Abstract
Hypoglossal nerve activity (HNA) controls the position and movements of the tongue. In persons with compromised upper airway anatomy, sleep-related hypotonia of the tongue and other pharyngeal muscles causes increased upper airway resistance, or total upper airway obstructions, thus disrupting both sleep and breathing. Hypoglossal nerve activity reaches its nadir, and obstructive episodes are longest and most severe, during rapid eye movement stage of sleep (REMS). Microinjections of a cholinergic agonist, carbachol, into the pons have been used in vivo to investigate the mechanisms of respiratory control during REMS. Here, we recorded inspiratory-modulated phrenic nerve activity and HNA and microinjected carbachol (25–50 nl, 10 mm) into the pons in an in situ perfused working heart–brainstem rat preparation (WHBP), an ex vivo model previously validated for studies of the chemical and reflex control of breathing. Carbachol microinjections were made into 40 sites in 33 juvenile rat preparations and, at 24 sites, they triggered depression of HNA with increased respiratory rate and little change of phrenic nerve activity, a pattern akin to that during natural REMS in vivo. The REMS-like episodes started 151±73 s (SD) following microinjections, lasted 20.3±4.5 min, were elicited most effectively from the dorsal part of the rostral nucleus pontis oralis, and were prevented by perfusion of the preparation with atropine. The WHBP offers a novel model with which to investigate cellular and neurochemical mechanisms of REMS-related upper airway hypotonia in situ without anaesthesia and with full control over the cellular environment.
Hypoglossal motor output regulates the position and movements of the tongue during various oropharyngeal behaviours, such as swallowing, grooming or vocalization (Travers & Jackson, 1992; for review see Gestreau et al. 2005). Prominent inspiratory-related discharge can be recorded in conditions of enhanced chemical drive for breathing from hypoglossal motoneurones and the XII cranial nerve (Haxhiu et al. 1987; Withington-Wray et al. 1988), following vagotomy (Bartlett & St John, 1988; Fregosi & Fuller, 1997), or in response to stimulants applied to hypoglossal motoneurones (Morrison et al. 2003). Patients suffering from the obstructive sleep apnoea syndrome exhibit enhanced tonic and inspiratory-modulated lingual muscle activity when compared with healthy persons (Suratt et al. 1988; Mezzanotte et al. 1992). This adaptation allows them to maintain the upper airway open during wakefulness, but becomes insufficient during sleep, when hypoglossal motor activity is reduced, and particularly so during rapid eye movement sleep (REMS; Sauerland & Harper, 1976). Thus, in obstructive sleep apnoea subjects, sleep-related decrease of the tone in the lingual and other pharyngeal muscles causes upper airway muscle hypotonia, increases upper airway resistance and leads to upper airway narrowing or a complete obstruction. Obstructive episodes are often longest and most severe during REMS (Remmers et al. 1978). For this reason, state-dependent changes of the hypoglossal motor output have been studied particularly extensively during REMS (Orem & Kubin, 2005; Horner, 2007).
Some fundamental findings relevant for respiratory rhythm generation have been made using in vitro models (Richter & Spyer, 2001). However, sleep–wake modulation of motor and respiratory outputs has not been investigated in in vitro preparations. Consequently, all studies of the interaction between sleep and breathing conducted to date employed freely behaving, acutely anaesthetized or decerebrate in vivo models. In decerebrate or anaesthetized rats or cats, microinjections of cholinergic agonists, especially carbachol, into the dorsomedial pontine reticular formation trigger an REMS-like state (for reviews see Kubin, 2001; Kubin & Fenik, 2004; Datta & MacLean, 2007). This approach is based on the findings in freely behaving animals that pontine carbachol injections effectively trigger and/or enhance a REMS-like state that shares many electrophysiological features with its natural counterpart (e.g. Baghdoyan et al. 1987; Vanni-Mercier et al. 1989; Bourgin et al. 1995; reviewed by Baghdoyan, 1997).
