAGE-overexpressing cardiomyocytes, S100B (100 nM) resulted in increased messenger RNA, protein and secretion of VEGF, and activation of nuclear factor (NF)-κB. Pretreatment of the RAGE-overexpressing cardiomyocytes with an inhibitor of NF-κB, caffeic acid phenethyl ester, blocked these effects. Similarly, introduction of dominant negative (DN) RAGE suppressed S100-mediated induction and secretion of vascular endothelial growth factor (VEGF). To determine if VEGF might impact myofibroblast function, adult myofibroblasts were treated with VEGF (10 ng/mL) and this resulted in induction of VEGFR-2 tyrosine kinase phosphorylation, ERK1/2 phosphorylation, and proliferation of myofibroblasts. The authors deduced that S100B release by cardiomyocytes might impact neighboring myofibroblasts by stimulation of proliferation, thereby providing a potential mechanism by which the RAGE pathway might be linked to scar formation in the infarcted myocardium [30].
It is important to note that some of the published studies in isolated cardiomyocytes have been performed in adult cardiomyocytes; others have been performed in neonatal cardiomyocytes. Although the precise implications of neonatal cardiomyocytes and their response to stresses relevant to the adult are not fully known, the key messages from these studies are 1) cardiomyocytes express RAGE and RAGE expression is upregulated in H/R; 2) stressed cardiomyocytes release RAGE ligands; 3) RAGE ligands signal in cardiomyocytes to augment death and stress pathways; 4) products of RAGE ligand (S100B)-stimulated cells such as cardiomyocytes may, in a paracrine manner, signal distinct cells to participate in damage pathways; and 5) blockade/deletion of RAGE and RAGE signaling in cardiomyocytes appears to be protective against these stresses that in the setting of infarction may lead to both immediate/acute damage (loss of functional myocardium) and late-stage damage (scarring and fibrosis post–myocardial infarction).
RAGE, Diabetes, and the Heart
In the studies discussed above, I/R induced in the isolated perfused diabetic heart resulted in greater indices of damage and loss of function compared to nondiabetic mice [24]. Work from Lefer’s group [31] has shown that diabetic (type 2) db/db mice subjected to in vivo myocardial infarction displayed significantly less survival compared to age- and sex-matched nondiabetic mice, and greater degrees of left ventricular dilatation, cardiac hypertrophy, and contractile dysfunction 28 days after 45 min of myocardial ischemia. Although the effects of RAGE modulation have yet to be reported in this setting, others have examined the effects of RAGE on innate cardiac dysfunction that accompanies diabetes in murine models.
Ma and colleagues [32] studied the impact of streptozotocin-induced diabetes on cardiac properties in FVB mice. Diabetes resulted in a significant increase in pre-AGE molecules, methylglyoxal (MG), AGEs, and RAGE levels in heart, especially in cardiomyocytes; in vivo short-interfering RNA knockdown of RAGE expression in the heart blocked these effects. In the context of function, diabetes decreased left ventricular contractility and this was prevented by knockdown of RAGE. Cardiomyocyte mechanical properties were studied in isolated cardiomyocytes from mice; AGEs induced prolongation of time to peak shortening (TPS) and time to 90 % relengthening (TR90); this was blocked by an antibody to RAGE. AGE-induced dysfunction of cardiomyocytes was linked to mitochondrial membrane depolarization and reduced GSK-3β inactivation, events that were prevented by RNA interference knockdown of RAGE expression [32].
Nielsen and colleagues [33] tested the effects of a blocking antibody to RAGE in db/db mice. Administration of this antibody was beneficial in the diabetic heart; RAGE blockade prevented the development of increased left ventricular diastolic chamber stiffness and blocked the reduction in cardiac systolic function. Further, the cardiac expression of collagen (col1a1), increased in diabetes, was reduced by approximately 45 % in the hearts of mice treated with RAGE antibody. These data suggest that profibrotic mechanisms in the diabetic heart may be mediated, at least in part, by RAGE action.
RAGE and Inflammatory Damage to the Heart
From the first studies on RAGE, it was clear that the receptor was expressed in a broad range of cell types; hence, in addition to cardiomyocytes, it was shown that RAGE was expressed in vascular cells and inflammatory cells [14]. Ramasamy and colleagues [24] showed that RAGE signaling in both endothelial cells (ECs) and mononuclear phagocytes contributed to the adverse impact of RAGE in the heart, particularly in diabetes. They employed transgenic mice expressing DN RAGE driven by the pre-proendothelin 1 promoter (PPET-1), thus driving DN RAGE expression in ECs (but not exclusively), and by the macrophage scavenger receptor-type (MSR-A) promoter, thus driving DN RAGE expression in mononuclear phagocytes. In each case, compared to wild-type littermates, induction of I/R in the isolated perfused heart resulted in less damage.
