Abstract
Comprehending high-imagery sentences like The number eight when rotated 90 degrees looks like a pair of eyeglasses involves the participation and integration of several cortical regions. The linguistic content must be processed to determine what is to be mentally imaged, and then the mental image must be evaluated and related to the sentence. A theory of cortical underconnectivity in autism predicts that the interregional collaboration required between linguistic and imaginal processing in this task would be underserved in autism. This functional MRI study examined brain activation in 12 participants with autism and 13 age- and IQ-matched control participants while they processed sentences with either high- or low-imagery content. The analysis of functional connectivity among cortical regions showed that the language and spatial centres in the participants with autism were not as well synchronized as in controls. In addition to the functional connectivity differences, there was also a group difference in activation. In the processing of low-imagery sentences (e.g. Addition, subtraction and multiplication are all math skills), the use of imagery is not essential to comprehension. Nevertheless, the autism group activated parietal and occipital brain regions associated with imagery for comprehending both the low and high-imagery sentences, suggesting that they were using mental imagery in both conditions. In contrast, the control group showed imagery-related activation primarily in the high-imagery condition. The findings provide further evidence of underintegration of language and imagery in autism (and hence expand the understanding of underconnectivity) but also show that people with autism are more reliant on visualization to support language comprehension.
Keywords: autism, cortical connectivity, high imagery, low imagery, functional MRI
Introduction
Language comprehension and visual imagery are two separable facets of cognition, but in everyday thinking, they are used concurrently rather often, particularly when language makes reference to spatial or physical objects. The combination of sentence comprehension and visual imagery processes seems a particularly apt object of investigation in the context of autism. For example, to evaluate a sentence like The number eight when rotated 90 degrees looks like a pair of spectacles, a reader must retrieve a mental image of a figure eight, mentally apply a rotation transformation to it, and then evaluate the resulting image. Several recent studies have suggested that there may be an underconnectivity among cortical areas in autism (Just et al., 2004a; Koshino et al., 2005), which could negatively impact or slow integration or communication among cortical regions involved in language and imagery processing. In addition, neuroimaging findings indicate a tendency in autism to use visual–spatial regions to compensate for higher-order cortical regions (Koshino et al., 2005).
The visuospatial system has traditionally been viewed as an area of intact if not enhanced ability in autism until relatively recently. This view has been based on the relatively high scores on the Block Design subtest of the Wechsler Intelligence Scales attained by people with autism (Shah and Frith, 1993; Siegel et al., 1996; Goldstein et al., 2001), their good performance on the embedded figures task (Joliffe and Baron-Cohen, 1997; Happe, 1999) and on the Raven’s Progressive Matrices (Dawson et al., 2005). Also, some studies have suggested that there is enhanced visuospatial processing in autism in domains such as language. For instance, in a semantic priming study, Kamio and Toichi (2000) found that people with autism performed better on a picture–word completion task than on a word–word completion task, suggesting an advantage of pictures over words in access to semantics in autism. Another acknowledgement of the important role of visual thinking in autism comes from treatment methods in common use that are based on picture exchange communication (Bondy and Frost, 1998, 2001) or visual organizers such as the TEACCH method (Schopler and Olley, 1982) according to which the use of pictures increases comprehension by eliminating reliance on abstract words and concepts. However, there have also been contrary reports of impairments on visuospatial tests involving complex stimuli like faces (e.g. Deruelle et al., 2004), and second-degree motion processing (Chubb and Sperling, 1988; Cavanagh and Mather, 1989; Bertone and Faubert, 1999, 2003; Bertone et al., 2005).
Anecdotal accounts of experiences of people with autism, such as the eloquent descriptions provided by Dr Temple Grandin in her autobiography Thinking in Pictures (1995), support the centrality of visual thinking. The opening words of this book are as follows:
I think in pictures. Words are like a second language to me. I translate both spoken and written words into full-color movies, complete with sound, which run like a VCR tape in my head. When somebody speaks to me, his words are instantly translated into pictures. Language-based thinkers often find this phenomenon difficult to understand, but in my job as an equipment designer for the livestock industry, visual thinking is a tremendous advantage.
In contrast to the relative integrity of the visual–spatial system, the language-processing system in autism exhibits obvious impairment. For example, people with high-functioning autism have more difficulty in following complex instructions (Minshew and Goldstein, 1998) or comprehending idioms (Dennis et al., 2001a), metaphors (Happe, 1993; Dennis et al., 2001b) and connected discourse (Tager-Flusberg, 1995). According to Tager-Flusberg (1996), when language is acquired in autism, verbal communication continues to be primarily limited to the expression of instrumental functions, or simple labelling. Results of psychometric tests such as the Detroit Test of Oral Directions suggest that the language deficit in high-functioning individuals with autism occurs primarily when higher order or emergent skills are required (Goldstein et al., 1994). A recent neuroimaging study of sentence comprehension (Just et al., 2004a) found that individuals with high-functioning autism exhibited lower levels of activation in Broca’s area (relative to controls) and higher levels of activation in Wernicke’s area. This different pattern was interpreted as a lesser reliance on integrative (syntactic and thematic) processing in autism, and a greater reliance on word-oriented (lexical) processing. This study also found a lower degree of synchronization of the time course of activation among the participating cortical areas in high-functioning autism compared with controls.
Electrophysiological findings also provide evidence for abnormal processing of language in autism. Selective attention to the meaning of words was impaired (less responsiveness to the N4 component) by semantic context in people with autism (Dunn et al., 1999). People with Asperger syndrome showed lower mismatch negativities and longer latencies of the negativities in a speech prosody discrimination task (Kujala et al., 2005). Overall, evidence from neuroimaging and electrophysiological studies suggests that there is good reason to think that language processing in autism is different from controls.
