Abstract
Anabaena sp. strain PCC 7120 is a filamentous cyanobacterium that can use inorganic compounds such as nitrate or ammonium as nitrogen sources. In the absence of combined nitrogen, it can fix N2 in differentiated cells called heterocysts. Anabaena also shows substantial activities of amino acid uptake, and three ABC-type transporters for amino acids have been previously characterized. Seven new loci encoding predicted amino acid transporters were identified in the Anabaena genomic sequence and inactivated. Two of them were involved in amino acid uptake. Locus alr2535-alr2541 encodes the elements of a hydrophobic amino acid ABC-type transporter that is mainly involved in the uptake of glycine. ORF all0342 encodes a putative transporter from the dicarboxylate/amino acid:cation symporter (DAACS) family whose inactivation resulted in an increased uptake of a broad range of amino acids. An assay to study amino acid release from Anabaena filaments to the external medium was set up. Net release of the alanine analogue α-aminoisobutyric acid (AIB) was observed when transport system N-I (a hydrophobic amino acid ABC-type transporter) was engaged in the uptake of a specific substrate. The rate of AIB release was directly proportional to the intracellular AIB concentration, suggesting leakage from the cells by diffusion.
Keywords: ABC-type transporters, amino acid diffusion, cyanobacteria, membrane transport
1. Introduction
Cyanobacteria are a group of prokaryotic microorganisms characterized by their ability to fix CO2 at the expense of oxygenic photosynthesis. They represent a coherent phylogenetic group in spite of showing a very diverse morphology and having colonized many different natural habitats. Some cyanobacteria are true multicellular organisms, growing as chains of cells that communicate between them and divide labor between specialized cells. Under combined nitrogen deprivation, heterocyst-forming cyanobacteria present two cell types: vegetative cells that perform oxygenic photosynthesis and heterocysts that carry out N2 fixation [1]. These specialized cells rely on each other: heterocysts require photosynthate that is provided by vegetative cells, and heterocysts provide vegetative cells with fixed nitrogen [2,3].
Three substrates that have been proposed as molecules transferred from vegetative cells to heterocysts are glutamate, sucrose, and alanine [2,4,5,6,7]. Sucrose is a universal vehicle of reduced carbon in plants [8], and it seems to be important also in cyanobacteria [9]; alanine can be an immediate source of reducing power in the heterocyst, where it is metabolized by alanine dehydrogenase [6]; and glutamate is mainly synthesized in vegetative cells by glutamate synthase and used in the heterocysts by glutamine synthetase to produce glutamine [2,10]. Heterocysts accumulate cyanophycin, a non-ribosomically synthesized peptide made of aspartate and arginine (multi-l-arginyl-poly [l-aspartic acid]) [11]. Cyanophycinase produces β-aspartyl-arginine, which is hydrolyzed by isoaspartyl dipeptidase in the vegetative cells [3]. Thus, glutamine and β-aspartyl-arginine (and perhaps also aspartate and arginine) appear to be nitrogen vehicles from heterocysts to vegetative cells [3,12].
Two possible pathways for intercellular molecular exchange in heterocyst-forming cyanobacteria have been discussed [1,13,14,15]. One involves structures at the septum between cells, septal junctions, which may consist of protein complexes that traverse septal peptidoglycan [16]. Septal junctions appear to allow the exchange of small, water-soluble compounds between cells [17,18]. Possible components of these cell–cell connecting structures are the SepJ, FraC, and FraD proteins [19,20]. The other route for cell–cell communication is the continuous periplasm present in the cyanobacterial filament [13,21]. The exchange of a substance through this route would require export to the periplasm in the producing cell, diffusion through this compartment, and import into the recipient cell. Interestingly, the outer membrane, which is continuous along the filament, appears to be relatively impermeable for compounds such as sucrose and glutamate that are exchanged between cells [22]. Independently of the pathway that might be used for nutrient exchange, cytoplasmic membrane transporters appear to be important in the diazotrophic physiology of heterocyst-forming cyanobacteria, since some amino acid transport mutants of the model heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120 (hereafter Anabaena) are impaired in diazotrophic growth [23].