Effects of RAGE Ligand HMGB1
Further insights into potential roles for inflammatory cell RAGE in cardiac dysfunction were identified in distinct experiments. Andrassy and colleagues [34] showed that in the in vivo model of LAD infarction/reperfusion, 30 min of ischemia followed by 48 h of reperfusion resulted in strikingly increased expression of RAGE ligand HMGB1, particularly in infiltrating leukocytes in the heart compared to sham controls. Consistent with pathogenic roles for HMGB1 in I/R injury, treatment of wild-type mice with the HMGB1 inhibitor, HMGB1 box A, resulted in smaller infarct size. In RAGE-null mice, HMGB1 box A afforded no further benefit to protection against I/R, and exogenously added recombinant HMGB1 did not increase I/R injury in the RAGE-null mice hearts [34].
Beyond the short-term 48-h end point, these same authors studied the effects of HMGB1 after 10 weeks of sustained hyperglycemia. Compared to nondiabetic wild-type mice, mice with diabetes displayed significantly increased HMGB1 in parallel with left ventricular hypertrophy and fibrosis in the areas adjacent to infiltrating inflammatory cells [35]. Enhanced mortality and loss of cardiac function in the diabetic mice was partially protected by HMGB1 box A and by RAGE deletion [35].
These authors also tested the role of HMGB1 and activation of NF-κB in a model of troponin-induced myocarditis. At 21 days posttroponin, they found significantly increased expression of HMGB1 in the heart as well as increased serum levels of HMGB1. The extent of HMGB1 elevation paralleled the degree of cardiac dysfunction and amplified activation of NF-κB in infiltrating inflammatory cells. In RAGE-null mice hearts, immunization with troponin resulted in significantly less infiltration of inflammatory cells [35].
Effects of S100A8/A9
Boyd and colleagues [36] discovered novel roles for members of the S100/calgranulin family, S100A8 and S100A9, in lipopolysaccharide (LPS)-induced cardiac dysfunction, and attributed the pathogenic roles of these S100s to RAGE signaling. In vivo, S100A8/9 co-immunoprecipitated with RAGE. Cardiac overexpression of S100A8 and S100A9 caused a decrease in calcium flux in isolated primary cardiomyocytes in a RAGE-dependent manner, and in the mouse, such overexpression of these molecules resulted in decreased cardiac ejection fraction, but knockdown of S100A9 in vivo resulted in attenuation of LPS-induced cardiac damage. When primary cardiomyocytes were exposed to LPS, upregulation of S100A8 and S100A9 occurred and caused a decrease in cellular contractility in a RAGE-dependent manner [36, 37]. It is important to note that S100A8 and S100A9 also have been reported to interact with toll-like receptor (TLR) 4 [38].
In addition to studies in animal models, evidence for RAGE axis activity is human heart failure is accruing, providing potential biomarkers for heart failure in the clinic.
RAGE and Heart Failure: Studies in Humans
RAGE Polymorphisms: Genetic Studies
Multiple polymorphisms of the gene encoding RAGE (AGER) have been reported [39]. Among the known RAGE polymorphisms, some are located in the promoter region of the gene, and another, the G82S (glycine/serine mutation at position 82), is of particular interest in that it lies within the V-type domain of the receptor, the site in which most of the known ligands have been shown to bind [40, 41]. We previously showed that RAGE G82S increased affinity for RAGE ligand S100B compared to the wild-type allele, and when expressed in mononuclear phagocyte–like cells, G82S enhanced inflammation and matrix metalloproteinase (MMP) activity consequent to S100B binding compared to cells expressing the wild-type allele [40]. Intriguingly, a genome-wide association study discovered very strong links of this polymorphism of RAGE to the decline of lung function in aging [42].
To date, this polymorphism has not yet been studied in heart failure. However, Poon and colleagues [43] studied three distinct polymorphisms of RAGE in the promoter region (T-429 C, T-374A, and the 63 base pair deletion spanning −407 to −345 nucleotides) for the potential relationship to cardiovascular disease in type 2 diabetic Chinese patients with overt nephropathy. These studies revealed that the 63 base pair deletion imparted a marginal benefit to cardiovascular event-free survival in nephropathy patients, and patients with any of the three mutations displayed a lower risk of ischemic heart disease [43].
In addition to polymorphisms, soluble levels of RAGE ligands and RAGE have been examined in patients with heart failure.
Soluble Levels of RAGE Ligands in Heart Failure
RAGE ligand AGEs are a heterogeneous group of molecules; on account of their negative charge, however, a number of members of the AGE family have been shown to interact with and bind RAGE [44]. One of the AGEs, pentosidine, has been studied in heart failure in humans. Serum pentosidine concentration was found to be an independent risk factor for heart failure; even among patients with heart failure, the highest levels were found in those with New York Heart Association (NYHA) class III/IV and were higher in patients with cardiac events versus event-free patients. The highest quartile of serum pentosidine was found to be associated with the highest risk for cardiac events [45].