When a task is amenable to either a visual or a verbal strategy, there is a suggestion that people with autism prefer a visual strategy. There are many informal reports that individuals with autism are predominantly visual thinkers (Grandin, 1995). In an fMRI letter n-back study, Koshino et al. (2005) found evidence (based on activation locations) of more visual coding of letters in autism compared with verbal coding strategy in the controls. Similar results were also found in an fMRI facial working memory task (H Koshino, RK Kana, TA Keller, VL Cherkassky, NJ Minshew, MA Just, under review). Whitehouse et al. (in press, 2006) found that children with autism had very limited use of inner speech and used a visuospatial strategy in a switching task that involved articulatory suppression. These studies indicate that there is a tendency in people with autism to use more visuospatial processing by recruiting posterior brain regions in accomplishing even language tasks. It has been suggested that autistic artists may think in visual–spatial ways, which might compensate for their language deficits (O’Connor and Hermelin, 1987; Hermelin and O’Connor, 1990).
The present study focused on the interplay between language and visuospatial systems. This study examined the performance of high-functioning individuals with autism in a task that requires the integration of two different domains of cognitive processing, the visuospatial (which sometimes is unimpaired, as described in the studies above) and the complex language-processing systems (which tend to be impaired). The participants had to judge sentences with mental imagery content as true or false (e.g. The number six can be rotated to make the number nine). Since the information about the content of the imagery is in the form of sentences, participants had to coordinate the language and visuospatial systems in accomplishing this task. At the neural level, brain regions underlying the visuospatial and language functions have to collaborate to accomplish this task. Since this task requires the integration of two somewhat separable neural systems, we hypothesized that high-functioning individuals with autism would show underconnectivity between these neural systems. It is evident from our previous studies (Just et al., 2004a; Koshino et al., 2005) that the underconnectivity (lower level of synchronization) in the autism group is largest (most discrepant from the control group) in the areas that perform the central and most demanding computation for the task at hand. In the visual imagery comprehension task, the prediction was that the underconnectivity would be most apparent in synchronizations between the frontal and parietal regions that are required in the integration of language and spatial processing (Just et al., 2004b).
Another prediction in this study concerned the way people with autism approach this task. In the low-imagery condition in the present study, the participants with autism might use more visual and spatial imagery to make sense of the sentences. As a result of this, there might be more activation in the autism group in regions such as parietal or occipital during the processing of low-imagery sentences. On the other hand, control participants should process the low-imagery sentences by recruiting more language areas and fewer visuospatial areas, as found in previous studies of such processing in normal college students (Just et al., 2004b).
A third prediction concerns the relation between the size of the corpus callosum and the functional connectivity between cortical regions that it connects. The functional connectivity in this sentence imagery task, which is known to engage prefrontal and parietal areas bilaterally (Just et al., 2004b), might well depend on the corpus callosum as part of the biological infrastructure that permits communication among brain areas. This study measured the size of the various segments of the corpus callosum of each participant in the functional imaging study, and we hypothesized that the sizes of key areas would be smaller in the participants with autism, following similar previous findings in purely morphometric studies (Egaas et al., 1995; Piven et al., 1997; Hardan et al., 2000). Moreover, this study tested for a correlation between the size of various corpus callosum segments and the interhemispheric functional connectivity between the cortical areas that they connect. The prediction was that in the participants with autism there would be a positive correlation, because the size of the corpus callosum is constraining the functional connectivity. In the control group, there should be no correlation because there is no constraint on information processing imposed by the size of the corpus callosum on neural connectivity. The neural resources and neural connectivity of participants without autism are hypothesized to be more than adequate to meet the task demands.
Material and methods
Participants
Twelve high-functioning individuals with autism [mean age = 22.5 years, standard deviation (SD) = 8.8] and 13 control participants (mean age = 20.3 years, SD = 4.0) were included in the analyses (Full-Scale and Verbal IQ scores of 80 or above). Participants were matched on the basis of age, Full-Scale and Verbal IQs (mean Full-Scale IQ: autism = 110.7, SD = 9.2; control = 113.2, SD = 9.2; mean Verbal IQ: autism = 109.7, SD = 10.8; control = 109.4, SD = 10.5). Among the 12 participants in the autism group, one was female and two were left-handed. In the control group, there was one female participant and two participants were left-handed. Data from six other participants were discarded owing to excessive head motion or other technical problems. The diagnosis of autism was established using two structured research diagnostic instruments, the ADI-R (Autism Diagnostic Interview—Revised, Lord et al., 1994) and the ADOS-G (Autism Diagnostic Observation Schedule-Generic, Lord et al., 2000), supplemented with confirmation by expert clinical opinion. Potential participants with autism were excluded on the basis of an associated disorder, such as fragile-X syndrome or tuberous sclerosis. Potential control participants and participants with autism were also excluded if found to have evidence of birth asphyxia, head injury or a seizure disorder. Other exclusionary criteria were based on neurological history and examination, physical examination, any type of anti-seizure medication and chromosomal analysis, or metabolic testing if indicated.
The control participants were community volunteers recruited to match the participants with autism on age, Full-Scale IQ, gender, race and family of origin socioeconomic status, as measured by the Hollingshead method. Potential control participants were screened by questionnaire, telephone, face-to-face interview and observation during screening psychometric tests. Exclusionary criteria, evaluated through these procedures, included current or past psychiatric and neurological disorders, birth injury, developmental delay, school problems, acquired brain injury, learning disabilities and medical disorders, with implications for the central nervous system or those requiring regular medication. Potential control participants were also screened to exclude those with a family history of autism, developmental cognitive disorder, affective disorder, anxiety disorder, schizophrenia, obsessive compulsive disorder or other neurological or psychiatric disorder thought to have a genetic component (in first-degree relatives or self), medications that affect the CNS, hypertension, diabetes, substance abuse (self, or first degree relative) and autism in first-, second- or third-degree relatives.
Each participant signed an informed consent that had been approved by the University of Pittsburgh and Carnegie Mellon University Institutional Review Boards. Before testing in the scanner, each participant was familiarized with the task, and had several practice sessions in an MRI simulator, a full-scale replica of the Siemens Allegra 3-T scanner used for this study, to assure their comfort in the MRI environment.
Experimental paradigm
This experiment assessed the brain activation and performance between autism and control groups during the processing of sentences involving mental imagery. In addition to a fixation baseline condition, there were two experimental conditions: a low-imagery condition and a high-imagery condition. The sentences appeared on the computer screen one at a time and the participant had to decide whether the sentence was true or false by pressing buttons in their right and left hands. There were three blocks each of high- and low-imagery stimuli, each block containing five sentences.