Here, we demonstrate that carbachol microinjection into the previously identified in vivo pontine REMS-triggering region reliably and repetitively elicits REMS-like suppression of hypoglossal nerve activity (HNA) in an in situ perfused working heart–brainstem preparation (WHBP) of juvenile rats. We demonstrate that, at the most effective injection sites, carbachol microinjections accelerate the central respiratory rate and increase its variability. A combination of depressed pharyngeal muscle activity with small changes in diaphragmatic activity, increased breathing frequency and increased interbreath variability are typical features of natural REMS (Remmers et al. 1976; Orem, 1986; Orem & Anderson, 1996). This novel in situ model should facilitate investigation of the mechanisms underlying the central respiratory rhythm changes and motoneuronal depression during REMS, including depression of activity in orofacial motoneurones that importantly protect the upper airway from collapse in patients with obstructive sleep apnoea.
Preliminary results have been published (Brandes et al. 2009).
Methods
Experiments were performed on juvenile rats (Wistar strain, n=33; 19–23 days old; body weight 70–100 g) of either sex obtained from the animal breeding facility of the University of Göttingen. All experimental procedures followed the European Community and National Institutes of Health guidelines for the care and use of laboratory animals, and were approved by the Ethical Committee of the Georg August University, Göttingen.
Working heart–brainstem preparation
We used the intra-arterially perfused brainstem preparation, as described previously (Paton, 1996; also see Dutschmann & Herbert, 2006). In brief, rats were deeply anaesthetized with isoflurane (1-chloro-2,2,2-trifluoroethyl-difluoromethylether; Abbott, Wiesbaden, Germany). Once breathing was profoundly depressed and the animal failed to respond to strong noxious pinch of the tail or toe, it was transected below the diaphragm, decerebrated at the precollicular level and cerebellectomized while it was simultaneously superfused with cold Ringer's solution gassed with 95% O2 and 5% CO2 (carbogen). The preparation was then transferred to a custom-made recording chamber, and the descending aorta was cannulated and perfused at a flow rate of 28–32 ml min−1 using a peristaltic pump (Watson & Marlow, Rommerskirchen, Germany) via a double-lumen catheter with carbogen-gassed Ringer solution containing Ficoll (1.25%; Sigma, Taufkirchen, Germany) and heated to 31°C. The perfusate was filtered and passed through a bubble trap that removed undissolved gas and dampened both the pump- and the heart-generated pulsations. The perfusate exiting from the preparation was collected, reoxygenated and recirculated. Rhythmic contractions of the diaphragm usually resumed within 2–5 min after the start of perfusion. Perfusion pressure within the aorta was monitored via one port of the double-lumen catheter using a pressure transducer, and the pressure was set at 70–90 mmHg by adjusting the flow rate. In these conditions, the preparations generated rhythmic respiratory discharge for at least 5 h.
The perfusate contained (mm): 125 NaCl, 24 NaHCO3, 2.5 CaCl2, 1.25 MgSO4, 4 KCl, 1.25 KH2PO4, 10 d-glucose and Ficoll to maintain osmotic pressure. The osmolarity of the medium was 298±5 mosmol l−1 and, on gassing with carbogen, the pH was 7.35±0.05. In some preparations, respiratory-related muscle activity was abolished by including vecuronium bromide in the perfusate (0.3 μg ml−1).
Recording neural output to respiratory muscles
In all experiments, the left phrenic and hypoglossal nerves were dissected and recorded using glass suction electrodes. The signals were amplified (Neurolog; Digitimer Ltd, Hydeway, UK), filtered (8 Hz to 3 kHz; Neurolog modules 104 and 125) and integrated (time constant 100 ms), digitally acquired at a sampling rate of 5 kHz (MacLab 8s; AD Instruments, Sydney, NSW, Australia) and displayed on a computer using Chart software (AD Instruments).
Experimental protocol
Pressure-microinjections of carbachol (10 mm in 0.9% NaCl; Sigma-Aldrich, Taufkirchen, Germany) or Pontamine Sky Blue dye (2%; Sigma-Aldrich) were made using a two-barrelled micropipette (tip diameter 30–40 μm) positioned in the dorsomedial pons. The injected volumes were measured by observing the movement of the meniscus through a monocular microscope fitted with a calibrated reticule. In some experiments, after recovery from the initial carbachol effects, an additional one or two carbachol injections were made at the same site with or without the muscarinic cholinergic antagonist, atropine (50 nm; Sigma-Aldrich) added to the perfusate.