Other ligands of RAGE have been tested in heart failure as well. Wang and colleagues [46••] studied the levels of HMGB1 in Chinese patients with heart failure with and without diabetes. Levels of serum HMGB1 were higher in diabetic and nondiabetic patients with heart failure compared to those without heart failure, and the levels of HMGB1 correlated positively with left ventricular end-diastolic and end-systolic volumes.
Studies in a European population examined levels of HMGB1 after myocardial infarction and found that levels were related to infarct size in both ST-elevation and non-ST elevation myocardial infarction, and levels of HMGB1 were predictive of ejection fraction 6 months after myocardial infarction in these patients [47]. The authors concluded that HMGB1 levels were a surrogate marker for infarction transmurality and for the prediction of residual left ventricular function.
Although levels of S100/calgranulins in plasma have been linked to a range of inflammatory and malignant disorders [48], there are no known reports linking soluble levels of these molecules to heart failure in humans.
Soluble Levels of RAGE in Heart Failure
In addition to measurement of RAGE ligand levels in the serum or plasma of human patients, multiple studies have reported the measurement of plasma or serum levels of sRAGE in human patients. There are two forms of sRAGE; the first is likely to be the extracellular form of RAGE cleaved from the cell surface via MMPs or ADAM10 molecules. The second form of sRAGE is called endogenous secretory RAGE (esRAGE) and results from a splice variant lacking the membrane/cytoplasmic domains. A novel stretch of amino acids in the C2 immunoglobulin domain of esR-AGE renders a unique epitope for antibody formation. Approximately 20 % of total sRAGE is accounted for by esRAGE [49].
Levels of sRAGEs have been measured in many of the RAGE-related diseases, including in heart failure. Raposeiras-Roubin and colleagues [50] examined patients with various levels of NYHA heart failure criteria; there was a correlation between sRAGE (total) and severity of heart failure. Specifically, in class I, mean levels were 741.9±88.9 pg/mL; in class II, mean levels were 1195.9±113.2 pg/mL; and in class III, mean levels were 1724.8±245 pg/mL. Hence, in these studies, levels of sRAGE rose as the degree of heart failure worsened.
These findings were similar to those by Wang and colleagues [46••]; they showed that total levels of sRAGE were higher with increasing degrees of heart failure, as assessed by NYHA functional class. Interestingly, however, their data revealed that esRAGE levels were lower in the heart failure patients versus the controls. The precise mechanisms underlying these findings regarding the different trends between total and esRAGE levels have yet to be determined.
In other studies in cardiovascular disease, others have reported disparate findings regarding the changes in sRAGE with extent of disease. For example, in one study of coronary artery disease in nondiabetic men in Italy, lower levels of sRAGE correlated with greater degrees of cardiovascular disease [51]; yet, in another study of the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial patients, higher levels of both total sRAGE and esRAGE were associated with greater cardiovascular disease [52]. The reasons for the disparities amongst the various trials in terms of the “direction” of sRAGEs with disease burden is not yet clear; certainly, it has been shown that renal disease results in higher levels of sRAGEs [53]. Hence, in all study populations, it is essential to note the status of renal function in interpretation of sRAGE.
Further, multiple studies have now shown that sRAGE levels may be modulated by drug treatments such as statins [54, 55], insulin [56], calcium channel blockers [57], angiotensin receptor blockers [58], thiazolidines [59], angiotensin-converting enzyme inhibitors [59], and certain nutraceuticals [59]; hence, in the aforementioned studies, the potential impact of timing of measurement of sRAGE with potential changes in medication program was not discussed.
Taken together, these studies suggest that in heart disease and in heart failure, measurement of total levels of sRAGE may be directly correlated with the degree of cardiac dysfunction; however, levels of esRAGE may be inversely correlated (at least in a Chinese population). These promising findings might set the stage for larger-scale studies in diverse populations to determine the predictiveness of sRAGE levels for the degree of heart failure and, perhaps, the potential prediction of the response to therapeutic intervention.
Perspectives
Innate Roles for RAGE Signaling in Homeostasis
A yet-to-be-established question in vivo is whether salutary roles for the RAGE axis are important in tissue repair. One of the first clues that the actions of RAGE may not all be deleterious were elucidated by experiments in sciatic nerve crush injury induced in nondiabetic mice. Administration of sRAGE or introduction of DN RAGE in vivo in transgenic mice expressing the signaling deficient RAGE mutant in neurons or in macrophages resulted in attenuation of repair after unilateral sciatic nerve crush, as assessed by myelinated fiber density quantification and by motor and sensory conduction velocities [60, 61]. In a distinct setting, systemic or topical administration of sRAGE to diabetic (db/db) mice with full-thickness excisional wounds resulted in acceleration of tissue repair and attenuation of excessive MMP activity in the wound itself [62].
