Abstract
The brain activation of a group of high-functioning autistic participants was measured using fMRI during the performance of a Tower of London task, in comparison to a control group matched with respect to IQ, age, and gender. The two groups generally activated the same cortical areas to similar degrees. However, there were three indications of underconnectivity in the group with autism. First, the degree of synchronization (i.e. the functional connectivity, or the correlation of the time series of the activation) between the frontal and parietal areas of activation was lower for the autistic than the control participants. Second, relevant parts of the corpus callosum, through which many of the bilaterally activated cortical areas communicate, were smaller in cross-sectional area in the autistic participants. Third, within the autism group but not within the control group, the size of the genu of the corpus callosum was correlated with frontal-parietal functional connectivity. These findings suggest that the neural basis of altered cognition in autism entails a lower degree of integration of information across certain cortical areas resulting from reduced intra-cortical connectivity. The results add support to a new theory of cortical underconnectivity in autism, which posits a deficit in integration of information at the neural and cognitive levels.
Keywords: autism, functional connectivity, fMRI, corpus callosum, executive function
Newly emerging theories of neurological functioning in autism are highlighting interregional functional and anatomical connectivity as a likely key feature of the pathophysiology. Several recent functional neuroimaging studies provide evidence of a lower degree of coordination among activated brain areas in autism. A recent study of sentence comprehension (Just et al., 2004) found that the brain activity was less synchronized across activated brain areas (i.e. there was reduced functional connectivity) in autism. Studies of social cognition (Castelli et al., 2002) and working memory (Luna et al., 2002) also suggest aberrant functional connectivity in the brains of individuals with autism. The cortical underconnectivity theory of autism (Just et al., 2004) provides an integrating framework for the new findings, and also provides useful extensions to previous theories of autism. Very briefly, underconnectivity theory proposes that autism is a cognitive and neurobiological disorder associated with underfunctioning of integrative circuitry, resulting in a deficit in integration of information at the neural and cognitive levels.
In addition to functional imaging studies, anatomical studies also present evidence for abnormal connectivity in autism. Courchesne et al. (2001) found an abnormal developmental trajectory of white matter in autism, such that 2–3 year old boys with autism had increased cerebral and cerebellar white matter volume. Herbert et al. (2004) found localized white matter enlargement in the outer radiate compartment of the white matter in children with autism. Herbert et al. suggested an ongoing postnatal process involving white matter in autism that primarily affects intrahemispheric and corticocortical connections. A diffusion tensor imaging study found reduced fractional anisotropy (indicating a lower degree of coherence of directionality) in white matter adjacent to the ventromedial prefrontal cortices, anterior cingulate gyri, temporoparietal junctions, and in the corpus callosum (Barnea-Goraly et al., 2004). At a much more fine-grained level, Casanova et al. (2002) found more numerous and abnormally narrow minicolumns in the frontal and temporal cortex in autism, creating an abundance of short connective fibers relative to long ones, which may indicate a deficiency in long distance (inter-regional) connectivity. The converging findings of functional connectivity abnormalities and white matter abnormalities in autism in several studies suggest that alterations in cortical connectivity and the communication among cortical regions may be part of the pervasive core processing deficits in autism (Herbert et al., 2004).
One of the anatomical regions that has emerged as a target of autism research is the corpus callosum, which mediates the inter-hemispheric communication among cortical areas underpinning higher level cognitive function. Several morphometric studies report abnormalities, especially reduction in size, in various subregions of the corpus callosum in autism (e.g. Piven et al., 1997; Manes et al., 1999; Hardan et al., 2000; Vidal et al., 2003). Chung et al. (2004) found lower white matter density (an index for neural connectivity) in the genu, rostrum and splenium of the corpus callosum in individuals with autism, and suggested that this reduction might result in impaired inter-hemispheric connectivity in frontal, temporal and occipital regions. The interhemispheric fibers from the inferotemporal and occipital lobes (posterior areas) traverse the splenium (the posterior part of the corpus callosum), whereas fibers from the frontal lobes traverse the genu and rostrum (Pandya & Seltzer, 1986). Therefore it is possible that abnormalities in the subregions of the corpus callosum could disrupt the functional connectivity among cortical regions in the two hemispheres.
The particular task used for examining brain activation in the current fMRI study, the Tower of London puzzle, is considered a test of executive function. Some of the strongest experimental evidence for executive dysfunction in autism so far involves the Tower of London (TOL), a task requiring planning and goal-management ability. Several investigations using Tower tasks have found significant impairments in autism relative to matched controls (Minshew et al., 2002; Ozonoff et al., 1991; Hughes et al., 1994; Bennetto et al., 1996; Ozonoff & Jensen, 1999; Ozonoff & McEvoy, 1994). The executive processing in the Tower of London task has been shown in normal individuals to evoke prominent activation bilaterally in prefrontal and parietal areas (Newman et al., 2003).
Brain activation during tests of executive function has not been widely investigated in autism (Hill & Frith, 2003). It is not known whether executive function deficits in autism are due to impairment within prefrontal cortex itself or to some other underlying system-wide deficit such as its connectivity to other regions. If there is general reduction in the functional connectivity among the brain regions in autism, as shown in a sentence comprehension task (Just et al., 2004), then one would expect reduced communication and integration among the brain regions to also undermine executive function in TOL.
Our study focused on the interdependence of functionally related brain regions during the performance of a TOL task. The theoretical rationale for this focus is that it is becoming clear that thinking is an emerging property of a large-scale network of collaborating cortical areas. Therefore, to characterize neural functioning in autism, it may be necessary to examine the cortical activation at a systems level rather than at the level of local brain regions (Just et al., 2004). One way to measure the synchronization among brain regions is to compute the correlation or covariance between the activation levels in two activated areas over some time period. This measure generally shows systematic synchronization between areas, modulated by a number of variables. The synchronization is taken as evidence of functional connectivity (Friston, 1994; Horwitz, Rumsey, & Donohue, 1998). The term functional connectivity has been used to describe the interdependence of functionally related brain regions. The synchrony of the blood flow fluctuations in the functionally related brain regions implies the existence of neuronal connections that facilitate coordinated activity. Functional connectivity between two brain regions is assessed as the correlation between pairs of measurements of cerebral blood flow (PET) or blood oxygenation level (fMRI). Castelli et al. (2002) used PET based correlation of activation levels between two regions of interest across the participants in a theory of mind study and predicted that the visual areas may not be properly connected with the cognitive areas in autism. Two older functional imaging studies using coarser-grain measures (e.g. Horwitz et al., 1988; Zilbovicius et al. 1995) implicated lower inter-regional brain connectivity in autism.
In fMRI studies, functional connectivity measurements are based on the correlation of the activation time-series in pairs of brain areas. The time series in this study included an observation every 3 sec (i.e. a TR of 3 sec) while participants were performing the TOL task. The general assumption is that the functioning of voxels whose activation levels rise and fall together is coordinated. The functional connectivity was measured between some of the key areas involved in executive processing, and then was compared between the autism and control participants. The main hypothesis was that there would be a lower level of functional connectivity among the autism participants in the frontal-parietal network.
The functional connectivity in the TOL task, which is known to engage prefrontal and parietal areas bilaterally (Newman et al., 2003), might well depend on the corpus callosum as part of the biological infrastructure that permits communication among brain areas. This study measured the size of the various segments of the corpus callosum of each participant in the functional imaging study, hypothesizing that the sizes of key areas would be smaller in the autistic participants, following similar previous findings in purely morphometric studies (Egaas et al., 1995; Hardan et al., 2000; Piven et al., 1997). Moreover, for the first time, this study tests for a correlation between the size of various corpus callosum segments and frontal-parietal functional connectivity. The secondary hypothesis was that in the participants with autism, there would be a positive correlation, because the size of the corpus callosum is constraining the functional connectivity. In the control group, there should be no correlation because there is no constraint on information processing imposed by the size of their corpus callosum and their neural connectivity. That is, their neural resources and neural connectivity are assumed to always be adequate to meet these task demands.