At the end of the experiment, the brainstem was removed and fixed for 1–2 days in 4% paraformaldehyde and 20% sucrose. For anatomical localization of the injection sites, 50-μm-thick coronal sections were cut serially through the pons using a freezing microtome. The sections were mounted and stained with Neutral Red. Those containing the injection sites marked with Pontamine Sky Blue were redrawn, and the centres of the marked locations were transferred onto the corresponding standard cross-sections adapted from a rat brain atlas (Paxinos & Watson, 2004).
Data analysis
All data analyses were performed off-line. The following respiratory parameters were derived from phrenic nerve activity (PNA): the respiratory cycle length (TTOT), duration of inspiration (TI), duration of expiration (TE; the period of PNA quiescence) and respiratory cycle variability (TVAR). The TVAR was calculated from the instantaneous TTOT as variance. The average value of each parameter was measured for 60 s before, during and after the effect of carbachol injection. The time of onset of depression of HNA following carbachol injection and the duration of the depression were measured from the integrated HNA. The maximal magnitude of the depression was measured as a percentage decrease relative to the peak inspiratory HNA prior to carbachol injection, and the same was done with the peak inspiratory PNA. The significance of the differences between the baseline, maximal carbachol effect and recovery was tested using repeated-measures ANOVA followed by Fisher's LSD post hoc tests. The effects of atropine on the carbachol-evoked depression of HNA were tested with a two-tailed Student's paired t test. All data are expressed as means±SD. Differences were regarded significant when P was less than 0.05.
Results
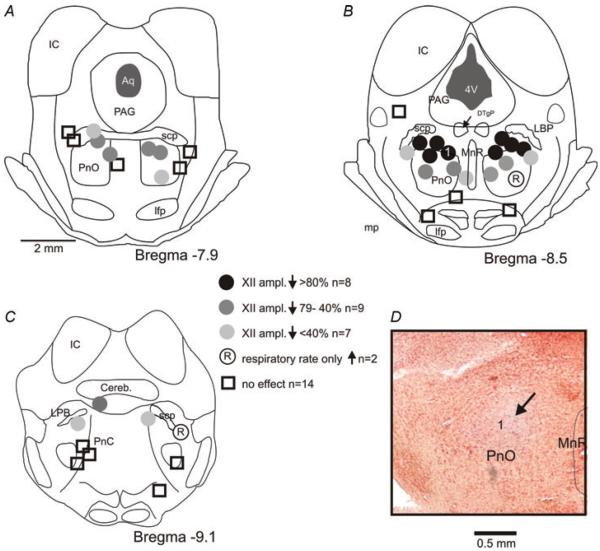
Pontine carbachol injections (20–50 nl; mean 46±28 nl) were made into 40 sites in 33 in situ perfused brainstem preparations. At 24 sites, carbachol elicited a significant depression of HNA, whereas the amplitude of PNA changed little. The remaining injections were either ineffective (n =14) or caused an increase of central respiratory rate without HNA depression (n =2). Histological analysis of the injection sites revealed that the injections made within the dorsomedial, rostral nucleus pontis oralis (PnO) produced the strongest HNA depression, whereas injections into other areas had weaker or no effect (Fig. 1).
Figure 1. Location of the effective and ineffective carbachol injection sites superimposed on standard coronal sections derived from a rat brain atlas (Paxinos & Watson, 2004).
D shows a highly effective injection site in a Neutral Red-stained section (arrow; the site is also marked as `1' in B). Pontamine Sky Blue staining became faint after tissue processing.
Abbreviations: Aq, aqueduct; Cereb., cerebellum; DTgP, dorsal tegmental nucleus; IC, inferior colliculus; lfp, longitudinal fasciculus of the pons; LPB, lateral parabrachial nuclei; MnR, median raphe nucleus; PAG, periaqueductal grey; PnC, pontine reticular nucleus, caudal part; PnO, pontine reticular nucleus, oral part; scp, superior cerebellar peduncle; and 4V, fourth ventricle.