Similar opposing findings were observed in the heart post–myocardial infarction. Whereas Andrassy and colleagues [34] showed that administration (systemic) of HMGB1 in murine myocardial infarction (I/R) accelerated cardiac damage, Limana and colleagues [63] showed that local administration of very small amounts of HMGB1 to the infarcted heart (permanent ligation without reperfusion) activated resident c-kit+cells that accelerated repair. Rossini and colleagues [64] recently followed up this work and showed that human cardiac fibroblasts express RAGE. They showed that HMGB1 significantly upregulated growth factor production in the medium of cardiac fibroblasts cells. In turn, this conditioned medium induced the migration and proliferation of cardiac stem cells. The authors concluded that HMGB1 created a microenvironment conducive to cardiac regeneration [64]. Importantly, these latter experiments were only performed in vitro and the specific interplay between cardiac stem cells and fibroblasts in vivo was not tested, nor was the specific role of RAGE in this process tested.
We predict that there are multiple factors that may influence whether ligands of RAGE impart beneficial versus adverse impact. First, the “dose” of the ligand in the local microenvironment must be critical. Indeed, even in the contrasting findings of Andrassy et al. [34] versus Limana et al. [63] referenced above, a key difference was the many-fold higher dose given by Andrassy systemically versus the much lower dose by Limana administered locally to the target heart tissue. Indeed, work has suggested that whereas low doses of S100B are adaptive, high doses of S100B exert cell death–provoking mechanisms [65].
Second, it is plausible that the microenvironment differentially regulates expression of the distinct classes of receptors for the ligands of RAGE; as indicated above, RAGE ligands also bind to toll-like receptors [38]. Perhaps, depending on the specific settings, RAGE versus non-RAGE receptors for these ligands might be more highly upregulated and active, thereby directing distinct signal transduction mechanisms and phenotypic outcomes.
Third, it is likely that distinct cell types may release RAGE ligands in the specific microenvironment and that the source of the ligand might determine, in part, the autocrine/paracrine implications of the interaction. For example, in the case of the heart, Andrassy and colleagues [34] showed that infiltrating inflammatory cells expressed HMGB1; others suggested that cardiomyocytes might be key sources of HMGB1.
Fourth, we predict that the local microenvironment is critical in potential modification of RAGE ligands; such modified ligands might gain or lose unexpected functions that either accelerate damage or forestall repair. Chen and colleagues [66] showed that in an oxidative stress environment, AGEs were generated that, via RAGE, suppressed endothelial progenitor cell function [66]. We propose that in chronic cellular stress environments, RAGE ligands might accumulate to higher levels (damage phenotype) and be modified, thereby activating distinct signaling pathways that exacerbate inflammation and block attempts at repair.
Clues that support the veracity of this hypothesis were revealed in our first studies on RAGE; we showed that AGEs resulted in activation of the proinflammatory transcription factor NF-κB in endothelial cells and in vivo in a RAGE- and oxidative stress–dependent manner [67, 68]. Further, it was shown that in contrast to acute stimuli, chronic RAGE-dependent activation in diabetes or in intestinal inflammation resulted in sustained activation of NF-κB [69, 70]. From this work, we hypothesized that RAGE did not critically participate in innate immune mechanisms that were essential for survival in severe stress, but, rather, that RAGE perpetuated inflammatory mechanisms that once set in motion not only blocked repair but also augmented tissue damage.
RAGE Is not Primarily Involved in Innate Immune Mechanisms but in Exacerbation of Inflammatory Stress
We recently reported the findings of our experiments in a murine model of severe liver injury. Compared to 70 % hepatectomy in mice, resection of 85 % of the liver is associated with dramatic mortality within the first 7 days after hepatectomy. We generated three distinct groups of mice to test the role of RAGE versus toll-like receptor signaling in massive liver resection; we employed mice devoid of RAGE, mice devoid of myeloid differentiating factor 88, (Myd88 [a major signal transduction adaptor for toll-like receptors such as 2 and 4]), and mice devoid of both RAGE and Myd88. Our data revealed that although Myd88 was absolutely essential for survival via regulation of NF-κB and tumor necrosis factor–α, only deletion of RAGE significantly improved survival compared to wild-type, Myd88-null, or RAGE-null/Myd88-null mice. Molecular analyses revealed that RAGE opposes Myd88 signaling by multiple mechanisms: first, by suppression of p65 levels, thereby reducing activation of NF-κB and consequent production of cyclin D1; and second, by suppression of interleukin 6–mediated phosphorylation of Stat3, thereby downregulating Pim1 and suppressing the hyperplastic response [71••]. These data affirmed that RAGE was not responsible for immediate survival mechanisms, but, rather, RAGE action amplified inflammation and effectively prevented regeneration in massive liver resection.