Methods
Participants
Eighteen high-functioning individuals with autism (mean age 27.1 years, SD = 11.9) and eighteen healthy participants (mean age 24.5 years, SD = 9.9) were included in the study (Full Scale and Verbal IQ scores of 80 or above, as shown in Table 1. The diagnosis of autism was established using the ADI-R (Autism Diagnostic Interview-Revised, Lord et al., 1994), the ADOS-G (Autism Diagnostic Observation Schedule-General, Lord et al., 2000), and confirmed by expert clinical diagnosis. Nine of the participants with autism were taking psychotropic medications. Of these, six were taking only one medication, a serotonin reuptake inhibitor, but not on the day of the scan. All participants were required to be in good medical health. Potential autistic participants were excluded if they had evidence of an associated infectious, genetic, or metabolic disorder, such as fragile-X syndrome or tuberous sclerosis. Potential control and autistic participants were also excluded if found to have evidence of birth asphyxia, head injury, or a seizure disorder. Exclusions were based on neurologic history and examination, physical examination, and chromosomal analysis or metabolic testing if indicated. Written informed consent was obtained from participants and/or their guardians, using procedures approved by the University of Pittsburgh Medical Center Institutional Review Board.
Table 1.
Age, IQ, Handedness and Gender of participants
| Autism | Control | ||
|---|---|---|---|
| Age (years) | Mean ± SD | 27.1 ± 11.9 | 24.5 ± 9.9 |
| VIQ | Mean ± SD | 112.2 ± 17.0 | 107.6 ± 10.9 |
| FSIQ | Mean ± SD | 109.3 ± 17.7 | 108.1 ± 13.8 |
| Handedness | Right : left | 15 : 3 | 16 : 2 |
| Gender | Male : female | 17 : 1 | 15 : 3 |
The control participants were community volunteers recruited to match the autistic participants on age, Full Scale IQ, gender, race, and family of origin socioeconomic status, as measured by the Hollingshead method (Hollingshead, 1957). Potential control participants were screened by questionnaire, telephone, face-to-face interview, and observation during screening psychometric tests such as the Wechsler Abbreviated Scales of Intelligence (WASI) (Wechsler, 1999) and The Wide Range Achievement Test 3 (WRAT3). Family history of developmental and neuropsychiatric disorders was obtained using a questionnaire specifically developed for the CPEA research program. Exclusionary criteria, evaluated through these procedures, included current or past history of psychiatric and neurologic disorders, birth injury, developmental delay, school problems, acquired brain injury, learning disabilities, and medical disorders with implications for the central nervous system or those requiring regular medication usage. Potential control participants were also screened to exclude those with a family history of autism, developmental cognitive disorder, learning disability, affective disorder, anxiety disorder, schizophrenia, obsessive compulsive disorder, or other neurologic or psychiatric disorder thought to have a genetic component. There were no statistically reliable differences between the autistic and control participants in age or IQ. All participants were Caucasian. Twelve of the participants with autism and 9 control participants were previously included in a study of functional connectivity in a sentence comprehension task (Just et al., 2004), and one participant with autism was previously included in a study of functional connectivity in a verbal working memory task (Koshino et al., 2005).
Task
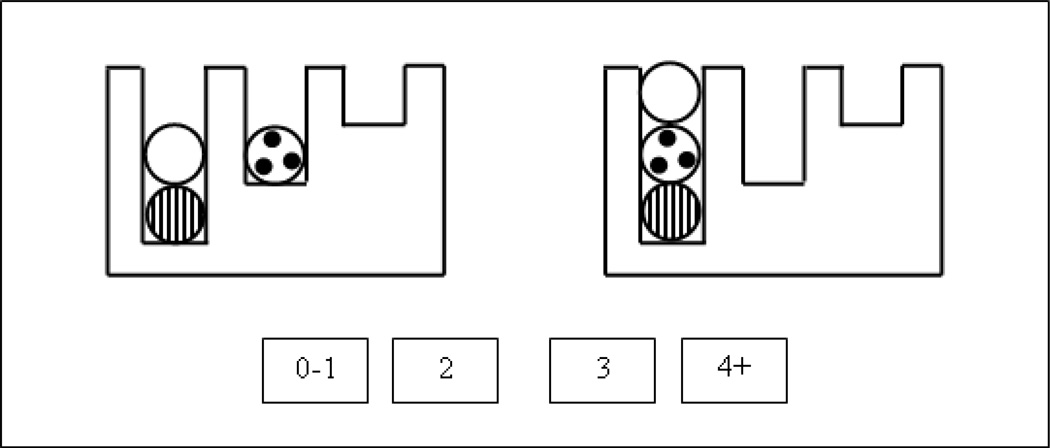
In the TOL task, the subject must rearrange the positions of three distinctive balls in three suspended pool pockets, until they match a specified goal configuration. Although some of the easier problems can be solved with a straightforward perceptual strategy, the harder problems require planning several moves ahead in order to satisfy various goals and subgoals. That is, more difficult problems require more executive processing (Newman et al., 2003). The standard TOL task was modified for use in the scanner, such that the participants did not move any physical ball, but indicated in a forced-choice response how many moves the optimal solution would require.
The left side of the display shows the initial state and the right side shows the goal state, as illustrated in Figure 1. On each trial, the subject is asked to work out how the balls could be rearranged in a sequence of moves such that the configuration on the right comes to be the same as the configuration on the left, in the minimum number of moves. The rules governing the movements of the balls are: only one ball can be moved at a time, a ball cannot be moved out of a pocket if another ball is on top of it, and a ball must be moved to the lowest unoccupied location in the destination pocket. In this example, the first move is to place the white ball in the rightmost pocket, then to move the spotted ball to the left pocket, and finally to move the white ball to the left pocket. Thus the answer to this problem is “3.” The participant indicates the minimum number of moves required, by pressing the appropriate button in the response panel and the next problem is presented. The study was implemented as a “block design” with two experimental conditions. The “easy” condition contained 70% 1-move problems and 30% 2-move problems, whereas the “hard” condition contained 70% 3-move problems and 30% 2-move problems.
Figure 1.
A sample TOL problem, with the start state on the left and the goal state on the right. The participant’s task is to indicate the number of moves required to solve the problem using the response buttons.
Data acquisition and analysis
Each fMRI scanning session consisted of a structural SPGR scan and functional echo-planar scan. The fMRI data were collected using GE Medical Systems 3.0T or 1.5T scanners (University of Pittsburgh Medical Center). An echo-planar pulse sequence with TR = 3000 ms, TE = 25 ms (50 ms at 1.5 T), flip angle = 90°, and a matrix of 128 × 64 (FOV = 40 × 20 cm) was used. Fourteen oblique-axial slices (5-mm thick, 1-mm gap, 3.125 × 3.125-mm in-plane resolution) were imaged. Structural images (124-slice SPGR volume scan with TR = 25 ms, TE = 4 ms, matrix 256 by 256; FOV = 24 × 24 cm, 1.5-mm slice thickness) were taken in the axial plane. Equal numbers of participants from both groups were tested at each field strength, but after preliminary analyses indicated similar results at 1.5 and 3.0T, the data from the two scanners were pooled.
Distribution of activation
To compare the participating groups in terms of the distribution of activation, the data were analyzed using SPM99. Images were corrected for slice acquisition timing, motion-corrected, normalized to the Montreal Neurological Institute (MNI) template, resampled to 2 × 2 × 2 mm voxels, and smoothed with an 8-mm Gaussian kernel to decrease spatial noise. Statistical analysis was performed on individual and group data by using the general linear model and Gaussian random field theory as implemented in SPM99 (Friston et al., 1995). Group analyses were performed using a random-effects model. Preliminary analyses indicated no reliable interaction between the effect of easy versus hard problems (although both groups showed increased activation with increased difficulty), so the data from the two conditions were combined into a single experimental condition that was contrasted with the fixation condition. Additionally, contrasts reflecting the complexity effects for each group, group by complexity interactions, and the group differences in the distribution of activation relative to fixation were computed. For the group differences contrasts, possible differences in deactivation (relative to fixation condition) were excluded. An uncorrected height threshold of p=0.005 and an extent threshold of 6 8-mm3 voxels were used.