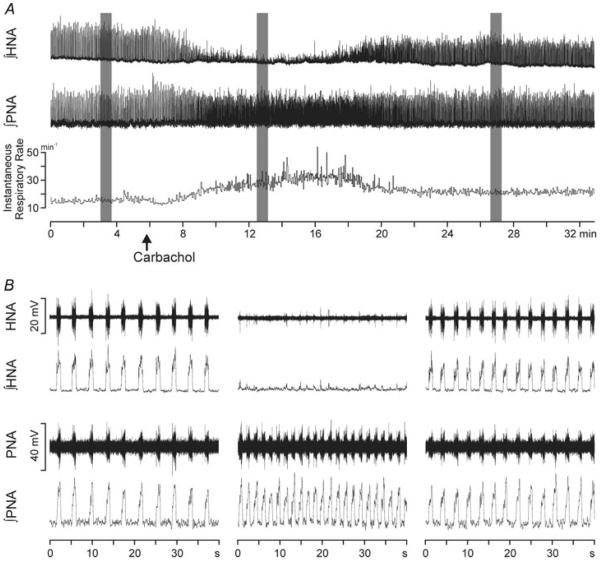
For the 24 effective injection sites, depression of HNA started 151±73 s after carbachol injection. The maximal depression, to 39.8±22.9% of the baseline (precarbachol) level of HNA, was significant [ANOVA, F(2, 23)=16.352; Fisher's LSD test, P <0.001]. The carbachol-induced HNA depression was accompanied by a significant increase of central respiratory rate, from 12±8 to 18±10 bursts min−1 [ANOVA, F(2, 23)=11.965; Fisher's LSD test, P <0.001]. The increase of respiratory rate was due to a reduced duration of the respiratory cycle (TTOT) from 7.0±4.0 to 4.7±3.0 s [ANOVA, F(2, 23)=8.952; Fisher's LSD test, P <0.001], which was mainly due to a significant decrease of the expiratory phase duration (TE) from 5.7±3.4 to 3.4±2.3 s [ANOVA, F(2, 23)=15.202; Fisher's LSD test, P <0.001], whereas the duration of inspiration (TI) was not significantly changed [1.2±0.8 versus 1.1±0.4 s; ANOVA, F(2, 23)=1.790, P =0.17]. PNA amplitude measured at the time The of maximal depression of HNA was not significantly changed [94.9±10.7% of the precarbachol level; ANOVA, F(2, 23)=1.423, P =0.27]. The HNA returned to its precarbachol level 20.3±4.5 min after the onset of the carbachol effects. However, the central respiratory rate determined at the time of full HNA recovery usually remained elevated [16±8 bursts min−1; ANOVA, F(2, 23)=8.952; Fisher's LSD test, P =0.067] compared with control values. In most cases, it appeared that the respiratory rate increase following carbachol comprised a component that was closely associated with the period of depression of HNA and another component that lasted considerably longer and accounted for the higher central respiratory rate after HNA recovery than before carbachol injection (Fig. 2A). An example of a typical effect of carbachol injection into a highly effective site within the dorsal part of the PnO is illustrated in Fig. 2.
Figure 2. Effect of pontine carbachol injection on hypoglossal nerve activity (HNA), phrenic nerve activity (PNA), respiratory rate and its variability.
In A, note the respiratory rate increase in association with the depression of HNA and that the respiratory rate remains partly elevated when HNA returns to the precarbachol level. B, expanded traces illustrating both raw and integrated PNA and HNA during the selected stages of the experiment (marked by grey shading in A).