Conclusions
Experiments in animal models in distinct forms of myocardial injury reveal key roles for the ligand-RAGE axis in the perpetuation of damage signals that yield tissue injury and not repair. Data in human patients suggest that RAGE might be a novel and predictive biomarker of the extent of heart failure in human patients.
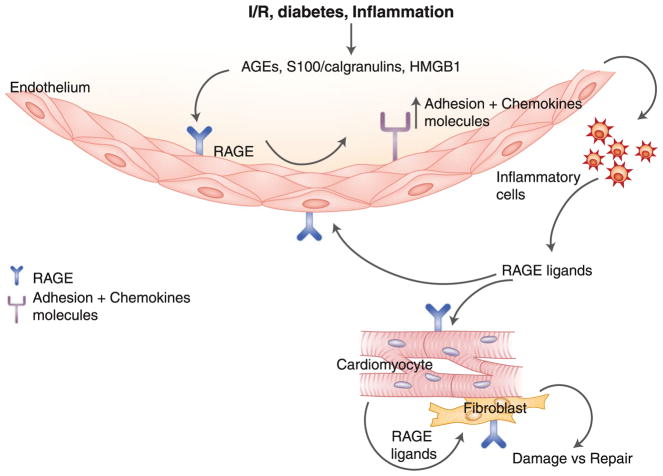
The key questions to address are to what extent will therapeutic antagonists of RAGE be beneficial in the failing heart, and are there settings in which blocking RAGE might be maladaptive in the context of cardiac regeneration? RAGE is expressed by multiple key cell types in the heart, including endothelial cells, infiltrating inflammatory cells, cardiomyocytes, and fibroblasts. We predict that the local interplay of these cell types, both producing and being impacted upon by RAGE ligands, sets the stage for damage versus repair. In I/R, diabetes, and inflammation, the evidence suggests that the dose, timing, and/or cell-specific expression and impact of RAGE ligands portend damage and blockade of repair (Fig. 1). We predict that RAGE blockade might forestall metabolic and fibrotic signaling that leads to functional failure and extensive fibrosis.
Fig. 1.
RAGE ligands-RAGE axis contributes to cardiac dysfunction and failure. The ligand-RAGE axis acts via both cell autonomous and cell non-autonomous mechanisms to enhance stress in the myocardium consequent to I/R, diabetes, or inflammation. We propose that the balance of RAGE action in these settings increases damage and blocks adaptive regeneration. Blockade of ligand-RAGE may be a targeted therapeutic strategy for strategies to salvage stressed myocardium. I/R ischemia/reperfusion; AGEs advanced glycation end products; HMGB1 high-mobility group protein B1; RAGE receptor for advanced glycation end products
As antagonists of RAGE are in development, we eagerly await the results of clinical studies that aim to establish the heart of the matter: is RAGE a safe and viable target for cardiopreservation in the failing heart?
Footnotes
Disclosures No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Kislinger T, Fu C, Huber B, et al. N(epsilon)-(carboxymethyl) lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–9. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 4.Yan SD, Chen X, Fu J, et al. RAGE and amyloid beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–91. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 5.Chavakis T, Bierhaus A, Al-Fakhri N, et al. The pattern recognition receptor RAGE is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–15. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He M, Kubo H, Morimoto K, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Reports. 2011;12:358–64. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan BH, Li X, Winkler AR, et al. Complement c3A, CpG oligos and DNA/C2a complex stimulate IFN-alpha production in a recpetor for advanced glycation end product dependent manner. J Immunol. 2010;185:4213–22. doi: 10.4049/jimmunol.1000863. [DOI] [PubMed] [Google Scholar]
- 8.Myint KM, Yamamoto Y, Doi T, et al. RAGE control of diabetic nephropathy in a mouse model: effects of RAGE gene disruption and administration of low molecular weight heparin. Diabetes. 2006;55:2510–22. doi: 10.2337/db06-0221. [DOI] [PubMed] [Google Scholar]
- 9.Staquicini FI, Cardo-Vila M, Kolonin MG, et al. Vacular ligand-receptor mapping by direct combinatorial selection in cancer patients. PNAS. 2011;108:18637–42. doi: 10.1073/pnas.1114503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz G. RAGE: a single receptor fits multiple ligands. Trends Biochem Sci. 2011;36:625–32. doi: 10.1016/j.tibs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Harja E, Bu DX, Hudson BI, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–94. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson BI, Kalea AZ, Del Mar Arriero M, et al. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283:34457–68. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Toure F, Qu W, et al. Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. J Biol Chem. 2010;285:23233–40. doi: 10.1074/jbc.M110.117457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brett J, Schmidt AM, Yan SD, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–712. [PMC free article] [PubMed] [Google Scholar]