Functional connectivity
The functional connectivity was computed (separately for each participant) as a correlation between the average time course of all the activated voxels in each member of a pair of regions of interest (ROIs). Fifteen ROIs were defined to encompass the main clusters of activation in the group activation map for each group in the TOL-Fixation contrast. Labels for these 15 ROIs [the medial frontal gyrus (MedFG), plus 7 bilateral ROIs, namely DLPFC (dorsolateral prefrontal cortex), IFG (inferior frontal gyrus), LG (lingual gyrus), IPS (intraparietal sulcus), PC (precuneus), FFG (fusiform gyrus), & MOG (middle occipital gyrus)] were assigned with reference to the parcellation of the Montreal Neurological Institute (MNI) single subject T1 weighted dataset carried out by Tzourio-Mazoyer and colleagues (Tzourio-Mazoyer et al., 2002). A sphere was defined for each cluster (with a radius from 10 to 12 mm) that best captured the cluster of activation in the map for each group. The ROIs used in the analysis were each the union of the two spheres – one encompassing the activation of the group with autism and the other encompassing the activation of the control group. This common set of 15 ROIs was used for the two groups.
The activation time course for each ROI was extracted separately for each participant, and was based on the normalized and smoothed images, which had been low-pass filtered and had the linear trend removed. Furthermore, the participant’s activation time course was based on only the activated voxels within the ROI. The correlation between the time courses of two ROIs was computed on only the images belonging to the experimental condition and excluded the fixation condition, so it reflects the interaction between the activation in two areas while the subject is performing the task. The analysis of an ROI pair eliminated any participant who had fewer than 23 activated (2 × 2 × 2mm) voxels in one of the ROIs. Fisher’s r to z’ transformation was applied to the correlation coefficients, and these transformed correlations were used in all reported analyses1. To carry out analyses of variance on various pairwise functional connectivities, the connectivity measures were aggregated within each lobe (frontal, parietal, temporal and occipital) and hemisphere (left and right) by averaging each participant’s z’-transformed correlations, resulting in eight large-scale regions and 28 connectivity measures for each participant. These connectivity measures were further categorized in two ways in the analyses of variance. In one analysis, functional connectivities were classified as either involving frontal-parietal connections (left frontal and left parietal, left frontal and right parietal, right frontal and left parietal, and right frontal and right parietal) or involving other possible connections. In a second analysis, frontal-parietal connectivities were further classified as either involving intra-hemispheric or inter-hemispheric connections.
Factor analysis
A factor analysis of the functional connectivities was performed to indicate the groupings of the 15 ROIs into networks based on the similarities of their time courses (Koshino et al., 2005). For each ROI-pair, mean z’-transformed values of the functional connectivity measures were computed across participants for each group. The mean z’-transformed values were then converted back to correlation coefficients, and a correlation matrix was constructed for each group. The functional ROIs in LIFG and RIFG were excluded from factor analysis, because only 50% of control subjects and 44% of subjects with autism showed enough activation (defined as having at least 23 2 × 2 × 2 mm activated voxels) in these areas for estimating the functional connectivity. The resulting connectivity matrices included 13 functional ROIs. An exploratory factor analysis (e.g., McLaughlin et al., 1992; Peterson et al., 1999) was then performed for each group separately. The logic behind the factor analyses was that each factor would correspond to a large-scale network of brain regions executing some high-level function (see Mesulam, 1990, 1998). Factor loadings represent the degree to which each of the ROIs correlates with each of the factors, and ROIs that had factor loadings of 0.4 or greater were taken into consideration in interpretation.
Corpus callosum morphometry
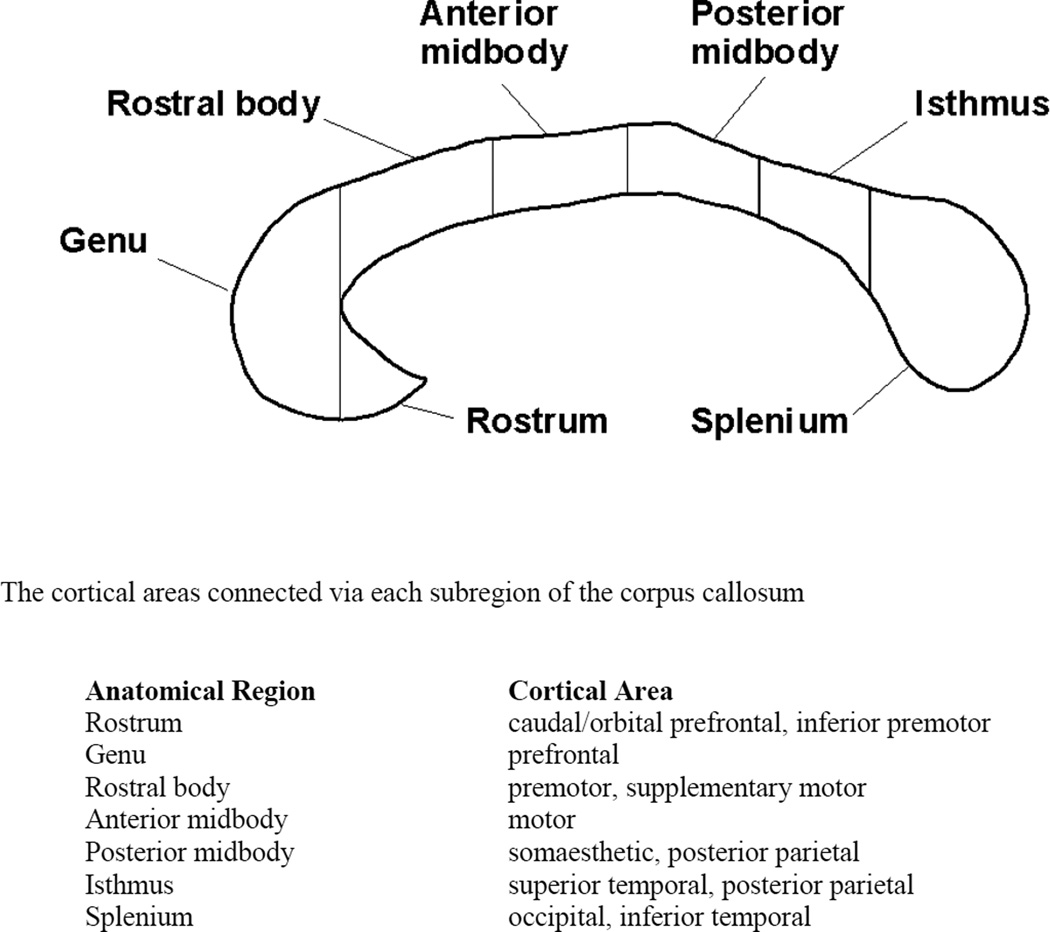
The cross-sectional area of the midsagittal slice of the corpus callosum was segmented (into rostrum, genu, rostral body, anterior midbody, posterior midbody, isthmus, and splenium) using the parcellation scheme described by Witelson (1989), as shown in Figure 2. For each participant, the corpus callosum’s outer contour was first manually traced in the midsagittal plane (with an inter-rater reliability of .87), and then the interior segmentation into the seven areas and the area computations were performed by image processing software. In addition, the gray matter, white matter, and cerebrospinal fluid (CSF) volumes of each participant were measured by segmenting the T1-weighted structural brain image into three masks using SPM2 routines. Because a preliminary analysis revealed a trend toward a larger total brain volumes in the participants with autism [Autism Mean = 1009 mm3, SE = 24 mm3, Control Mean = 946 mm3, SE = 23 mm3, t(17) = 1.92, P=0.072], the corpus callosum area measurements were normalized (divided by) by the total brain volume (exclusive of CSF) for each participant.
Figure 2.
Subdivisions of the midsagittal slice of the human corpus callosum (adapted from Witelson, 1989).
Results
Overview
High functioning individuals with autism showed a distribution of brain activation that was spatially similar to the control participants in most of the brain regions of interest. However, the functional connectivity among brain regions was consistently lower for participants with autism in the frontal-parietal network, a pathway central to the performance of the Tower of London task. In addition, two segments of the corpus callosum were smaller in the autism group (the genu and the splenium). Finally, for the autism group only, the functional connectivity between frontal-parietal activated cortical regions was reliably correlated with the size of the genu of the corpus callosum, which is likely to provide at least some of the anatomical connectivity. In controls, by contrast, there was no such systematic correlation.