The increase of respiratory rate variability was clear in the experiments with strong depression of HNA (Fig. 2). When analysed for the entire data set comprising all 24 effective sites, carbachol effects on the variability of respiratory rate were not significant. However, when the experiments were grouped according to the magnitude of HNA depression into three categories [(Fig. 1): (i) HNA reduction by more than 80% (n =8); (ii) reduction by 40–79% (n =9); and (iii) reduction by less than 40% (n =7)], carbachol injections that caused a larger than 80% depression were accompanied by a significant increase of TVAR [85±74 ms2 prior to carbachol, 250±175 ms2 during maximal depression of HNA, and 145±96 ms2 after recovery of HNA; ANOVA, F(2, 7)=3.3; Fisher's LSD test, P <0.05]. In the two remaining groups, TVAR was not significantly increased during the carbachol-induced depression of HNA (for group 79–40%, 97±62, 154±109 and 101±52 ms2, respectively, Fisher's LSD tests, P >0.3; and for group <40%, 98±51, 138±115 and 101±41 ms2, respectively, Fisher's LSD tests, P >0.6). While TVAR was increased, we did not observe `fractionations' of inspiratory activity similar to those occurring in cats during natural REMS (e.g. Orem & Anderson, 1996).
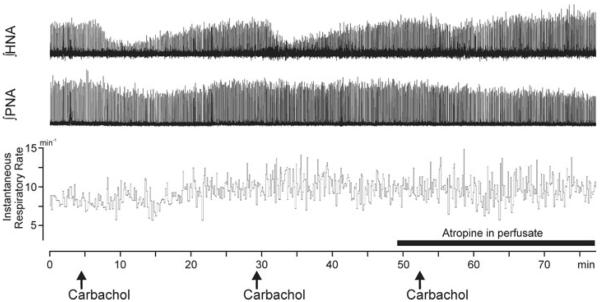
In three preparations, carbachol injections were made three times at the same site, twice in the normal conditions and for the third time with the cholinergic antagonist, atropine, added to the perfusate prior to carbachol injection. The effects of the first two injections were highly reproducible, thus showing that carbachol could repeatedly trigger HNA depression from the same site (Fig. 3). Both the timing and the magnitude of the carbachol-evoked HNA depression varied little between the first and the second injection (Fig. 3). When carbachol was then injected with simultaneous perfusion of the preparation with atropine, HNA depression was significantly attenuated (by 77±10% without atropine and by 31.7±0.05% with atropine; Student's paired t test, P <0.05; Fig. 3).
Figure 3. Integrated HNA and PNA in an experiment with three successive carbachol microinjections at a single site located in the dorsal pontine reticular nucleus.
Note the reproducibility of the effects of the first two carbachol injections and that the effects of the third injection are nearly abolished by perfusion of the preparation with the muscarinic cholinergic antagonist, atropine (filled bar above the time scale).
Discussion
The present study shows that carbachol microinjections into a distinct region located in the dorsomedial part of the rostral oral pontine reticular nucleus trigger REMS-like changes in the respiratory motor output in the WHBP of juvenile rats. The changes include profound suppression of HNA, which provides motor innervation to the genioglossus and other muscles of the tongue, acceleration of the central respiratory rate and small changes of PNA. Both the similarity of the location of the most effective sites to the sites from which carbachol elicits an REMS-like state in vivo and the similarity of the pattern of respiratory motor output and respiratory rhythm changes to the changes in the respiratory system during natural REMS suggest that the episodes triggered by pontine carbachol in the WHBP represent an in situ activation of at least a subset of the neural network that is also being activated during natural REMS.
The WHBP is a validated experimental model for systemic and cellular studies of cardiorespiratory control (Paton, 1996; Richter & Spyer, 2001). In contrast to in vitro models, the entire pontomedullary brainstem of the WHBP is oxygenated via the circulatory system and generates a eupnoeic respiratory pattern characterized by co-ordinated discharges in the cranial and spinal respiratory motor outputs (Paton, 1996; Dutschmann & Paton, 2002). The WHBP also displays physiological responses to various afferent inputs important for cardiorespiratory control (reviewed by Dutschmann et al. 2004; Paton et al. 2006). The preparation has been successfully used for preclinical screening of drug effects (Dutschmann et al. 2009; Manzke et al. 2009). Thus, the WHBP shows cardiorespiratory activity similar to that of an unanaesthetized, decerebrate preparation in vivo. However, to date, cardiorespiratory changes akin to those related to the sleep–wake cycle have not been investigated in this preparation.