Anabaena is able to take up from the external medium a broad range of amino acids independently of whether it has been grown with combined nitrogen or under diazotrophic conditions [23,24,25,26,27,28]. This ability has been ascribed to the activity of several transport systems, termed N-I, N-II, and Bgt, which show different specificities for amino acids [27,28]. N-I recognizes 20 proteinogenic amino acids except for aspartate and is the main transporter responsible for the uptake of hydrophobic amino acids, especially proline, although it also transports some other amino acids including glutamine and glutamate. N-II recognizes and transports mainly acidic (aspartate and glutamate) and neutral polar amino acids, again including glutamine. Finally, the Bgt system is a basic amino acid transporter that also contributes to the uptake of glutamine, an amino acid that can therefore be transported by the three identified transporters of Anabaena. These three systems are ABC-type uptake transporters, and their constituents are summarized in Table 1. Of note, the bgtA gene encodes an ATP-binding subunit that energizes transport by the N-II and Bgt systems [28].
Table 1.
ABC-type amino acid uptake transporters of Anabaena sp. strain PCC 7120. The Transporter Classification Database (TCDB) family number [29,30] is indicated. The order in which the transported amino acids are presented for each transporter reflects the contribution of the corresponding transporter to the total uptake of the indicated amino acids by nitrate-grown Anabaena filaments; bold face, preferred amino acids. ATPase: ATP-binding and hydrolyzing protein; Orn: ornithine; PSB: periplasmic substrate-binding protein; TM: transmembrane (permease) protein. Cys, Trp, and Tyr have not been investigated in transport assays in Anabaena.
| Transporter | TCDB # | Transported amino acids | ORF | Gene | Predicted gene product | Ref. |
|---|---|---|---|---|---|---|
| N-I | 3.A.1.4 | Pro, Phe, Leu, | all1046 | natA | ATPase | [27] |
| Gly, Thr, Ala, | alr1834 | natB | PSB | |||
| Ser, Met, Asn, | all1047 | natC | TM | |||
| His, Orn, Gln, | all1248 | natD | TM | |||
| Glu | all2912 | natE | ATPase | |||
| N-II | 3.A.1.3 | Asp, Glu, Asn, | alr4164 | natF | PSB | [28] |
| Gln, Met, Thr, | alr4165 | natG | TM | |||
| Ala, Ser, Gly, | alr4166 | natH | TM | |||
| His | alr4167 | bgtA | ATPase | |||
| Bgt | 3.A.1.3 | Lys, Arg, Orn, | alr4167 | bgtA | ATPase | [28] |
| His, Gln | alr3187 | bgtB | PSB and TM | |||
| N-III | 3.A.1.4 | Gly, Pro, Glu, | alr2535 | natI | PSB | This work |
| Phe, Leu, Ala, | alr2536 | natJ | TM | |||
| Gln | alr2538 | natK | TM | |||
| alr2539 | natL | ATPase | ||||
| alr2541 | natM | ATPase |
Some cyanobacterial mutants have been reported to accumulate amino acids in the culture medium [6,23,27,28,31,32]. In Anabaena, release of hydrophobic amino acids is a general feature of strains mutated in neutral amino acid transporters [23,27,28]. This is especially relevant under diazotrophic conditions, where alanine released from strains mutated in the N-I and N-II systems reaches a concentration of 0.1 mM in the medium [28].
In this work we have investigated additional Open Reading Frames (ORF) of the Anabaena genome encoding proteins that show homology with amino acid transporters. We generated inactivation mutants of these ORFs and studied their amino acid uptake activity. Moreover, with the aim of clarifying aspects of the loss of amino acids to the external medium, we set up an assay to quantify the release from the cells of an alanine analogue that cannot be metabolized.
2. Experimental Section
2.1. Bacterial Strains and Growth Conditions
Anabaena sp. (also known as Nostoc sp.) strain PCC 7120 was grown in BG11 (which contains NaNO3) [33] or BG110 (free of combined nitrogen) medium at 30 °C in the light (25–75 μmol m−2 s−1), in shaken (80–90 rpm) liquid cultures or in medium solidified with 1% Difco agar. When indicated, the medium was supplemented with 10 mM NaHCO3 and the cultures were bubbled with 1% CO2 (BG11C or BG110C). For the mutants described below, antibiotics were used at the following concentrations: streptomycin sulfate (Sm), 2–5 μg mL−1; spectinomycin dihydrochloride pentahydrate (Sp), 2–5 μg mL−1 and neomycin sulfate (Nm), 5–30 μg mL−1 for liquid cultures; and Sm, 5 μg mL−1; Sp, 5 μg mL−1 and Nm, 40 μg mL−1 for solid cultures. DNA from Anabaena sp. was isolated by the method of Cai and Wolk [34].