Behavioral results
Low error rates in both groups indicated that the participants were able to perform the task proficiently. The percentage of errors was slightly greater in the autism group in both easy (5%) and hard (12%) conditions compared to the control group (2% and 8%, respectively). A 2 (group) × 2 (difficulty) mixed ANOVA showed that neither the main effect of group nor the interaction were significant, although there was a reliable main effect of problem difficulty [F (1, 34) = 26.79, P < .01]. For the response times there was also no significant main effect of group, and there was a reliable main effect of difficulty [F(1, 34) = 68.73, P < . 01] and a reliable group by difficulty interaction [F(1, 34) = 5.56, P < . 05], resulting from the control participants responding faster than those with autism only in the hard condition.
Brain activation
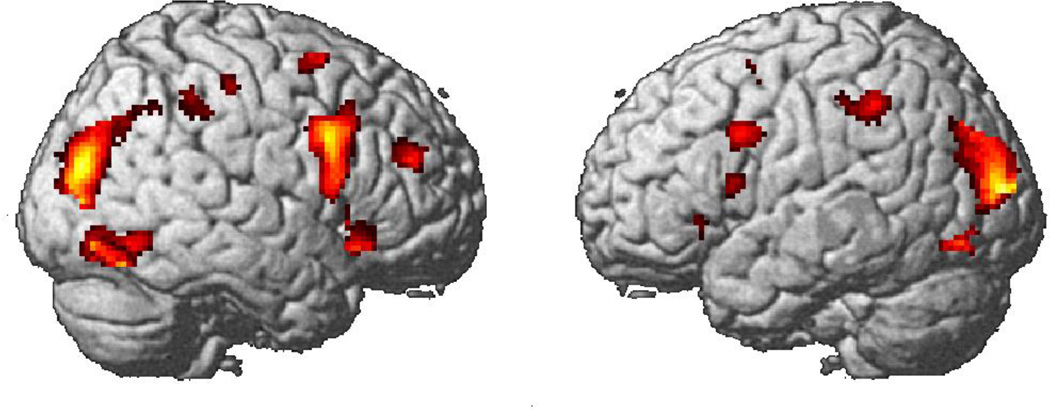
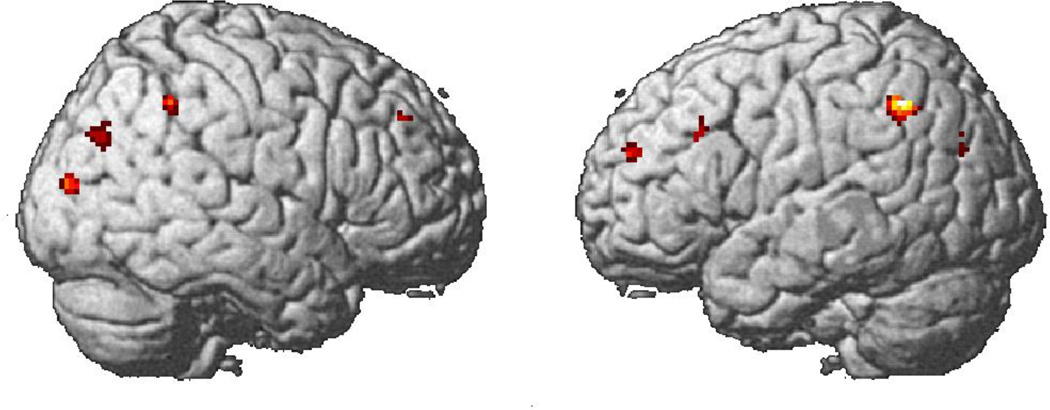
The activation in the group with autism and the control group occurred in similar areas to those reported for normal subjects in previous TOL studies (e.g. Newman et al., 2003). Figure 3 displays the activation for the group with autism (for the contrast between the Tower of London task and the fixation condition). Table 2 contains the results for both groups. The autism group and the control group generally showed similar areas of activation, especially in frontal brain areas such as dorsolateral prefrontal cortex (DLPFC), which was found to play a key role in the Tower of London task in previous studies. Alongside these overall activation similarities, there were also a few group differences. The autism group activated approximately the same parts of the cortex as the control group, but the autism group did so with a greater number of smaller noncontiguous clusters of activation. The statistical subtraction between the two groups revealed only a small number of areas where the controls had reliably greater activation: bilaterally in inferior and superior parietal areas, angular gyri, superior and mid occipital areas, middle frontal gyri, and the right precentral gyrus, superior frontal and the left inferior frontal gyri, as shown in Figure 4. The autism group showed more activation than the control group in left and right hippocampus, thalamus and the left lingual gyrus.
Figure 3.
Activation in the autism group in the Tower of London task (contrast with fixation condition).
Table 2.
Areas of activation for the contrast of the Tower of London task with fixation for the two groups
| Cluster | MNI Coordinates | ||||
|---|---|---|---|---|---|
| Location of peak activation | Size | t(34) | x | y | z |
| Participants with Autism | |||||
| Bilateral lingual, inferior occipital, fusiform, calcarine, and cerebellum; left middle occipital and superior occipital; right inferior temporal |
4285 | 9.34 | −24 | −96 | 10 |
| R superior occipital, middle occipital, and inferior parietal |
990 | 7.88 | 30 | −74 | 24 |
| R postcentral and inferior parietal | 24 | 4.79 | 56 | −24 | 52 |
| R inferior parietal | 85 | 3.81 | 42 | −38 | 40 |
| L inferior parietal | 340 | 6.32 | −38 | −40 | 38 |
| L inferior parietal | 17 | 3.37 | −58 | −30 | 46 |
| Bilateral superior medial frontal, middle cingulate, and supplementary motor |
277 | 5.82 | 6 | 28 | 40 |
| R middle frontal, inferior frontal, and precentral |
753 | 4.82 | 56 | 22 | 38 |
| R middle frontal and inferior frontal | 104 | 4.17 | 48 | 44 | 26 |
| R middle frontal and superior frontal | 74 | 3.85 | 28 | 6 | 60 |
| R insula and inferior orbital frontal | 289 | 6.77 | 34 | 22 | 2 |
| L middle frontal, inferior frontal, and precentral |
135 | 4.02 | −48 | 10 | 32 |
| L middle frontal and superior frontal | 28 | 3.87 | −24 | 0 | 52 |
| L inferior frontal | 41 | 3.45 | −58 | 14 | 10 |
| L insula and inferior frontal | 40 | 3.83 | −34 | 24 | 2 |
| R thalamus, hippocampus, and lingual | 139 | 5.02 | 24 | −24 | −8 |
| R thalamus | 29 | 3.92 | 10 | −14 | 10 |
| L thalamus, hippocampus, and lingual | 111 | 5.75 | −20 | −28 | −2 |
| L thalamus | 7 | 3.31 | −12 | −20 | 10 |
| Normal Control Participants | |||||
| Bilateral lingual, inferior occipital, middle occipital, superior occipital, fusiform, calcarine, cerebellum, and inferior parietal |
11206 | 12.65 | −28 | −74 | 30 |
| Bilateral precuneus | 10 | 3.61 | 2 | −64 | 44 |
| Bilateral cuneus | 16 | 3.28 | 4 | −86 | 32 |
| Bilateral superior medial frontal, middle cingulate and supplementary motor area |
561 | 6.31 | 0 | 14 | 52 |
| R middle frontal, inferior frontal, and precentral |
2146 | 6.98 | 26 | 2 | 52 |
| R insula and inferior frontal | 87 | 4.01 | 32 | 26 | −2 |
| L middle frontal, inferior frontal, and precentral |
925 | 8.86 | −42 | 42 | 32 |
| L middle frontal, superior frontal, and precentral |
206 | 5.52 | −24 | −2 | 54 |
| L middle frontal and superior frontal | 21 | 4.28 | −32 | 64 | 8 |
| L insula and inferior frontal | 84 | 4.24 | −32 | 28 | 0 |
| Bilateral cerebellum and right lingual | 13 | 3.21 | 6 | −42 | 0 |
| R thalamus | 44 | 4.51 | 20 | −28 | 10 |
| L thalamus | 47 | 4.37 | −20 | −30 | 8 |
Notes: The threshold for significant activation was p < .005 for a spatial extent of at least 6 voxels, uncorrected for multiple comparisons. Region labels apply to the entire extent of the cluster.
t-values and MNI coordinates are for the peak activated voxel in each cluster only.