In vivo carbachol models of an REMS-like state have been successfully used with many modifications to study various aspects of the central neural regulation during REMS. Carbachol injections into the rostral, dorsomedial pons trigger such hallmarks of REMS as the atonia of postural muscles, cortical and hippocampal activation and depression of respiratory motor output (reviewed by Baghdoyan, 1997; Kubin, 2001; Kubin & Fenik, 2004; Datta & MacLean, 2007). In decerebrate or anaesthetized rats and cats, carbachol reliably triggers depression of hypoglossal motor output when injected at sites analogous to those explored in the present study (Kimura et al. 1990; Taguchi et al. 1992; Lu et al. 2007).
The present study demonstrates that carbachol injections into the dorsomedial pons cause REMS-like changes in respiratory motor outputs in the WHBP, thus in the in situ conditions. We found the most effective injection sites in the dorsomedial part of the rostral nucleus reticular pontis oralis, a location consistent with previous in vivo studies in anaesthetized rats (Fenik & Kubin, 2009). Importantly, in the WHBP, the carbachol-induced depression of HNA occurred with a simultaneous increase of the central respiratory rate and, at least at some sites, with increased variability of the duration of the respiratory cycle. The last two features distinguish the effects of pontine carbachol in the WHBP from the previously described models (anaesthetized or decerebrate), in which respiratory rate was typically decreased and no respiratory rate variability was observed. The absence of respiratory rate acceleration or respiratory variability has been previously interpreted as related to the inability of pontine carbachol injections to fully mimic the rapid changes in acetylcholine release that occur naturally during REMS (Kimura et al. 1990), or as an observation supportive of the concept that respiratory variability during REMS reflects the contents of dreams that require the presence of the forebrain (Orem & Kubin, 2005). The respiratory rate acceleration and increased respiratory rate variability found following pontine carbachol injections in the WHBP bears similarity to the changes in breathing during natural REMS in mammals (Remmers et al. 1976; Orem & Anderson, 1996) and suggests that some not yet fully understood aspects of the baseline state of different animal models determine the pattern of respiratory changes following pontine carbachol.
Similar to the previous carbachol studies in decerebrate rats and cats in vivo (Kimura et al. 1990; Taguchi et al. 1992), the duration of carbachol-triggered REMS-like episodes in the WHBP was considerably longer than the typical duration of naturally occurring episodes of REMS in rats (about 2 min on average) or the duration of REMS-like episodes elicited in forebrain-intact, urethane-anaesthetized rats (3–4 min; Kubin, 2001; Kubin & Fenik, 2004; Lu et al. 2007). This difference provides further support to the earlier suggestion that REMS episodes are initiated or triggered in the pons, but their proper termination requires an intact forebrain (Kubin, 2001; Lu et al. 2007). The experimental conditions with temperature of the brainstem around 31°C causing slower metabolism may also contribute to the difference in the temporal patterns of REMS-like episodes observed in vivo and in situ.
The respiratory rate increase elicited by carbachol in the WHBP comprised two components. One was temporarily associated with the depression of HNA, whereas the other one was longer, resulting in an incomplete return of the respiratory rate to baseline after the injections. It is likely that this second, longer-lasting component was due to the effects of carbachol not directly related to its ability to trigger REMS-like episodes. It could be due to activation of dorsal pontine noradrenergic neurones (Koyama & Kayama, 1993), as this is known to accelerate respiratory rate (Errchidi et al. 1990). It could also be due to the spread of carbachol to the parabrachial region (Bonis et al. 2010) or the ventral pontine reticular formation (Fenik et al. 2005). If the spread of carbachol beyond the most effective region for triggering of REMS-like episodes is the explanation, the use of smaller injection volumes may help to better separate what appears to be two different components of the respiratory rate increase identified in this study.