Stimulus examples
Low Imagery, True: Addition, subtraction and multiplication are all math skills. Low Imagery, False: Animals and minerals are both alive, but plants are not. High Imagery, True: The number eight when rotated 90 degrees looks like a pair of eyeglasses. High Imagery, False: Oranges, pineapples and coconuts are all triangular in shape.
Each participant practised the task once or twice before the scanning session. The practice consisted of three high-imagery sentences and three low-imagery sentences that were not used in the main task.
Participants made all responses with two 1-button mice, held in their left and right hands. The display of each sentence lasted 8500 ms, and responses were accepted for 8500 ms from the onset of the display of the sentence. There was an 8500 ms rest between each block of five sentences. In addition, a 24-s fixation condition was presented after every two blocks, for a total of four, to provide a baseline measure of brain activation with which to compare each experimental condition. In this fixation condition, participants fixated on a centred asterisk without performing any task. In addition, two sentences (one high imagery and one low imagery) were presented at the beginning, as practice trials to re-familiarize the participant with the task.
Functional MRI parameters
The imaging was carried out at the Brain Imaging Research Center (BIRC), University of Pittsburgh and Carnegie Mellon University, on a 3-T Siemens Allegra scanner using a circularly polarized transmit/receive head coil. The stimuli were rear-projected onto a translucent plastic screen and participants viewed the screen through a mirror attached to the head coil. For the functional imaging a gradient echo, echo-planar pulse sequence was used with repetition time (TR) = 1000 ms, echo time (TE) = 30 ms and a flip angle of 60°. Sixteen adjacent oblique-axial slices were acquired in an interleaved sequence, with 5-mm slice thickness, 1-mm slice gap, a 20 × 20 cm field of view (FOV) and a 64 × 64 matrix, resulting in in-plane resolution of 3.125 × 3.125 mm. A 160-slice 3D MPRAGE volume scan with TR = 200 ms, TE = 3.34 ms, flip angle = 7, FOV = 25.6 cm, 256 × 256 matrix size and 1-mm slice thickness was acquired at the same orientation as the oblique-axial functional images for each participant. This structural scan was used for making measurements of corpus callosum size.
Distribution of activation
To compare the participating groups in terms of the distribution of activation, the data were analysed using SPM99. Images were corrected for slice acquisition timing, motion-corrected, normalized to the Montreal Neurological Institute (MNI) template, re-sampled to 2 × 2 × 2 mm voxels and smoothed with an 8-mm Gaussian kernel to decrease spatial noise. Statistical analysis was performed on individual and group data by using the general linear model as implemented in SPM99 (Friston et al., 1995). Group analyses were performed using a random-effects model. Contrasts reflecting the imagery effects for each group, the group differences in the distribution of activation relative to fixation and group by imagery interactions were computed. For the group difference contrasts, possible differences in deactivation (relative to fixation condition) were excluded. An uncorrected height threshold of P = 0.005 and an extent threshold of six voxels were used.
Functional connectivity
The functional connectivity was computed (separately for each participant) as a correlation between the average time course of signal intensity of all the activated voxels in each member of a pair of regions of interest (ROIs). Twenty-one functional ROIs were defined to encompass the main clusters of activation in the group activation map for each group in both the high imagery-fixation and the low imagery-fixation contrasts. Labels for these 21 ROIs [the medial frontal gyrus (MedFG), plus 10 bilateral ROIs, namely IFG (inferior frontal gyrus), IFG2 (there was a second cluster of activation defined here since the first one did not cover the whole activation), MFG (middle frontal gyrus), IPS (intraparietal sulcus), SPL (superior parietal lobule), IPL (inferior parietal lobule), IT (inferior temporal), MTG (middle temporal gyrus), IOG (inferior occipital gyrus) and MOG (middle occipital gyrus)] were assigned with reference to the parcellation of the MNI single-subject T1-weighted dataset carried out by Tzourio-Mazoyer et al. (2002). A sphere was defined for each cluster (with a radius ranging from 5 to 10 mm) that best captured the cluster of activation in the map for each group. The ROIs used in the analysis were each the union of the four spheres defined for the two groups in each of the two conditions. The activation time course extracted for each participant over the activated voxels within the ROI originated from the normalized and smoothed images that were low-pass filtered and had the linear trend removed. A subject was excluded from further analysis (such as correlation analysis) if the number of voxels activated for that subject in either of the ROIs (constituting the pair) was <12. The correlation was computed on the images belonging only to the low-imagery and high-imagery conditions, so it reflects the interaction between the activation in two areas while the participant is performing the task and not during the baseline condition. Fisher’s r to z transformation was applied to the correlation coefficients for each participant before averaging and statistical comparison of the two groups.
Functional connectivity was measured for each participant in each group for both low and high-imagery conditions using the 21 functional ROIs described above. In order to summarize where reliable group differences in functional connectivity were obtained, these 21 ROIs were grouped into larger regions on the basis of lobe (frontal, parietal, temporal or occipital), and then functional connectivity measures for these groups of ROIs were obtained for each participant by averaging the connectivities of all of the relevant ROI pairs. This resulted in 10 networks for which connectivities were aggregated: six inter-lobe connectivities (frontal–parietal, frontal–temporal, frontal–occipital, parietal–temporal, occipital–temporal and parietal–occipital) and four intra-lobe connectivities (within frontal, temporal, parietal and occipital).
Corpus callosum morphometry
The cross-sectional area of the midsagittal slice of the corpus callosum was measured using the parcellation scheme described by Witelson (1989). The seven subregions of the corpus callosum include the rostrum, genu, rostral body, anterior midbody, posterior midbody, isthmus and splenium. In addition, there were two linear measurements: the distance between the anterior-most and posterior-most point, which is considered the length of the corpus callosum, and the callosum width at the midpoint. The corpus callosum size was normalized (divided by) by the total grey and white matter volume for each participant. The grey matter, white matter and cerebrospinal fluid volumes were measured for each subject by segmenting the T1-weighted structural brain image into three masks. The segmentation was performed by SPM2 routines. The outer contour of the corpus callosum was manually traced (with an inter-rater reliability of 0.87), and then interior segmentation, area and length computations were performed by image processing software.