Figure 4.
Group contrast showing areas where control participants have more activation than the autism group in the Tower of London task.
Both the autism and the control group showed the difficulty effect between easy and hard conditions of the Tower of London task (i.e., more activation in the condition where more moves were required). Cortical areas of common activation across groups for the contrast between hard and easy conditions included left and right parietal regions, left and right superior, middle, and inferior frontal regions, and left hemisphere pre-and post-central gyri. In order to assess whether these difficulty effects interacted with group membership, hard vs. easy contrasts between the groups were directly compared in a random effects model. This analysis indicated that only a small cluster of 8 voxels in the right middle occipital gyrus showed a larger difficulty effect in the group with autism [peak F(1,34) = 7.72; P < 0.01]. There were no areas that showed a larger difficulty effect for the control group in this analysis. The two difficulty levels were subsequently collapsed in the remaining analyses.
Functional connectivity
Since the functional connectivity hypothesized to be most affected by autism in the TOL task was between frontal and parietal areas, a 2 (group) by 2 (connection type) mixed ANOVA was conducted with ROI pairs separated into frontal-parietal vs. other. This analysis thus contrasted the mean functional connectivities between frontal and parietal areas (left frontal and left parietal, left frontal and right parietal, right frontal and right parietal, and right frontal and left parietal), with the mean connectivities among all other pairs of regions. This analysis indicated reliably lower functional connectivities in the autism group [F(1,34) = 4.45, P < .05), a reliable main effect of connection type [F(1, 34) = 22.29, P < .0001], and a reliable interaction [F(1, 34) = 6.30, P < .02). Tests of the simple main effect of group within each type of connection showed that the mean frontal-parietal connectivity was lower for the group with autism (Mean = 0.37) than for controls [Mean = 0.51, F(1, 34) = 6.98, P < .02], but there was no reliable group difference for the means of the other connections [Autism Mean = 0.51, Control Mean = 0.55, F(1, 34) = 1.18, P = .28]. These results reveal a functional underconnectivity in the autism group during the performance of the TOL task focused in the frontal-parietal network, the network believed to underpin the planning and problem-solving.
To determine whether autism differentially affected inter-hemispheric versus intra-hemispheric frontal-parietal functional connectivity (particularly in light of the corpus callosum group differences reported below), another 2 (group) by 2 (connection type) mixed ANOVA was conducted, but this time with connections categorized as inter-hemispheric or intra-hemispheric. There was a reliable main effect of group [F(1, 34) = 6.00, P < .02], with the autism group having lower overall frontal-parietal connectivity (Mean = 0.39) than the control group (Mean = 0.52), repeating the main result in a slightly different statistical design. However, there was no suggestion of a group by connection type interaction [F(1, 34) = 0.00], indicating that autism similarly affects inter- and intra-hemispheric functional connectivity in this task. Intra-hemispheric functional connectivities were marginally higher than inter-hemispheric functional connectivities across groups [F(1, 34) = 3.48, P = 0.071)].
The factor analysis yielded another perspective on the functional underconnectivity in autism, by grouping the areas that had similar time courses into separate factors The factor analysis revealed three factors for the autism group (explaining 66% of the variance) but only two factors for the control group (62% of the variance), indicating a lower degree of synchronization among the activation clusters in autism. The striking difference between the two groups was in the connectivity patterns among frontal-parietal areas. In the autism group, the frontal and parietal ROIs were distributed over two Factors (F2 and F3), whereas in the control group, the frontal and parietal ROIs were included in a single factor (F1), as shown in Table 3. (Each group had yet another factor, F1 for autism and F2 for controls that had approximately the same composition of inferior temporal and occipital ROIs for the two groups). In other words, the frontal and parietal ROIs functioned within a single coordinated system in the control group, but within two separate networks for the autism group. Hence, the factor analysis also reflects a lack of integrative connectivity or underconnectivity in the frontal-parietal network in autism.
Table 3.
Results of the factor analysis
| Autism Group | Control Group | ||||
|---|---|---|---|---|---|
| Region | F1 | F2 | F3 | F1 | F2 |
| L dorsolateral prefrontal cortex | . | . | 0.73 | 0.69 | . |
| L fusiform gyrus | 0.72 | . | . | . | 0.69 |
| L intra parietal sulcus | . | 0.66 | 0.42 | 0.79 | . |
| L lingual gyrus | 0.85 | . | . | . | 0.82 |
| L middle occipital gyrus | 0.55 | 0.61 | . | 0.51 | 0.65 |
| L precuneus | . | 0.77 | . | 0.72 | . |
| Medial frontal gyrus | . | . | 0.80 | 0.6 | . |
| R dorsolateral prefrontal cortex | . | . | 0.61 | 0.64 | . |
| R fusiform gyrus | 0.61 | . | . | . | 0.65 |
| R intraparietal sulcus | . | 0.73 | . | 0.77 | . |
| R lingual gyrus | 0.78 | . | . | . | 0.82 |
| R middle occipital gyrus | 0.58 | 0.6 | . | 0.45 | 0.69 |
| R precuneus | . | 0.79 | . | 0.72 | . |
| F1: inferior temporal and occipital bilaterally |
F1: frontal, parietal, and occipital bilaterally |
||||
| F2: parietal and occipital bilaterally |
F2: inferior temporal and occipital bilaterally |
||||
| F3: frontal bilaterally and left IPS |
|||||
Further data explorations of the functional connectivity group differences
The lower functional connectivity in autism in the frontal-parietal connections could be due to several characteristics of the data, and several hypotheses concerning such differences were investigated in the data, but rejected. For example, the time courses were not more variable in autism. More generally, detailed quantitative comparisons of the activation time courses for left dorsolateral prefrontal cortex and the right and left precuneus revealed very similar patterns across the regions for the two groups. Nor was there any indication of there being a phase shift (delayed correlation) of one of the time courses in autism. (Additional functional connectivity measures computed for these regions with positive or negative lags between the regions showed that the correlations between regions were highest for both groups with no lag, and that the correlations decreased similarly and monotonically for both groups as the lag was increased). Furthermore, there was no evidence that low versus high frequency components of the time course contributed differentially to the group difference in functional connectivity. (To test the hypothesis, the time course data were temporally filtered with a Gaussian low-pass filter (FWHM = 3 s) or high-pass filter (FWHM = 10 s), and the resulting connectivity measures showed that both the low- and high-frequency components of the time courses contributed to the difference in connectivity between the groups). Similar analyses into the basis for the underconnectivity were performed on another task in which the imaging data were acquired at a higher temporal resolution and hence provided more detail about the time course (TR = 1 s rather than the present TR = 3 s) and again similar results were obtained. The current analyses indicate that the decreased synchronization of activation between frontal and parietal areas is not due to some abnormality in the time course of the activation of either area, but in the time courses being less coordinated between regions.
There were no differences in the activation between autistic participants on medication and those not on medication in this sample nor in our previous published fMRI studies of functional connectivity in different subject samples. From a theoretical perspective, functional connectivity likely relates to the quality of structural connections as well as the capacity to dynamically bring different systems online to address task demands. The effect of medications that reduce anxiety and enhance cognitive function, if they impact functional connectivity at all, might be expected to improve functional connectivity rather than reduce it. The medications would not be expected to impact structural connectivity.
Corpus callosum size
The normalized size of the seven midsagittal subregions of the corpus callosum was compared between the two groups in a 2 (group) by 7 (segment) ANOVA. This analysis revealed a marginal main effect of group [F(1, 34) = 4.05, P < .1], with a smaller mean segment size in autism (Mean = 0.089, SE = 0.003)] than in the control group (Mean = 0.100, SE = 0.003), and a reliable group by segment interaction [F(6, 204) = 2.55, P < .05]. (There was also a main effect of segment, [F(6, 204) = 425.05, P < .0001]). Tests of the simple main effect of group within each segment indicated that the genu (the most anterior region) and the splenium (the most posterior region) were reliably smaller in the autism group than in the control group, as shown in Table 4. Genu fibers are presumed to connect prefrontal cortical areas (Witelson, 1989) and hence are likely to be involved in frontal-parietal anatomical connectivity. These anatomical results are in general agreement with previous studies (Hardan et al., 2000; Manes et al., 1999; Piven et al., 1997; Saitoh et al., 1995; Egaas et al., 1995), but here the anatomical connection differences occur in the context of reduced functional connectivity between the relevant cortical regions in the autism group.