Outlook and conclusion
Carbachol microinjections into a restricted region of the dorsomedial pontine reticular formation trigger REMS-like alterations of respiratory motor output to the upper airway and of the respiratory rate in situ. Remarkably, the observed changes in breathing are similar to those observed during natural REMS. Thus, the WHBP offers a novel experimental model with which to study the cellular and network mechanisms of REMS-related upper airway hypotonia in experimental conditions that offer the ability to stringently control the extracellular environment of the brainstem network. The model of REMS-like state elicited by carbachol in the WHBP can significantly contribute to the understanding of physiological mechanisms underlying REMS, including those that affect breathing. With obstructive sleep apnoea being one of the most common sleep disorders, the ability to study REMS-related control of upper airway motor output in situ may help to develop new treatment strategies for this disorder.
Acknowledgements
The study was supported by the Bernstein Centre for Computational Neurosciences, Göttingen (BCCN, 01GQ0432) and grant HL-47600 to L.K. The authors thank Ms Anne Bischoff for her expert technical assistance.
References
- Baghdoyan HA. Cholinergic mechanisms regulating REM sleep. In: Schwartz WJ, editor. Sleep Science: Integrating Basic Research and Clinical Practice. Karger; Basel: 1997. pp. 88–116. [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987;414:245–261. doi: 10.1016/0006-8993(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Bartlett D, Jr, St John WM. Influence of lung volume on phrenic, hypoglossal and mylohyoid nerve activities. Respir Physiol. 1988;73:97–109. doi: 10.1016/0034-5687(88)90130-2. [DOI] [PubMed] [Google Scholar]
- Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Qian B, Pan LG, Forster HV. A role for the Kölliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. J Appl Physiol. 2010;109:159–170. doi: 10.1152/japplphysiol.00933.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin P, Escourrou P, Gaultier C, Adrien J. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat. NeuroReport. 1995;6:532–536. doi: 10.1097/00001756-199502000-00031. [DOI] [PubMed] [Google Scholar]
- Brandes IF, Stettner G, Kubin L, Dutschmann M. Carbachol injections in the nucleus pontis oralis elicit REM sleep-like depression of XII nerve activity in the rat in situ perfused working heart-brainstem preparation (WHBP) FASEB J. 2009;23:960.9. [Google Scholar]
- Datta S, MacLean RR. Neurobiological mechanisms for the regulation of mammalian sleep–wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Mörschel M, Kron M, Herbert H. Development of adaptive behaviour of the respiratory network: implications for the pontine Kölliker-Fuse nucleus. Respir Physiol Neurobiol. 2004;143:155–165. doi: 10.1016/j.resp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Paton JFR. Inhibitory synaptic mechanisms regulating upper airway patency. Respir Physiol Neurobiol. 2002;131:57–63. doi: 10.1016/s1569-9048(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Waki H, Manzke T, Simms AE, Pickering AE, Richter DW, Paton JFR. The potency of different serotonergic agonists in counteracting opioid evoked cardio-respiratory disturbances. Phil Trans R Soc Lond B Biol Sci. 2009;364:2611–2623. doi: 10.1098/rstb.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errchidi S, Hilaire G, Monteau R. Permanent release of noradrenaline modulates respiratory frequency in the newborn rat: an in vitro study. J Physiol. 1990;429:497–510. doi: 10.1113/jphysiol.1990.sp018269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik VB, Kubin L. Differential localization of carbachol- and bicuculline-sensitive pontine sites for eliciting REM sleep-like effects in anesthetized rats. J Sleep Res. 2009;18:99–112. doi: 10.1111/j.1365-2869.2008.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik VB, Ogawa H, Davies RO, Kubin L. Carbachol injections into the ventral pontine reticular formation activate locus coeruleus cells in urethane-anesthetized rats. Sleep. 2005;28:551–559. doi: 10.1093/sleep/28.5.551. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol. 1997;110:295–306. doi: 10.1016/s0034-5687(97)00095-9. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol. 2005;147:159–176. doi: 10.1016/j.resp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, van Lunteren E, Mitra J, Cherniack NS. Comparison of the response of diaphragm and upper airway dilating muscle activity in sleeping cats. Respir Physiol. 1987;70:183–193. doi: 10.1016/0034-5687(87)90049-1. [DOI] [PubMed] [Google Scholar]