- 15.Iskratsch T, Lange S, Dwyer J, et al. Formin follows function: a muscle specific isoform of FHOD3 is regulated by CK2 phosphorylation and promotes myofibril maintenance. J Cell Bio. 2010;191:1159–72. doi: 10.1083/jcb.201005060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simm A, Casselmann C, Schubert A, et al. Age associated changes of AGE-receptor expression: RAGE upregulation is associated with human heart dysfunction. Exp Gerontol. 2004;39:407–13. doi: 10.1016/j.exger.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Moss SE, Klein R, Klein BE. Cause-specific mortality in a population-based study of diabetes. Am J Public Health. 1991;81:1158–62. doi: 10.2105/ajph.81.9.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burke AP, Kolodgie FD, Zieske A, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–71. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 19.Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation end products. Nat Med. 1998;4:1025–31. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 20.Wendt T, Harja E, Bucciarelli L, et al. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis. 2006;185:70–7. doi: 10.1016/j.atherosclerosis.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Soro-Paavonen A, Watson AM, Li J, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–9. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L, Ishida T, Yasuda T, et al. RAGE mediates oxidized LDL-induced pro-inflammatory effects and atherosclerosis in non-diabetic LDL receptor-deficient mice. Cardiovasc Res. 2009;82:371–81. doi: 10.1093/cvr/cvp036. [DOI] [PubMed] [Google Scholar]
- 23•.Bu DX, Rai V, Shen X, et al. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ Res. 2010;106:1040–51. doi: 10.1161/CIRCRESAHA.109.201103. This work shows that diabetes was associated with an upregulation of profibrotic mechanisms in the vasculature of atherosclerosis-vulnerable apoE null mice and that genetic deletion of RAGE prevented upregulation of these damage pathways in the cardiovasculature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucciarelli LG, Ananthakrishnan R, Hwang YC, et al. RAGE and modulation of ischemic injury in the diabetic myocardium. Diabetes. 2008;57:1941–51. doi: 10.2337/db07-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bucciarelli LG, Kaneko M, Ananthakrishnan R, et al. Receptor for advanced glycation end products: key modulator of myocardial ischemic injury. Circ. 2006;113:1226–34. doi: 10.1161/CIRCULATIONAHA.105.575993. [DOI] [PubMed] [Google Scholar]
- 26••.Aleshin A, Ananthakrishnan R, Li Q, et al. RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model. Am J Physiol Circ Physiol. 2008;294:H1823–32. doi: 10.1152/ajpheart.01210.2007. This work showed that in a murine model of myocardial infarction, administration of soluble RAGE or genetic deletion of RAGE was strikingly protective against the degree of infarct volume and the loss of cardiac function induced by transient ligation and reperfusion of the left anterior descending coronary artery. [DOI] [PubMed] [Google Scholar]
- 27.Tsoporis JN, Izhar S, Leong-Poi H, et al. S100B interaction with the receptor for advanced glycation end products (RAGE): a novel receptor mediated mechanism for myocyte apoptosis postinfarction. Circ Res. 2010;106:93–101. doi: 10.1161/CIRCRESAHA.109.195834. [DOI] [PubMed] [Google Scholar]
- 28.Lu L, Zhang Q, Xu Y, et al. Intra-coronary administration of soluble receptor for advanced glycation end products attenuates cardiac remodeling with decreased transforming growth factor beta 1 expression and fibrosis in minipigs with ischemia-reperfusion injury. Chin Med J. 2010;123:594–8. [PubMed] [Google Scholar]
- 29.Shang L, Ananthakrishnan R, Li Q, et al. RAGE modulates hypoxia/reoxygenation injury in adult murine cardiomyocytes via JNK and GSK-3beta signaling pathways. PLoS One. 2010;5:e10092. doi: 10.1371/journal.pone.0010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsoporis JN, Izhar S, Proteau G, Slaughter G, Parker TG. S100B-RAGE dependent VEGF secretion by cardiac myocytes induces myofibroblast proliferation. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.08.015. In press. [DOI] [PubMed] [Google Scholar]
- 31.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the diabetic db/db mouse. Am J Physiol Heart Circ Physiol. 2006;290:H146–53. doi: 10.1152/ajpheart.00583.2005. [DOI] [PubMed] [Google Scholar]
- 32.Ma H, Li SY, Xu P, et al. Advanced glycation end product (AGE) accumulation and AGE receptor (RAGE) upregulation contribute to the onset of diabetic cardiomyopathy. J Cell Mol Med. 2009;13:1751–64. doi: 10.1111/j.1582-4934.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Nielsen JM, Kristiansen SB, Norregaard R, et al. Blockage of receptor for advanced glycation end products prevents development of cardiac dysfunction in db/db type 2 daibetic mice. Eur J Heart Fail. 2009;11:638–47. doi: 10.1093/eurjhf/hfp070. [DOI] [PubMed] [Google Scholar]