Table 4.
Areas of the midsagittal slice of the corpus callosum normalized by the total gray plus white matter volume
| Corpus Callosum Midsagittal Slice Area |
Autism group |
Control group |
F(1, 134) |
|---|---|---|---|
| Rostrum | 0.03 | 0.02 | 0.05 |
| Genu | 0.12 | 0.14 | 5.19* |
| Rostral body | 0.10 | 0.12 | 3.62 |
| Anterior midbody | 0.08 | 0.08 | 0.09 |
| Posterior midbody | 0.07 | 0.08 | 0.60 |
| Isthmus | 0.05 | 0.06 | 0.87 |
| Splenium | 0.19 | 0.21 | 12.12* |
Notes: F-values are for tests of the simple main effect of group. Denominator degrees of freedom are adjusted using Satterthwaite’s approximation.
P < 0.05.
Relation between functional connectivity and corpus callosum size
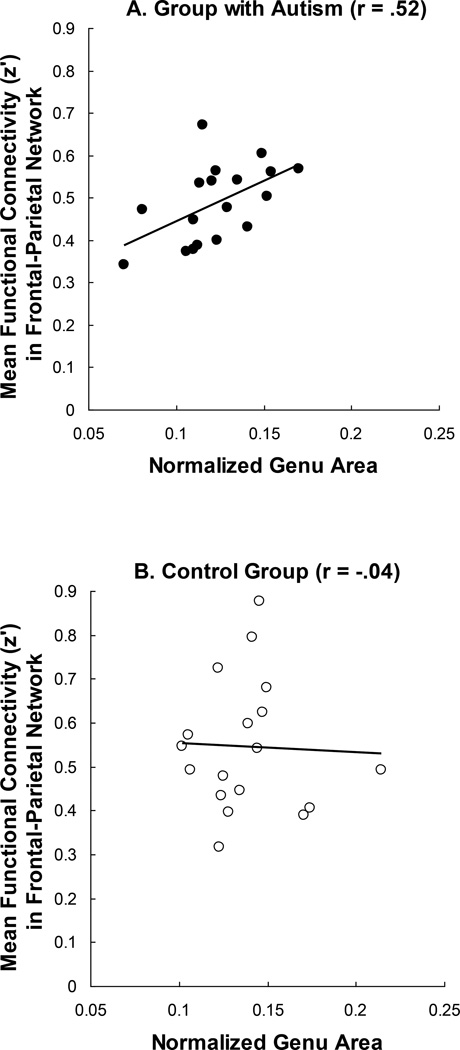
The results above establish that the group with autism had lower functional connectivity between frontal and parietal areas and also smaller corpus callosum areas. If the size of the corpus callosum imposes a constraint or upper bound on the functional connectivity between regions, one might predict that the autism group’s (lower) functional connectivity measures in frontal-parietal ROI pairs would be positively correlated with their (smaller) genu sizes. There in fact was a reliable positive correlation between the frontal-parietal connectivity and the size of the genu in the group with autism [r = .52, t(16) = 2.47, P < .02, one-tailed test], as shown in Figure 5. In contrast, among control participants there was no relationship between these measures, consistent with the idea that the size of the corpus callosum does not constrain their functional connectivity. Furthermore there was a reliable difference between the correlations in the two groups (z = 2.66, P < .01). The pattern of correlations is suggestive of a constraint on functional connectivity in autism imposed by some anatomical property of the corpus callosum.
Figure 5.
Correlation between the midsagittal area of the genu portion of the corpus callosum and the mean functional connectivity between frontal and parietal areas for the Autism group (A) and the Control group (B).
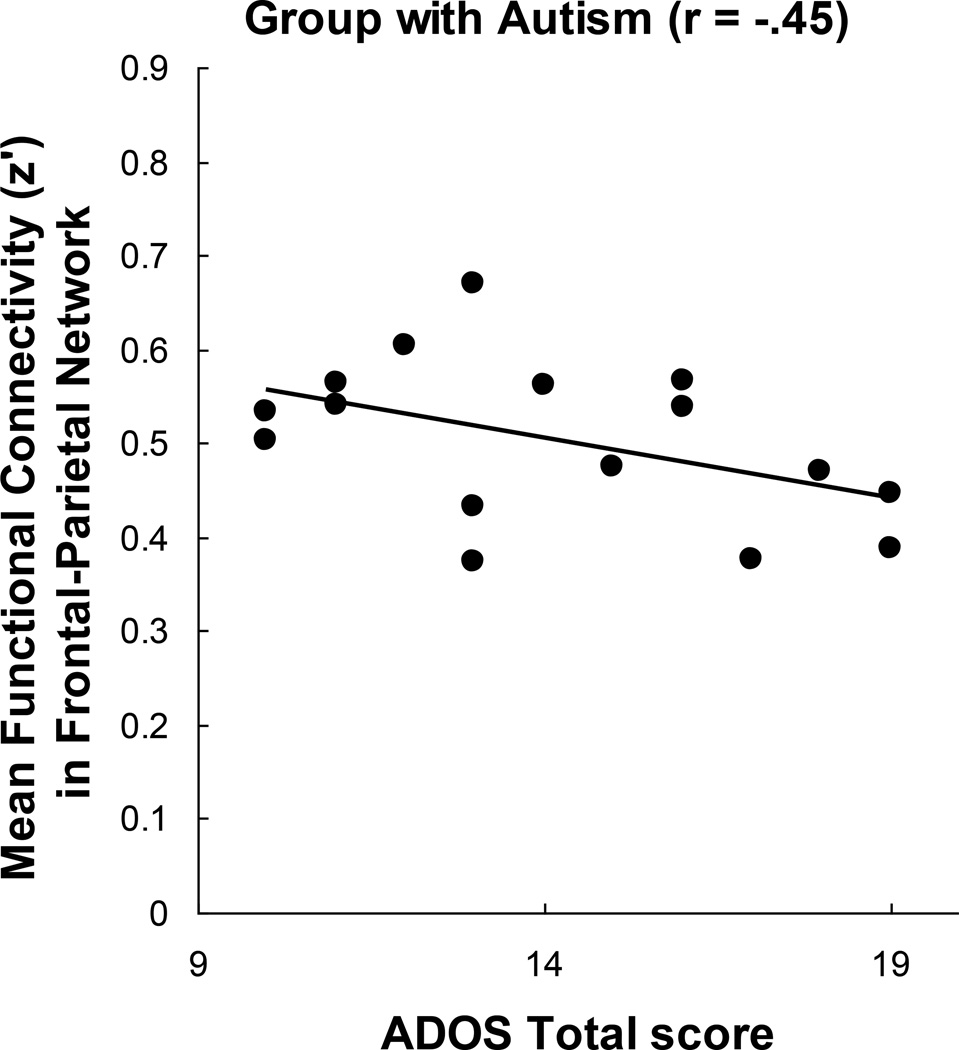
Relation between functional connectivity and ADOS scores
If the decreased frontal-parietal connectivity in the group with autism is related to the severity of autism, one would predict a negative relationship between this measure and ADOS scores. Frontal-parietal functional connectivity was indeed negatively correlated with total ADOS scores among the participants with autism as expected [r = −.45, t(14) = −1.91, P < .05, one-tailed test], as shown in Figure 6. (Two of the participants with autism whose ADOS-based diagnosis was obtained from a different site were excluded from the analysis because their precise ADOS scores could not be obtained). The correlation indicates that autistic participants with lower frontal-parietal functional connectivity scores tend to have higher ADOS scores. Although ADOS scores are not intended to provide a measure of the severity of autism nor to be used as a psychometric measure, it is intriguing to consider that a brain activation measure of functional connectivity may be related to the best current research-based measure of autism.
Figure 6.