- Horner RL. Respiratory motor activity: influence of neuromodulators and implications for sleep disordered breathing. Can J Physiol Pharmacol. 2007;85:155–165. doi: 10.1139/y06-089. [DOI] [PubMed] [Google Scholar]
- Kimura H, Kubin L, Davies RO, Pack AI. Cholinergic stimulation of the pons depresses respiration in decerebrate cats. J Appl Physiol. 1990;69:2280–2289. doi: 10.1152/jappl.1990.69.6.2280. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Kayama Y. Mutual interactions among cholinergic, noradrenergic and serotonergic neurons studied by ionophoresis of these transmitters in rat brainstem nuclei. Neuroscience. 1993;55:1117–1126. doi: 10.1016/0306-4522(93)90325-a. [DOI] [PubMed] [Google Scholar]
- Kubin L. Carbachol models of REM sleep: recent developments and new directions. Arch Ital Biol. 2001;139:147–168. [PubMed] [Google Scholar]
- Kubin L, Fenik V. Pontine cholinergic mechanisms and their impact on respiratory regulation. Respir Physiol Neurobiol. 2004;143:235–249. doi: 10.1016/j.resp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Lu JW, Fenik VB, Branconi JL, Mann GL, Rukhadze I, Kubin L. Disinhibition of perifornical hypothalamic neurones activates noradrenergic neurones and blocks pontine carbachol-induced REM sleep-like episodes in rats. J Physiol. 2007;582:52–67. doi: 10.1113/jphysiol.2007.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Dutschmann M, Schlaf G, Mörschel M, Koch UR, Ponimaskin E, Bidon O, Lalley PM, Richter DW. Serotonin targets inhibitory synapses to induce modulation of network functions. Phil Trans R Soc Lond B Biol Sci. 2009;364:2589–2602. doi: 10.1098/rstb.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABAA receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J Physiol. 2003;548:569–583. doi: 10.1113/jphysiol.2002.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orem JM. Respiratory neuronal activity in sleep. In: Edelman NH, Santiago TV, editors. Breathing Disorders of Sleep. Churchill Livingstone; New York: 1986. pp. 19–44. [Google Scholar]
- Orem J, Anderson CA. Diaphragmatic activity during REM sleep in the adult cat. J Appl Physiol. 1996;81:751–760. doi: 10.1152/jappl.1996.81.2.751. [DOI] [PubMed] [Google Scholar]
- Orem JM, Kubin L. Respiratory physiology: central neural control. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th edn Elsevier-Saunders; Philadelphia: 2005. pp. 213–223. [Google Scholar]
- Paton JFR. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Nalivaiko E, Boscan P, Pickering AE. Reflexly evoked coactivation of cardiac vagal and sympathetic motor outflows: observations and functional implications. Clin Exp Pharmacol Physiol. 2006;33:1245–1250. doi: 10.1111/j.1440-1681.2006.04518.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th edn Academic Press; San Diego: 2004. [Google Scholar]
- Remmers JE, Bartlett D, Putnam MD. Changes in the respiratory cycle associated with sleep. Respir Physiol. 1976;28:227–238. doi: 10.1016/0034-5687(76)90041-4. [DOI] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. TINS. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am Rev Respir Dis. 1988;137:889–894. doi: 10.1164/ajrccm/137.4.889. [DOI] [PubMed] [Google Scholar]
- Taguchi O, Kubin L, Pack AI. Evocation of postural atonia and respiratory depression by pontine carbachol in the decerebrate rat. Brain Res. 1992;595:107–115. doi: 10.1016/0006-8993(92)91458-q. [DOI] [PubMed] [Google Scholar]
- Travers JB, Jackson LM. Hypoglossal neural activity during licking and swallowing in the awake rat. J Neurophysiol. 1992;67:1171–1184. doi: 10.1152/jn.1992.67.5.1171. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Sakai K, Lin JS, Jouvet M. Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch Ital Biol. 1989;127:133–164. [PubMed] [Google Scholar]
- Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience. 1988;25:1041–1051. doi: 10.1016/0306-4522(88)90057-7. [DOI] [PubMed] [Google Scholar]