- 34.Andrassy M, Volz HC, Igwe JC, et al. High mobility group box-1 in ischemia-reperfusion injury of the heart. Circ. 2008;117:3216–26. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 35.Volz HC, Kaya Z, Katus HA, Andrassy M. The role of HMGB1/RAGE in inflammatory cardiomyopathy. Sem Thromb Hemostasis. 2010;36:185–94. doi: 10.1055/s-0030-1251503. [DOI] [PubMed] [Google Scholar]
- 36.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dyfunction via the receptor for advanced glycation end products. Circ Res. 2008;102:1239–46. doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- 37.Feuerstein GZ. Cardiac RAGE in sepsis. Call TOLL free for anti-RAGE. Circ Res. 2008;102:1153–4. doi: 10.1161/CIRCRESAHA.108.177261. [DOI] [PubMed] [Google Scholar]
- 38.Vogl T, Tenbrock K, Ludwig S, et al. Mrp8 and Mrp14 are endogenous activators of toll-like recpetor 4, promoting lethal endotoxin shock. Nat Med. 2007;13:1042–9. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 39.Kalea AZ, Schmidt AM, Hudson BI. RAGE: a novel biological and genetic marker for vascular disease. Clin Sci (London) 2009;116:621–37. doi: 10.1042/CS20080494. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann MA, Drury S, Hudson BI, et al. RAGE and arthritis: the G82S polymorphsim amplifies the inflammatory response. Gene Immun. 2002;3:123–35. doi: 10.1038/sj.gene.6363861. [DOI] [PubMed] [Google Scholar]
- 41.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793:993–1007. doi: 10.1016/j.bbamcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Repapi E, Sayers I, Wain LV, et al. Genome-wide association study identifies five loci associated with lung function. Nat Genet. 2010;42:36–44. doi: 10.1038/ng.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poon PY, Szeto CC, Chow KM, Kwan BC, Li PK. Relation between polymorphisms of receptor for advanced glycation end products (RAGE) and cardiovascular diseases in Chinese patients with diabetic nephropathy. Clin Nephrol. 2010;73:44–50. doi: 10.5414/cnp73044. [DOI] [PubMed] [Google Scholar]
- 44.Xue J, Rai V, Singer D, et al. Advanced glycation end product recognition by the receptor for advanced glycation end products. Structure. 2011;19:722–32. doi: 10.1016/j.str.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koyama Y, Takeishi Y, Arimoto T, et al. High serum level of pentosidine, an advanced glycation end product, is a risk factor of patients with heart failure. J Card Fail. 2007;13:199–206. doi: 10.1016/j.cardfail.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 46••.Wang LJ, Lu L, Zhang FR, et al. Increased serum high mobility group box 1 and cleaved receptor for advanced glycation end products and decreased endogenous secretory receptor for advanced glycation end product levels in diabetic and non diabetic patients with heart failure. Eur J Heart Fail. 2011;13:440–9. doi: 10.1093/eurjhf/hfq231. This work showed that levels of RAGE ligand HMGB1 in the plasma of patients was increased in parallel with the degree of heart failure and the levels of total soluble RAGE also were increased in parallel with the severity of heart failure. This work strongly suggests that the RAGE ligand-RAGE axis may be a biomarker of the degree of heart failure in humans. [DOI] [PubMed] [Google Scholar]
- 47.Andrassy M, Volz HC, Riedle N, et al. HMGB1 as a predictor of infarct transmurality and functional recovery in patients with myocardial infarction. J Intern Med. 2011;270:245–53. doi: 10.1111/j.1365-2796.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 48.Foell D, Frosch M, Roth J. Phagocyte specific calcium binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004;344:37–51. doi: 10.1016/j.cccn.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 49.Yan SF, Ramasamy R, Schmidt AM. Soluble RAGE: therapy and biomarker in unraveling the RAGE axis in chronic disease and aging. Biochem Pharmacol. 2010;79:1379–86. doi: 10.1016/j.bcp.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raposeiras-Roubin S, Rodino-Janeiro BK, Grigorian-Shamagian L, et al. Soluble receptor of advanced glycation end products levels are related to ischaemic aetiology and extent of coronary disease in chronic heart failure patients, independent of advanced glycation end products levels: new roles for soluble RAGE. Eur J Heart Fail. 2010;12:1092–100. doi: 10.1093/eurjhf/hfq117. [DOI] [PubMed] [Google Scholar]