Correlation between the ADOS total score and the functional connectivity in the frontal-parietal network
Discussion
The central contributions of this study were to 1. document new evidence of functional underconnectivity in autism between frontal and parietal areas in an executive processing task; 2. replicate previous findings of anatomically smaller corpus callosum sizes in autism; and 3. establish a relation between the functional and anatomical connectivity measures. These findings add support to the cortical underconnectivity theory of autism first proposed on the basis of similar evidence observed in an fMRI study of sentence comprehension (Just et al., 2004) and more recently extended on the basis of results from a verbal working memory task (Koshino et al., 2005). The frontal-parietal underconnectivity observed during the TOL task is provocative because a frontal-parietal network has been found to be involved not only in Tower of London problem-solving (Newman et al., 2003), but in many other executive function tasks (Schneider, 1999). Thus, executive dysfunction in autism, which has been observed in a number of behavioral studies (Ozonoff et al., 1991; Hughes & Russell, 1993; Hughes et al., 1994), might be the result of frontal-parietal underconnectivity. In this new perspective, executive dysfunction is just one of many possible consequences of cortical underconnectivity.
The lower functional connectivity in autism in an executive function task suggests that the communication between certain cortical areas is less effective in autism, affecting how the cortically distributed components of thinking are coordinated. The lowered functional connectivity can be thought of as a reduced inter-area communication bandwidth. An underconnected system would be particularly disruptive to those complex or higher order psychological functions with a heavy dependence on the coordination of brain regions, such as social, language and problem solving functions. These and other complex psychological functions require the concurrent coordination of many different types of information processing, explaining why symptomatic disruptions of such divergent psychological functions might co-occur in autism to form a syndrome. Underconnectivity theory is consistent with the broad yet circumscribed range of disruption of cognitive and social functioning in autism. Deficits in theory of mind (Baron-Cohen et al., 1985), face processing (Schultz et al., 2000; Pierce et al., 2001; Critchley et al., 2000), executive function (Ozonoff et al., 1991; Hughes & Russell, 1993; Hughes et al., 1994), language (Harris et al., in press; Just et al., 2004) and other seemingly unrelated deficits could all be the result of a deficit in integrating types of information processing. Even postural deficits have been reported in autism (Minshew et al, 2004). Autism appears to be a neural systems disorder, and underconnectivity theory provides a framework for the accumulating empirical evidence concerning the nature of the disorder.
The newly discovered relationship between the lower functional connectivity and the reduced size of the genu in the group with autism opens new avenues of investigation. The smaller the genu was, the lower was the functional connectivity between the frontal and parietal regions. This correlation in the autism group may reflect a constraint on the functional connectivity imposed by anatomical properties of the corpus callosum. We interpret the reduced corpus callosum sizes in autism as an index of white matter abnormality, whose nature and impact are not currently understood. In control participants, by contrast, there was no such correlation, and presumably no such constraint. Note that although the mean sizes of the genu and splenium tend to be smaller in autism, there is considerable overlap in the distributions of the two groups’ regional measurements that probably fails to reflect larger underlying differences in microstructure and function.
The results suggest that abnormalities in major interhemispheric tracts such as the corpus callosum may contribute to diminished functional connectivity patterns in autism (Quigley et al., 2001). The majority of callosal fibers are thought to originate from association cortices and subserve higher order functions (Innocenti, 1986; Pandya and Seltzer, 1986). A smaller corpus callosum in autism might reflect lower interhemispheric connectivity. Recent findings also indicate intrahemispheric white matter abnormalities in autism (see Chung et al., 2004; Herbert et al., 2003, 2004; Carper et al., 2002; Courchesne et al., 2001). Note that the white matter abnormalities in autism include not only smaller white matter volumes in some regions but also dysregulation and larger white matter volumes in other regions (Herbert et al., 2002; Courchesne et al., 2001). Moreover, our measure of white matter volume in the present study provided an index only of interhemispheric anatomical connectivity. (The results of the functional connectivity analyses provided no evidence that interhemispheric temporal synchronization of activation was more affected in autism than intrahemispheric synchronization.) It is important to keep in mind that it is not known how the white matter volume abnormalities in autism are related to the functioning of the white matter. Converging results from white matter analyses and from functional imaging results may establish the relation between white matter volume abnormalities and functional abnormalities. Our laboratory is currently collecting functional connectivity and diffusion-tensor imaging data on the same participants with a goal of providing a converging measure of the relationship between functional connectivity and intrahemispheric and interhemispheric anatomical connectivity.
How general is the underconnectivity?
The newly-reported cortical underconnectivity in executive processing raises the question of how general the underconnectivity in autism might be. Does it occur in all tasks? Does it affect all pairs of regions? Does it occur in other special populations?
First consider the generality over tasks. Functional underconnectivity has previously been observed in autism using fMRI in a sentence comprehension task (Just et al., 2004) and in a letter n-back working memory task (Koshino et al., 2005). Functional underconnectivity in autism (between occipital and temporo-parietal regions) was also reported in a PET study (and hence measured at a more molar level) in a mental state attribution (Theory of Mind) task (Castelli et al., 2002). In sensory tasks, electrophysiological studies have reported functional underconnectivity in autism (although with a measurement in a different time scale) among association areas but normal functional connectivity among sensory areas (Mottron et al., 2001; Mottron et al., 2003). The new TOL results demonstrate that reduced functional connectivity occurs in executive function tasks. There is thus a convergence of findings based on tasks involving reasoning, language and social judgment, all the major symptom domains that define the syndrome of autism, supporting the idea of functional underconnectivity as a general characteristic of the neurobiology of neural systems in autism.
Second, consider whether the lower functional connectivity in autism applies to all pairs of cortical areas. The group difference in functional connectivity in the TOL was reliable only in the frontal-parietal network, namely the network that constitutes the main neural underpinning of cognitive functioning in this task. The functional connectivity was lower in the autism group in other networks as well (such as the frontal-temporal and temporal-occipital networks, where the difference between groups in functional connectivity was marginally reliable). However, frontal-parietal networks are not necessarily the manifestation of underconnectivity in autism in other tasks. In some language tasks, the greatest degree of underconnectivity may occur in a frontal-temporal network. The generalization concerning localization to date is that underconnectivity affects connections between the association areas that are most activated in a task, particularly affecting connectivity with frontal areas. Further fMRI studies of a variety of tasks will determine how cortical underconnectivity is localized. It is important to keep in mind that functional connectivity is a dynamic property in which different regions become activated and coordinated on an as-needed basis, depending on the task.
Third, it is interesting that autism is not the only disorder in which disconnection among brain areas has been observed or proposed. For example, Lawrie et al. (2002) proposed a disconnection syndrome in schizophrenia. The symptoms of autism show considerable overlap with the negative symptoms of schizophrenia, suggesting corresponding overlap in the neural systems disruptions. (Not surprisingly, the term “autism” was borrowed from the schizophrenia literature and for decades autism was classified as a childhood psychosis, despite the absence of psychotic symptoms). It should not be surprising if a complex system like the brain consisting of interacting subsystems could be susceptible to disruption of the inter-subsystem communication in more than one way. The lowered functional connectivity could vary in different pathologies, such as being limited to affecting the connections between particular brain regions (as has been proposed for example, for dyslexia). It would be particularly interesting to examine functional connectivity in participants with complete or partial agenesis of the corpus callosum but who nevertheless show relatively unimpaired cognitive functioning, to determine how callosal absence affects inter-hemispheric functional connectivity and presumably, the degree of coordination between hemispheres. Underconnectivity could be a part of several syndromes.
Previous theories
The underconnectivity theory has a straightforward relation to predecessor approaches to autism that pointed in a similar direction. Major cognitive theories in autism such as the complex information processing theory (Minshew et al., 1997) and the weak central coherence theory (Frith, 1989) suggest the possibility of underdeveloped connections in the brain in autism. The information processing theory focused on autism as a disorder of processing complex information. This approach attributed the disorder to a fundamental abnormality in the handling of information in high level tasks, particularly those requiring abstraction. Moreover, Minshew and Goldstein (1998) proposed that autism was a non-focal, systemic disorder of the brain, a distributed neural systems disorder. Underconnectivity theory enriches Minshew’s previous theory with the new findings from fMRI, linking the information processing abnormalities to a specific neurobiologic phenomenon, the brain connectivity itself. Frith’s (1989) theory of weak central coherence deals with a tendency to focus on details at the expense of configural information, which has been proposed as a cognitive style in autism. According to this view, autistic individuals fail in integrated representation. This is consistent with the underconnectivity approach. In more recent work with her colleagues, particularly in neuroimaging research, Frith has attempted to apply the concept of weak central coherence to the brain activity level. Hill and Frith (2003) mentioned that the central coherence account referred to poor connectivity throughout the brain between more basic perceptual processes and top-down modulating processes, perhaps due to failure of pruning. Underconnectivity theory specifies a particular underlying biological mechanism and goes on to predict similar impairments in motor functions, memory, and expressive nonverbal language, and to virtually all cortically mediated functions.