Results
Overview
Participants with high-functioning autism tended to evoke visuospatial imagery in comprehending both low- and high-imagery sentences (suggested by activation in parietal and occipital regions) even when there was no necessity for imagery. This result supports the hypothesis that people with autism are more likely to use visual thinking strategies. Moreover, participants with autism showed reduced functional connectivity between frontal and parietal areas, key brain regions for the performance of this task. In addition, the anterior section of the corpus callosum was marginally smaller in the autism group. More importantly, the size of the corpus callosum was positively correlated with the functional connectivity in participants with autism, whereas the control participants showed no such correlation.
Behavioural results
The behavioural results demonstrated similar performance between the autism and control groups. A Group × Imagery Condition ANOVA (analysis of variance) indicated no significant difference between groups in either the reaction time or error rate, nor any significant interaction between group and imagery condition for either measure. The high-imagery condition had reliably longer response times [mean low imagery = 3.96 s, mean high imagery = 4.36 s, F(1,23) = 22.06, P < 0.0001] and there was no reliable difference in error rate (mean low = 7%, mean high = 6%) between the conditions.
Group differences in brain activation
Unlike the control participants who showed a clear differentiation of the imagery effect in terms of their selective activation of imagery-related regions in only the high-imagery condition, the participants with autism showed similar activation in these regions in both low- and high-imagery conditions. The effect of sentence imagery (the contrast between the high-imagery and low-imagery conditions) in each participant group is shown in Fig. 1. The participants with autism seemed to process high- and low-imagery sentences similarly (as described below, they were recruiting the visuospatial areas that support visual imagery in both conditions). The control group showed a large difference between the two conditions; in particular, one of the regions in which the control group prominently showed more activation in the high-imagery condition is the IPS area, which has previously been strongly associated with visual imagery in sentence comprehension (Just et al., 2004b). Note that the autism group also had a large amount of activation in this region, but it was approximately equal in magnitude in the two conditions, so it does not appear in the contrast presented in Fig. 1. Another cluster of activation composing the control group’s imagery effect is located in the IT area, another region associated with visual imagery. A third cluster of the control group’s imagery effect occurs in the left inferior frontal area, possibly associated with verbal rehearsal of the sentences as they are being transformed into visual images. The control group also showed more activation in the high- than the low-imagery condition in the right hemisphere in the superior parietal, IT and precentral regions. The autism group showed no areas of more activation in the high- compared with the low-imagery condition in the right hemisphere.
Fig. 1.
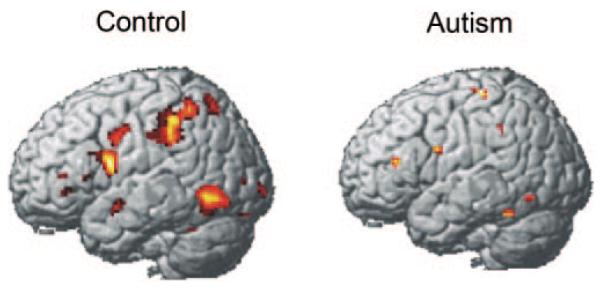
Within-group contrasts showing greater activation for high imagery than low imagery. The participants with autism showed little difference between the high- and low-imagery conditions, in contrast to the control participants, who showed more activation in the high-imagery condition than the low-imagery condition.
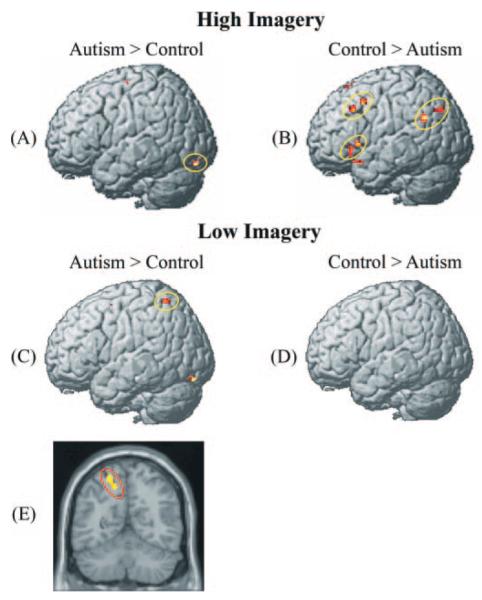
Following the analysis above assessing the imagery effect in each group, other analyses directly contrasted the two participant groups with each other. The group comparison in the high-imagery condition showed that the autism group had less activation than the control group in the left IFG and left angular gyrus, two areas that activate in the context of verbal rehearsal (Awh et al., 1996). There was also less activation for the autism group than controls in the left MFG, which could be the site of the strategic control of the verbal rehearsal. This effect is shown in Fig. 2B and Table 1, where the two groups are directly compared with each other in the two conditions. This result (more activation in the high-imagery condition for the control group than the autism group in left IFG, left angular gyrus and left MFG) may be indicative of the autism group needing less verbal rehearsal for the process of transforming sentence information into a visual image, consistent with the notion that they are more practised at translation of language into visual thinking.
Fig. 2.
Between-group contrasts of activation in each condition. (A) In the high-imagery condition, the autism group showed more activation than controls only in lingual gyrus. (B) In the high-imagery condition, the control group showed higher brain activation in left inferior frontal, middle frontal and left angular regions than the participants with autism. (C) In the low-imagery condition, the autism group showed more activation in L IPS and in left lingual gyrus in comparison with controls. (D) In the low-imagery condition, the control group did not show higher activation than the autism group; and (E) coronal view of higher activation in autism in L IPS than controls in the low-imagery condition.
Table 1.