Escherichia coli DH5α was used for plasmid constructions. This strain and strains HB101 and ED8654, used for conjugation to Anabaena, were grown in LB medium, supplemented when appropriate with antibiotics at standard concentrations [35].
2.2. Plasmid Construction and Genetic Procedures
For inactivation of alr2536, all0342, and alr3429, internal fragments of 552 bp, 596 bp, and 469 bp, respectively, were amplified by PCR using DNA from strain PCC 7120 as a template and primers alr2536-7120-1 and alr2536-7120-2 for alr2536, all0342-7120-1 and all0342-7120-2 for all0342, and alr3429-7120-1 and alr3429-7120-2 for alr3429 (all primers contain BamHI restriction sites in their 5' ends and are listed in Table 2). Amplified fragments were cloned into vector pGEM-T (Promega) in the case of alr2536 and all0342 or pGEM-T Easy (Promega) for alr3429, producing pCSR4, pCSR1, and pCSR19, respectively, and then transferred as BamHI-ended fragments to BamHI-digested pRL424 [36] producing pCSR17, pCSR13, and pCSR23, respectively (Nmr).
Table 2.
Oligodeoxynucleotide primers used in this work. Introduced restriction enzyme cutting sites are indicated in boldface.
| Primer | Sequence (5'→3') |
|---|---|
| alr2536-7120-1 | GGA TCC GCT AAC GCT ACT TTG CCG |
| alr2536-7120-2 | GGA TCC GCA ACC CAA AGC CAA TC |
| all0342-7120-1 | GGA TCC GTT GAC CAA TAC CCT CAT GGC |
| all0342-7120-2 | GGA TCC GCT TGG AAG GTT ACA GGC |
| alr3429-7120-1 | GGA TCC GGG GTT TAA AGA TGC TGA CGG |
| alr3429-7120-2 | GGA TCC GAG GAT GTT CTC TCA CCC |
| all1189-1 | GGA TCC GGA AAC TCA CAG |
| all1189-2 | GCG GAT CCA GGA TAA TAG |
| alr1538-1 | GGA TCC TGG CTG TGT ATT TAG |
| alr1538-2 | GGA TCC TTT GGG CAG AAG |
| all3551-1 | GGA TCC AGC CCA ATA GTT G |
| all3551-2 | GGA TCC CTG CCA AAG AC |
| AA-1 | GAG CCA TAC AAG CTC TGA TTC ATG G |
| AA-2 | ACG CGA TCG CTG ACT CCT GCC |
For inactivation of alr1519, a 2.3 kb DNA fragment carrying the full ORF and flanking regions was amplified from genomic DNA from strain PCC 7120 using primers AA-1 and AA-2. The amplified fragment was inserted into vector pGEM-T (Promega) producing pCSS1. The C.S3 cassette (encoding Smr Spr) was excised from plasmid pRL463 [37] with BamHI and inserted into the BclI-digested pCSS1 to interrupt the ORF alr1519, obtaining plasmid pCSS2. The construct was transferred as a PvuII-ended fragment to the suicide plasmid pRL278 [38] digested with NruI producing pCSS4 (Smr Spr Nmr).
For inactivation of all1189, alr1538, and all3551, internal fragments of 439 bp, 440 bp, and 417 bp, respectively, were amplified by PCR using DNA from strain PCC 7120 as a template and primers all1189-1 and all1189-2 for all1189, alr1538-1 and alr1538-2 for alr1538, and all3551-1 and all3551-2 for all3551 (all primers contain BamHI restriction sites in, or close to, their 5' ends). Amplified fragments were cloned into vector pMBL-T (MBL) producing pCSVM1, pCSVM2, and pCSVM3, respectively, and then transferred as BamHI-ended fragments to BamHI-digested pCSV3 producing, respectively, pCSVM4, pCSVM5, and pCSVM6 (Smr Spr).