In summary, normal brain function has been construed here as a collaboration of a confederation of processing centers. The new fMRI and MRI findings suggest that in autism, the confederation is loosened or underfunctioning. In this study, the underconnectivity theory is extended to new levels by linking it with white matter abnormalities. The new theory frames a number of research questions about the scope and nature of the underconnectivity in autism, which await investigation with converging methods.
Acknowledgements
This research was supported by the University of Pittsburgh-Carnegie Mellon Collaborative Program of Excellence in Autism (CPEA), Grant U19-HD35469 from the National Institute of Child Health and Human Development. We thank Dr. Diane Williams for her helpful comments on the manuscript. We would also like to express our appreciation to the individuals and families who gave generously of their time and courage to participate in these imaging studies.
Abbreviations
- fMRI
functional Magnetic Resonance Imaging
- L DLPFC
Left Dorsolateral Prefrontal Cortex
- ROI
Region of Interest
- TOL
Tower of London
Footnotes
- z’ = (0.5) ln (1+r/1−r)
References
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Bio Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a "theory of mind"? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, Rogers SJ. Intact and impaired memory functions in autism. Child Dev. 1996;67:1816–1835. [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. NeuroImage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger Syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Chung MK, Dalton KM, Alexander AL, Davisdon RJ. Less white matter concentration in autism: A 2D voxel-based morphometry. NeuroImage. 2004;23:242–251. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Second Edition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- Courchesne E, Karns CM, Davis HR, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, et al. The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. Reduced size of the corpus callosum in autism. Arch Neurol. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- Fisher RA. On the probable error of a coefficient of correlation deduced from a small sample. Metron. 1921;1:3–32. [Google Scholar]
- Friston K, Ashburner J, Frith C, Poline J-B, Heather J, Frackowiak R. Spatial registration and normalization of images. Hum Brain Map. 1995;2:165–189. [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Hum Brain Map. 1994;2:56–78. [Google Scholar]
- Frith U. Autism: Explaining the enigma. Oxford, UK: Blackwell; 1989. [Google Scholar]
- Goldstein G, Williams D, Minshew NJ. A consideration of complex cognitive abilities in high functioning autism. Manuscript submitted for publication. 2004 [Google Scholar]
- Hardan AY, Minshew NJ, Keshavan MS. Corpus callosum size in autism. Neurology. 2000;55:1033–1036. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, McGrath L, Condouris K, Tager-Flusberg H. Brain and Cognition. In press. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, et al. Abnormal asymmetry in language association cortex in autism. Annals Neurol. 2002;52:588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, et al. Dissociation of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, et al. Localization of white matter volume increase in autism and developmental language disorder. Annals Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hill EL, Frith U. Understanding autism: Insights from mind and brain. Phil Trans Royal Soc London. 2003;358:281–289. doi: 10.1098/rstb.2002.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. New Haven, CT: Yale University Department of Sociology; 1957. [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci USA. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism: Intercorrelations of regional glucose utilization. Arch Neurol. 1988;45:749–755. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J. Autistic children’s difficulty with mental disengagement from an object: Its implications for theories of autism. Dev Psychol. 1993;29:498–510. [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32:477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Innocenti GM. General organization of callosal connections. In: Jones EG, Peters A, editors. Cereb Cortex. New York: Plenum Press; 1986. [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky V, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Bio Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Lord C, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luna B, Minshew NJ, Garver KE, et al. Neocortical system abnormalities in autism. Neurology. 2002;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- Manes F, Piven J, Vrancic D, Nanclares V, Plebst C, Starkstein SE. An MRI study of the corpus callosum and cerebellum in mentally retarded autistic individuals. J Neuropsychiatry Clin Neurosci. 1999;11:470–474. doi: 10.1176/jnp.11.4.470. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Steinberg B, Christensen B, Law I, Parving A, Friberg L. Potential language and attentional networks revealed through factor analysis of rCBF data measured with SPECT. J. Cereb Blood Flow Metab. 1992;12:535–545. doi: 10.1038/jcbfm.1992.77. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Large-scale neurocognitive networks and distributed processing for attention, language and memory. Annals Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G. Autism as a disorder of complex information processing. Ment Retard Dev Disabil Res Rev. 1998;4:129–136. [Google Scholar]
- Minshew NJ, Goldstein G, Siegel D. Neuropsychologic functioning in autism: Profile of a complex information processing disorder. J Internat Neuropsychological Soc. 1997;3:303–316. [PubMed] [Google Scholar]
- Minshew NJ, Meyer J, Goldstein G. Abstract reasoning in autism: A dissociation between concept formation and concept identification. Neuropsychology. 2002;16:327–334. doi: 10.1037//0894-4105.16.3.327. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63:2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Mottron L, Burack JA. Enhanced perceptual functioning in the development of autism. In: Burack JA, Charman T, Yirmiya N, Zelazo P, editors. The development of autism: Perspectives from theory and research. Mahwah, NJ: Erlbaum; 2001. pp. 131–148. [Google Scholar]
- Mottron L, Burack J, Iarocci G, Belleville S, Enns J. Locally oriented perception with intact global processing among adolescents with high-functioning autism: evidence from multiple paradigms. J Child Psychology and Psychiatry. 2003;44:904–913. doi: 10.1111/1469-7610.00174. [DOI] [PubMed] [Google Scholar]
- Newman SD, Carpenter PA, Varma S, Just MA. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. 2003;41:1668–1682. doi: 10.1016/s0028-3932(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Jensen J. Specific executive function profiles in three neurodevelopmental disord ers. J Autism and Dev Disorders. 1999;29:171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, McEvoy RE. A longitudinal study of executive function and theory of mind: Development in autism. Dev Psychopathology. 1994;6:415–431. [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high-functioning autistic individuals: Relationship to theory of mind. J Child Psychology and Psychiatry. 1991;32:1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. The topography of commissioned fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two hemispheres--one brain: Functions of the corpus callosum. New York: Liss; 1986. pp. 47–73. [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: Evidence for cingulate subregions subserving multiple distributed attentional systems. Soc Biol Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller R-A, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: Evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Piven J, Bailey J, Ranson BJ, Arndt S. An MRI study of the corpus callosum in autism. Am J Psychiatry. 1997;154:1051–1056. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- Quigley M, Cordes D, Wendt G, et al. Effect of focal and nonfocal cerebral lesions on functional connectivity studied with MR imaging. Am J Neuroradiology. 2001;22:294–300. [PMC free article] [PubMed] [Google Scholar]
- Saitoh O, Courchesne E, Egaas B, Lincoln AJ, Schreibman L. Cross-sectional area of the posterior hippocampus in autistic patients with cerebellar and corpus callosum abnormalities. Neurology. 1995;45:317–324. doi: 10.1212/wnl.45.2.317. [DOI] [PubMed] [Google Scholar]
- Schneider W. Working memory in a multi-level hybrid connnectionist control architectue (CAPS) In: Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. Cambridge, MA: Cambridge University Press; 1999. pp. 340–374. [Google Scholar]
- Schultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger Syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vidal CN, De Vito T, Hayashi KM, Drost DJ, Williamson PC, Craven-Thuss B, Herman D, Sui Y, Toga AW, Nicolson R, Thompson PM. Mapping corpus callosum deficits in autistic children using novel computational anatomy algorithms. Proceedings of the Human Brain Mapping Conference; June 2003; New York, NY. 2003. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scales of Intelligence (WASI) San Antonio TX: Psychological Corporation; 1999. [Google Scholar]
- Wilkinson WS. The Wide Range Achievement Test 3 (WRAT3) Wilmington, DE: Wide Range; 1993. [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum: A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Garreau B, Samson Y, et al. Delayed maturation of the frontal cortex in childhood autism. Am J. Psychiatry. 1995;152:248–252. doi: 10.1176/ajp.152.2.248. [DOI] [PubMed] [Google Scholar]