Areas of activation differences between autism and control groups for the high-imagery condition
| Location of peak activation | High-imagery condition |
MNI coordinates |
||||
|---|---|---|---|---|---|---|
| Brodmann’s area |
Cluster size |
t(23) | x | y | z | |
| Areas in which control participants showed more activation than autism participants | ||||||
| L IFG–orbital | 47 | 40 | 3.84 | −34 | 20 | −18 |
| L IFG–triangular | 47 | 33 | 3.24 | −42 | 24 | −2 |
| L IFG–triangular 2 | 45,47 | 28 | 3.16 | −52 | 16 | 2 |
| R IFG–opercular | 47 | 49 | 3.32 | 48 | 18 | 2 |
| L MFG | 9 | 34 | 4.57 | −46 | 24 | 38 |
| L MFG 2 | 8 | 30 | 4.14 | −48 | 12 | 46 |
| R MFG | 11 | 45 | 3.5 | 24 | 46 | −2 |
| L supplementary motor area | 6 | 34 | 4.05 | −8 | 8 | 58 |
| R supplementary motor area | 6 | 21 | 4.29 | 14 | 18 | 66 |
| L angular gyrus | 40 | 89 | 4.13 | −50 | −54 | 28 |
| L angular gyrus 2 | 39 | 55 | 4.01 | −44 | −74 | 36 |
| L anterior cingulate gyrus | 32 | 303 | 4.29 | 0 | 28 | 30 |
| L thalamus | 81 | 4.03 | −10 | −8 | 8 | |
| Areas in which autism participants showed more activation than control participants | ||||||
| L lingual gyrus | 18 | 32 | 3.88 | −18 | −84 | −16 |
The threshold for significant activation was P < 0.005 for a spatial extent of at least six voxels, uncorrected for multiple comparisons.
Region labels apply to the entire extent of the cluster. t-Value scores and MNI coordinates are for the peak activated voxel in each cluster only.
In the comparison between the two participant groups in the low-imagery condition, the autism group showed higher activation than the control participants in posterior brain regions such as left intraparietal sulcus (L IPS), right SPL, bilateral cuneus and precuneus, and bilateral lingual gyrus, as shown in Figs 2B, C, and E and Table 2. The higher activation in the parietal and occipital regions in the autism group even in this low-imagery condition indicates their tendency to recruit visuospatial processes when they are comprehending sentences that do not refer to spatial objects or relations. This provides evidence of there being a greater predisposition towards visual thinking in autism. Controls on the contrary seem to process the low-imagery sentences by using more inferior frontal language regions and rely much less on parietal regions, similar to the activation pattern found among college students in their processing of such sentences (Just et al., 2004b).
Table 2.
Areas of activation differences between the autism and control groups for the low-imagery condition
| Location of peak activation | Low-imagery condition |
MNI coordinates |
||||
|---|---|---|---|---|---|---|
| Brodmann’s area |
Cluster size |
t(23) | x | y | z | |
| Areas in which control participants showed more activation than autism participants | ||||||
| L superior frontal gyrus medial | 21 | 21 | 3.15 | −4 | 28 | 32 |
| Areas in which autism participants showed more activation than control participants | ||||||
| L MFG | 6 | 30 | 3.15 | −26 | −2 | 54 |
| R precentral gyrus | 6 | 48 | 3.53 | 44 | −8 | 52 |
| L superior parietal lobe | 7 | 79 | 3.68 | −20 | −56 | 60 |
| R superior parietal lobe | 5 | 43 | 3.62 | 30 | −48 | 60 |
| L precuneus | 30 | 41 | 3.31 | 0 | −50 | 18 |
| R precuneus | 31 | 20 | 3.57 | 18 | −60 | 24 |
| L cuneus | 25 | 4.16 | −22 | −56 | 22 | |
| R cuneus | 7 | 29 | 3.19 | 18 | −78 | 44 |
| L lingual gyrus | 18 | 73 | 3.98 | −18 | −84 | −16 |
| R lingual gyrus | 18 | 60 | 4.19 | 20 | −88 | −10 |
| R middle occipital gyrus | 19 | 86 | 4.79 | 26 | −84 | 16 |
The threshold for significant activation was P < 0.005 for a spatial extent of at least six voxels, uncorrected for multiple comparisons.
Region labels apply to the entire extent of the cluster. t-Value scores and MNI coordinates are for the peak activated voxel in each cluster only.
Several previous studies have shown more posterior brain activation in autism in tasks such as embedded figures (Ring et al., 1999) and theory of mind (Castelli et al., 2002). At the same time, several previous studies have found that high-functioning individuals with autism may have a sparing or even an advantage in visuospatial processing (Shah and Frith, 1983; Ring et al., 1999; Mottron and Burack, 2001; Caron et al., 2004). The current results may help relate these two previous observations to each other. At least in the present sentence comprehension task, the autism group’s greater activation in more posterior parietal and occipital areas may be a manifestation of the greater reliance on visuospatial processing and a lesser reliance on linguistic processing.
Functional connectivity
The main functional connectivity finding was that the autism group showed the largest decrease in functional connectivity compared with control participants in the frontal–parietal network.
An ANOVA of the mean inter-lobe connectivities compared the mean functional connectivities of the two participant groups in pairs of ROIs that were in different cortical lobes, thus comparing the groups across six possible pairings (networks) of the four lobes: frontal–parietal, frontal–temporal, frontal–occipital, temporal–parietal, temporal–occipital and parietal–occipital. This 2 (Group) × 6 (Network) × 2 (Condition) mixed ANOVA revealed a main effect of pairing [F(5,115) = 16.78, P < 0.0001], but no reliable main effect of Group or Condition or interaction effects. The frontal–parietal network showed the largest group difference in functional connectivity, with the participants with autism having lower connectivity in this network (mean = 0.52, SE = 0.03) than the control participants (mean = 0.60, SE = 0.03), as shown in Table 3.
Table 3.
Mean functional connectivities between inter-lobe and intra-lobe networks by group and the group differences in the visual imagery task
| Inter-lobe networks | Autism | Control | Group difference |
|---|---|---|---|
| Frontal–parietal | 0.52 | 0.60 | 0.08 |
| Frontal–temporal | 0.45 | 0.45 | 0.00 |
| Frontal–occipital | 0.42 | 0.45 | 0.03 |
| Parietal–temporal | 0.44 | 0.47 | 0.03 |
| Parietal–occipital | 0.48 | 0.52 | 0.04 |
| Occipital–temporal | 0.44 | 0.42 | 0.02 |
|
| |||
| Intra-lobe networks | Autism | Control | Group difference |
|
| |||
| Frontal–frontal | 0.56 | 0.61 | 0.05 |
| Parietal–parietal | 0.82 | 0.87 | 0.05 |
| Temporal–temporal | 0.49 | 0.51 | 0.02 |
| Occipital–occipital | 0.67 | 0.66 | 0.02 |
On the basis of the prediction that the frontal–parietal network would show the largest underconnectivity for the autism group relative to the controls, a planned contrast compared the group difference in functional connectivity in the frontal–parietal network versus the group difference in the mean of the other five inter-lobe networks (frontal–temporal, frontal–occipital, parietal–temporal, parietal–occipital and occipital–temporal). The results of this contrast confirmed that the underconnectivity in autism between frontal and parietal areas (relative to controls) was larger than in the other networks [F(1,23) = 4.49, P < 0.05].