- 51.Falcone C, Emanuele E, D’Angelo A, et al. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25:1032–7. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 52.Colhoun HM, Betteridge DJ, Durrington P, et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 2011 doi: 10.2337/db11-0291. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalousová M, Hodková M, Kazderová M, et al. Soluble receptor for advanced glycation end products in patients with decreased renal function. Am J Kidney Dis. 2006;47:406–11. doi: 10.1053/j.ajkd.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 54.Tam HL, Shiu SW, Wong Y, et al. Effects of atorvastatin on serum soluble receptors for advanced glycation end-products in type 2 diabetes. Atherosclerosis. 2010;209:173–7. doi: 10.1016/j.atherosclerosis.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 55.Santilli F, Bucciarelli L, Noto D, et al. Decreased plasma soluble RAGE in patients with hypercholesterolemia: effects of statins. Free Radic Biol Med. 2007;43:1255–62. doi: 10.1016/j.freeradbiomed.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Arabi YM, Dehbi M, Rishu AH, et al. SRAGE in diabetic and non-diabetic critically ill patients: effects of intensive insulin therapy. Crit Care. 2011;15:R203. doi: 10.1186/cc10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura T, Sato E, Fujiwara N, et al. Calcium channel blocker inhibition of AGE and RAGE axis limits renal injury in nondiabetic patients with stage I or II chronic kidney disease. Clin Cardiol. 2011;34:372–7. doi: 10.1002/clc.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grossin N, Boulanger E, Wautier MP, Wautier JL. The different isoforms of the receptor for advanced glycation end products are modulated by pharmacological agents. Clin Hemorheol Microcirc. 2010;45:143–53. doi: 10.3233/CH-2010-1292. [DOI] [PubMed] [Google Scholar]
- 59.Lanati N, Emanuele E, Brondino N, Geroldi D. Soluble RAGE modulating drugs: state of the art and future perspectives for targeting vascular inflammation. Curr Vasc Pharmacol. 2010;8:86–92. doi: 10.2174/157016110790226642. [DOI] [PubMed] [Google Scholar]
- 60.Rong LL, Trojaborg W, Qu W, et al. Antagonism of RAGE suppresses peripheral nerve regeneration. FASEB J. 2004;18:1812–7. doi: 10.1096/fj.04-1899com. [DOI] [PubMed] [Google Scholar]
- 61.Rong LL, Yan SF, Wendt T, et al. RAGE modulates peripheral nerve regeneration by recruitment of inflammatory and axonal outgrowth pathways. FASEB J. 2004;18:1818–25. doi: 10.1096/fj.04-1900com. [DOI] [PubMed] [Google Scholar]
- 62.Goova MT, Li J, Kislinger T, et al. Blockade of receptor for advanced glycation end products restores effective wound healing in daibetic mice. Am J Pathol. 2001;159:513–25. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Limana F, Germani A, Zacheo A, et al. Exogenous high mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac c-kit+cell proliferation and differentiation. Circ Res. 2005;97:73–83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 64.Rossini A, Zacheo A, Mocini D, et al. HMGB1 stimulated human primary cardiac fibroblasts exert a paracrine action on human and murine cardiac stem cells. J Mol Cell Cardiol. 2008;44:683–93. doi: 10.1016/j.yjmcc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Businaro R, Leone S, Fabrizi C, et al. S100B protects LAN-5 neuroblastoma cells against abeta amyloid-induced neurotoxicity via RAGE engagement at low doses but increases abeta amyloid neurotoxicity at high doses. J Neurosci Res. 2006;83:897–906. doi: 10.1002/jnr.20785. [DOI] [PubMed] [Google Scholar]
- 66.Chen J, Song M, Yu S, et al. Advanced glycation end products alter functions and promote apoptosis in endothelial progenitor cells through receptor for advanced glycation end products mediate overexpression of cell oxidant stress. Mol Cell Biochem. 2010;335:137–46. doi: 10.1007/s11010-009-0250-y. [DOI] [PubMed] [Google Scholar]
- 67.Yan SD, Schmidt AM, Anderson GM, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–97. [PubMed] [Google Scholar]
- 68.Schmidt AM, Hori O, Chen JX, et al. Advanced glycation end products interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bierhaus A, Schiekofer S, Schwaninger M, et al. Diabetes-associated sustained activation for the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 70.Andrassy M, Igwe J, Autschbach F, et al. Posttranslationally modified proteins as mediators of sustained intestinal inflammation. Am J Pathol. 2006;169:1223–37. doi: 10.2353/ajpath.2006.050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71••.Zeng S, Zhang QY, Huang J, et al. Opposing roles of RAGE and Myd88 signaling inextensive liver resection. FASEB J. 2011 doi: 10.1096/fj.11-192997. In press. This work established firmly that RAGE is not involved in innate responses to severe stress such as that induced by massive hepatectomy, but rather that RAGE propagates inflamamtory mechanisms that sustain injury and prevent regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]