A separate 2 (Group) × 4 (Network) × 2 (Condition) mixed ANOVA for the four intra-lobe connectivities (within frontal, temporal, parietal and occipital) revealed no interaction with group. This result indicates that local intra-lobe functional connectivity in this task was not reliably different in autism compared with controls, in contrast to the underconnectivity seen for frontal–parietal inter-lobe connections. In fact, none of the outcomes of this ANOVA reflected on any group differences. The outcomes were a main effect of Network [F(3,69) = 48.43, P < 0.0001], and a reliable Condition × Network interaction [F(3,69) = 2.92, P < 0.05]. Tests of the simple effect of Condition within each Network revealed that only the parietal–parietal network showed differential connectivity between conditions [F(1,24) = 5.33, P < 0.05)], with higher connectivity for the high-imagery condition (mean = 0.89, SE = 0.03) than the low-imagery condition (mean = 0.80, SE = 0.04).
Corpus callosum size
Although the participants with autism had a smaller mean corpus callosum size than the controls, this effect was not statistically reliable. The anterior section of the corpus callosum, which includes rostrum, genu, rostral body and anterior midbody, was marginally smaller [t(23) = 1.88, P = 0.07] in the autism group compared with the controls. In addition, the anterior subregion, the genu, of the corpus callosum was marginally smaller [t(23) = 2.07, P = 0.05] in the autism group than in the control group. There was no statistically reliable difference in any other subregions between the two groups, even though all subregions had lower mean sizes in the autism group than in controls.
Corpus callosum size and functional connectivity
The size of the entire corpus callosum as well as some subregions was correlated with some of the functional connectivity measures in the low-imagery condition in both groups. Participants with autism had reliable positive correlations between the size of some subregions and the functional connectivity between relevant brain areas, whereas the control group showed no such correlations. The reliable differences in correlation were found mainly in the connections of the anterior and the posterior subregions of the corpus callosum.
The size of the anterior-most subregion, the genu, of the corpus callosum was reliably positively correlated with the functional connectivity between left frontal and right parietal regions in the autism group [r = 0.69, t(8) = 2.67, P < 0.05], while the control group showed no such correlation (r = −0.16, ns). Interestingly, left inferior frontal and right parietal regions are associated with language and spatial processing, respectively. Studies have found that fibres from the inferior frontal and parietal regions course through the rostrum and genu of the corpus callosum (de Lacoste et al., 1985). This result of positive correlation between the genu size and the functional connectivity in autism is similar to the findings in our previous study on executive functioning in autism (Just et al., in press, 2006).
The posterior midbody of the corpus callosum was reliably positively correlated with the functional connectivity between left middle temporal and right superior parietal regions in autism [r = 0.74, t(8) = 3.14, P < 0.05]. The controls showed no reliable correlation (r = 0.32, ns) here. Interestingly, the left middle temporal and right superior parietal regions are likely to be involved in language and spatial processing, respectively. There was also a reliable positive correlation in the autism group between the size of the posterior region of the corpus callosum and the functional connectivity between left and right occipital and left and right middle temporal regions. Here also the control group showed no reliable correlation. Thus the hypothesis of cortical underconnectivity in autism is consistent with the correlation between brain structure and brain function across individuals.
Discussion
Participants with autism showed more activation in parietal and occipital regions, suggesting that they were using visual imagery more widely than control subjects to support understanding of sentences. In Temple Grandin’s terms, they were probably ‘thinking in pictures’ much of the time (Grandin, 1995). The greater recruitment of the L IPS area during sentence comprehension among participants with autism than control participants was clear in the low-imagery condition. IPS activation has been previously associated with functions such as visual imagery, visual attention and spatial transformation (Alivisatos and Petrides, 1997; Vandenberghe et al., 2001; Just et al., 2004b). Greater activation in autism in the L IPS for this condition is particularly salient because comprehending these sentences does not typically require the use of visual imagery processes. The result suggests that the autism group might routinely recruit visual imagery for comprehending sentences rather than comprehending them on a purely linguistic basis.
Another result consistent with this interpretation is the autism group’s greater lingual gyrus activation (than controls) during the processing of low-imagery sentences. The lingual gyrus is an occipital brain region primarily involved in visual processing. This activation also suggests the autism group’s greater reliance on visualization to comprehend language. This result is reminiscent of Gaffrey et al.’s (2004) recent finding of greater than normal occipital activation in autism during a semantic decision task, suggesting an increased use of visual processes to comprehend language in autism.
The situation is slightly different in the comprehension of sentences involving high imagery, where the control group was expected to use visual imagery. Because participants with autism are presumed to be relatively unimpaired in visuospatial processing, they were expected to have no overactivation nor underactivation relative to the control group in the high-imagery condition. These expectations were borne out by the results.
What was unexpected but consistent with the other results was that the control participants showed overactivation consistent with more verbal rehearsal (in left angular gyrus, left IFG and left MFG). The angular gyrus and IFG form a verbal rehearsal loop in conjunction with the MFG, and angular gyrus activation is additionally known to occur when participants are asked to visualize a scene derived from a written text (Mellet et al., 2002). The higher activation in control participants in the left angular gyrus and in left IFG could be due to the control group’s use of a rehearsal loop to support the integration of linguistic and visuospatial information. For the control participants relative to those with autism, generating a mental image in the course of linguistic comprehension may be a less automatic aspect of sentence comprehension, requiring additional storage support provided by a rehearsal loop. In contrast to the control group, the autism group may be more facile with a translation from a linguistic to a visuospatial information format, and hence may require less such rehearsal.
The functional connectivity analyses indicate that the autism group showed cortical underconnectivity compared with controls in this task. The autism group’s underconnectivity relative to the control group was greatest in the frontal–parietal network. This finding is important for several reasons: (i) the task in this study demands the integration of language and spatial thinking, which involves frontal and parietal regions. Therefore, the finding of lower functional connectivity in the frontal–parietal network in autism indicates that integration between these two major neural systems may be compromised in autism; (ii) this finding might be evidence for abnormalities in the long-distance communication and coordination of brain regions in autism; and (iii) underconnectivity in the frontal–parietal network in autism may be a more general phenomenon. Our previous study on executive functioning in autism in a Tower of London task also found lower functional connectivity in the frontal–parietal network in participants with high-functioning autism (Just et al., in press, 2006).
The finding of functional underconnectivity in autism was further illuminated by the positive correlation of the size of corpus callosum and interhemispheric functional connectivity in participants with autism. The genu of the corpus callosum was positively correlated with functional connectivity between left frontal and right parietal regions in autism. Our previous study on executive functioning (Just et al., in press, 2006) found similar results. It should be noted here that the genu size in the autism group was only marginally smaller than the control group. The size of the corpus callosum in people with autism may be an index of some abnormality that may impose a constraint on the functional connectivity, whereas in controls, there is no such constraint and hence no positive correlation. Abnormalities in white matter tracts in autism (Courchesne et al., 2001; Carper et al., 2002; Herbert et al., 2003, 2004; Chung et al., 2004) might be contributing to the reduced functional connectivity in autism as Quigley et al. (2001) has suggested in the case of callosal agenesis.
Underconnectivity theory attributes many of the widespread abnormalities in psychological functioning in autism to a deficit in the coordination and communication between key brain processing centres. This study replicates the cortical underconnectivity in autism found in other types of cognitive tasks, such as sentence comprehension (Just et al., 2004a), and working memory (Koshino et al., 2005). One of the main predictions of underconnectivity theory is that any facet of psychological and neurological function that is dependent on the coordination or integration of brain regions is susceptible to disruption in autism, particularly when the computational demand of the coordination is large. In the current study, the task demanded the coordination and integration of two spatially remote brain centres that controlled language and visuospatial functions, and the results indicated functional underconnectivity in the participants with autism, as well as a correlation between corpus callosum segment sizes and the functional connectivities of some of the contralateral pairs of cortical areas that the segments connect. Thinking in pictures during sentence comprehension may be an adaptation to the underconnectivity in autism, making greater use of parietal and occipital areas and relying less on frontal regions for linguistic processing, possibly because the connections between the frontal and parietal regions are compromised.
Acknowledgements
This research was supported by the Collaborative Program of Excellence in Autism (CPEA) Grant HD35469 from the National Institute of Child Health and Human Development, and the Cure Autism Now Young Investigator Award. The authors would like to thank Sarah Schipul and Stacey Becker for assistance with the data collection and data analysis, and Diane Williams for providing useful comments on an earlier version of the manuscript.
Abbreviations
- IFG
inferior frontal gyrus
- IPS
intraparietal sulcus
- MFG
middle frontal gyrus
- MNI
Montreal Neurological Institute
- ROI
regions of interest
References
- Alivisatos B, Petrides M. Functional activation of the human brain during mental rotation. Neuropsychologia. 1997;35:111–8. doi: 10.1016/s0028-3932(96)00083-8. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: evidence from positron emission tomography. Psychol Sci. 1996;7:25–31. [Google Scholar]
- Bertone A, Faubert J. Motion sensitivity of first and second-order translating, expanding/contracting and rotation patterns. Invest Ophthalmol Vis Sci. 1999;40:2245. [Google Scholar]
- Bertone A, Faubert J. How is complex second-order motion processed? Vision Res. 2003;43:2591–601. doi: 10.1016/s0042-6989(03)00465-6. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuospatial information processing in autism depends on stimulus complexity. Brain. 2005;128:2430–41. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Bondy A, Frost L. The picture exchange communication system. Seminars Speech Lang. 1998;19:373–89. doi: 10.1055/s-2008-1064055. [DOI] [PubMed] [Google Scholar]
- Bondy A, Frost L. The picture exchange communication system. Behav Mod. 2001;25:725–44. doi: 10.1177/0145445501255004. [DOI] [PubMed] [Google Scholar]
- Caron M-J, Mottron L, Rainville C, Chouinard S. Do high functioning persons with autism present superior spatial abilities? Neuropsychologia. 2004;42:467–81. doi: 10.1016/j.neuropsychologia.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–51. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Mather G. Motion: the long and short of it. Spatial Vis. 1989;4:103–29. doi: 10.1163/156856889x00077. [DOI] [PubMed] [Google Scholar]
- Chubb C, Sperling G. Drift-balanced random stimuli: a general basis of studying non-Fourier motion perception. J Optical Soc Am A. 1988;5:1986–2007. doi: 10.1364/josaa.5.001986. [DOI] [PubMed] [Google Scholar]
- Chung MK, Dalton KM, Alexander AL, Davidson RJ. Less white matter concentration in autism: 2D voxel-based morphometry. Neuroimage. 2004;23:242–51. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–54. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Dawson M, Mottron L, Jelenic P, Soulières I. Superior performance of autistics on RPM and PPVT relative to Wechsler scales provides evidence for the nature of autistic intelligence. Poster presented at the International Meeting for Autism Research; Boston, MA. May, 2005. [Google Scholar]
- de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. J Neuropathol Exp Neurol. 1985;44:578–91. doi: 10.1097/00005072-198511000-00004. [DOI] [PubMed] [Google Scholar]
- Dennis M, Lazenby AL, Lockyer L. Inferential language in high-function children with autism. J Autism Dev Disord. 2001a;31:47–54. doi: 10.1023/a:1005661613288. [DOI] [PubMed] [Google Scholar]
- Dennis M, Purvis K, Barnes MA, Wilkinson M, Winner E. Understanding of literal truth, ironic criticism and deceptive praise following childhood head injury. Brain Lang. 2001b;78:1–16. doi: 10.1006/brln.2000.2431. [DOI] [PubMed] [Google Scholar]
- Deruelle C, Rondan C, Gepner B, Tardif C. Spatial frequency and face processing in children with autism and Asperger syndrome. J Autism Dev Disord. 2004;34:199–210. doi: 10.1023/b:jadd.0000022610.09668.4c. [DOI] [PubMed] [Google Scholar]
- Dunn M, Vaughan H, Kreuzer J, Kurtzberg D. Electrophysiological correlates of semantic classification in autistic and normal children. Dev Neuropsychol. 1999;16:79–99. [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. Reduced size of the corpus callosum in autism. Arch Neurol. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Map. 1995;2:189–210. [Google Scholar]
- Gaffrey MS, Kleinhans N, Haist F, Akshoomoff N, Campbell A, Courchesne E, et al. Reduced left inferior frontal and enhanced occipital activation during semantic decision in autism. Int Meeting Autism Res. 2004;123:S3.2.1. [Google Scholar]
- Goldstein G, Minshew NJ, Siegel DJ. Age differences in academic achievement in high-functioning autistic individuals. J Clin Exp Neuropsychol. 1994;16:671–80. doi: 10.1080/01688639408402680. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Beers SR, Siegel DJ, Minshew NJ. A comparison of WAIS-R profiles in adults with high-functioning autism or differing subtypes of learning disability. Appl Neuropsychol. 2001;8:148–54. doi: 10.1207/S15324826AN0803_3. [DOI] [PubMed] [Google Scholar]
- Grandin T. An inside out view of autism. In: Schopler E, Mesibov GB, editors. High functioning individuals with autism. Plenum Press; New York: 1992. pp. 105–26. [Google Scholar]
- Grandin T. Thinking in pictures: and other reports from my life with autism. Doubleday; New York: 1995. [Google Scholar]
- Happe FGE. Communicative competence and theory of mind: a test of relevance theory. Cognition. 1993;48:101–19. doi: 10.1016/0010-0277(93)90026-r. [DOI] [PubMed] [Google Scholar]
- Happe FGE. Autism: cognitive deficit or cognitive style. Trends Cogn Sci. 1999;3:216–22. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Keshavan MS. Corpus callosum size in autism. Neurology. 2000;55:1033–6. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Bakardjiev A, Hodgson J, Adrien KT, et al. Larger brain and white matter volumes in children with developmental language disorder. Dev Sci. 2003;6:F11–F22. [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–40. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hermelin B, O’Conner N. Art and accuracy: the drawing ability of idiot-savants. J Child Psychol Psychiatry. 1990;31:217–28. doi: 10.1111/j.1469-7610.1990.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the embedded figures test? J Child Psychol Psychiatry. 1997;38:527–34. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation, synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004a;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Newman SD, Keller TA, McEleney A, Carpenter PA. Imagery in sentence comprehension: an fMRI study. Neuroimage. 2004b;21:112–24. doi: 10.1016/j.neuroimage.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y, Toichi M. Dual access to semantic in autism: is pictorial access superior to verbal access? J Child Psychol Psychiatry. 2000;41:859–67. [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–21. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kujala T, Lepisto T, Wendt TN, Naatanen P, Naatanen R. Neurophysiological evidence for cortical discrimination impairment of prosody in Asperger syndrome. Neurosci Lett. 2005;383:260–5. doi: 10.1016/j.neulet.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism diagnostic interview—revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- Mellet E, Bricogne S, Crivello F, Mazoyer B, Denis M, Tzourio-Mazoyer N. Neural basis of mental scanning of a topographic representation built from a text. Cereb Cortex. 2002;12:1322–30. doi: 10.1093/cercor/12.12.1322. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G. Autism as a disorder of complex information processing. Mental Retard Dev Disabil Res Rev. 1998;4:129–36. [Google Scholar]
- Mottron L, Burack JA. Enhanced perceptual functioning in the development of autism. In: Burack JA, Charman N, Yirmiya N, Zelazo PR, editors. The development of autism: perspectives from theory and research. Erlbaum; Princeton, NJ: 2001. pp. 131–48. [Google Scholar]
- O’Connor N, Hermelin B. Visual and graphic abilities of the idiot-savant artist. Psychol Med. 1987;17:79–90. doi: 10.1017/s0033291700013003. [DOI] [PubMed] [Google Scholar]
- Piven J, Bailey J, Ranson BJ, Arndt S. An MRI study of the corpus callosum in autism. Am J Psychiatry. 1997;154:1051–6. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- Quigley M, Cordes D, Wendt G, Turski P, Moritz C, Haughton V, et al. Effect of focal and nonfocal cerebral lesions on functional connectivity studied with MR imaging. Am J Neuroradiol. 2001;22:294–300. [PMC free article] [PubMed] [Google Scholar]
- Ring HA, Baron-Cohen S, Wheelwright S, Williams SCR, Brammer M, Andrew C, et al. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance. Brain. 1999;122:1305–15. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- Schopler E, Olley JG. Comprehensive educational services for autistic children: the TEACCH model. In: Reynolds CR, Gutkin TR, editors. The handbook of school psychology. Wiley; New York: 1982. pp. 629–43. [Google Scholar]
- Shah A, Frith U. An islet of ability in autistic children: a research note. J Child Psychol Psychiatry. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the block design task? J Child Psychol Psychiatry. 1993;34:1351–64. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Siegel DJ, Minshew NJ, Goldstein G. Wechsler IQ profiles in diagnosis of high-functioning autism. J Autism Dev Disord. 1996;26:389–406. doi: 10.1007/BF02172825. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. ‘Once upon a ribbit’: stories narrated by autistic children. Br J Dev Psychol. 1995;13:45–59. [Google Scholar]
- Tager-Flusberg H. Brief report: current theory and research on language and communication in autism. J Autism Dev Disord. 1996;26:169–72. doi: 10.1007/BF02172006. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Gitelman DR, Parrish TB, Mesulam M-M. Location- or feature-based targeting of peripheral attention. Neuroimage. 2001;14:37–47. doi: 10.1006/nimg.2001.0790. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJO, Maybery MT, Durkin K. Inner speech impairments in autism. J Child Psychol Psychiatry. 2006 doi: 10.1111/j.1469-7610.2006.01624.x. in press. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum: a postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]