Conjugation of Anabaena sp. strain PCC 7120 with E. coli HB101 carrying plasmids pCSR17, pCSR13, pCSR23, pCSS4, pCSVM4, pCSVM5, or pCSVM6 with helper and methylation plasmid pRL623 was effected by the conjugative plasmid pRL443, carried in E. coli ED8654, and performed as described previously [39]. Exconjugants were selected for their resistance to Sm and Sp, or to Nm; for the generation of the mutant CSS4, double recombinants were selected for their resistance to sucrose. The genetic structure and segregation of selected clones were studied by Southern analysis using as probes the cloned fragments from the corresponding mutated gene (see above). The probes were labeled with Ready-To-Go DNA Labeling Beads (−dCTP) kit (GE Healthcare). The mutants, which were homozygous for the mutated chromosomes in all cases, were named as follows: CSR6 from plasmid pCSR17, CSR1 from plasmid pCSR13, CSR3 from plasmid pCSR23, CSS4 from plasmid pCSS4, CSVM1 from plasmid pCSVM4, CSVM2 from plasmid pCSVM5, and CSVM3 from plasmid pCSVM6.
2.3. Growth Tests and Sample Preparation
Growth tests were carried out by spotting 2–7 µL of cell suspension containing 2.5–10 µg Chl mL−1 on agar plates of BG11 or BG110 medium. The plates were incubated at 30 °C in the light (25–40 µmol m−2 s−1) and observed over a period of two weeks. Growth rates in liquid medium were determined as previously described [23]. The growth rate constant (µ = ln2/td, where td is the doubling time) was calculated from the increase of protein content determined in 0.2 mL of samples of shaken liquid cultures. Protein concentration was determined by a modified Lowry procedure [40]. Chlorophyll a (Chl) content of cultures was determined by the method of Mackinney [41].
2.4. Substrate Transport Assays
Amino acid uptake assays were carried out at 30 °C in the light (175 μmol m−2 s−1). Amino acid uptake was determined as described previously [23] in 10-min transport assays with the indicated concentration of l-[U-14C]amino acid, in filaments grown in BG11 medium (supplemented with the appropriate antibiotic in the case of the mutants), washed and suspended in 25 mM N-tris(hydroxymethyl)-methylglycine (Tricine)-NaOH buffer (pH 8.1). Significance of the difference of uptake between a mutant and the wild type was assessed by unpaired Student’s t tests assuming a normal distribution of the data. Differences with P ≤ 0.05 are considered statistically significant.
For solute release experiments, filaments grown in BG11 or BG110 medium were washed, suspended in 25 mM Tricine-NaOH buffer (pH 8.1), and incubated with 3 μM α-[1-14C]aminoisobutyric acid (AIB) for 40 or 90 min at 30 °C in the light (175 μmol m−2 s−1). At the time indicated, an excess of unlabeled substrate at a concentration of 1 mM was added. Samples were removed at different times after the addition of the unlabeled substrate and cell-associated radioactivity was determined after filtration and washing with about 3–5 mL of 5 mM Tricine-NaOH buffer (pH 8.1).
To determine metabolites produced from the labeled substrate and released from the cells at the end of the experiments, samples of 0.2 mL of the cell suspension were centrifuged at 14,000× g for 1 min and supernatants were centrifuged again. Samples of the resulting solutions were applied to 0.1-mm-thick cellulose thin-layer chromatography (TLC) plates (20 cm × 20 cm; Merck, Darmstadt, Germany). Two-dimensional separation of amino acids was effected by using the following solvents: the first dimension solvent consisted of n-butanol-acetone-ammonium hydroxide-water (20:20:10:4, v/v/v/v), and the second dimension solvent consisted of isopropanol-formic acid-water (20:1:5, v/v/v). TLC plates were analyzed by electronic autoradiography using a two-dimensional scanner for β particles (Cyclone Plus Phosphor Imager, PerkinElmer, Waltham, MA, USA).
3. Results
3.1. Predicted ABC-Type Amino Acid Transporters
Blast searches of the Anabaena genome, using as queries the amino acid sequences of the permease subunits of the Anabaena ABC-type transporters N-I and N-II (Table 1), identified a new cluster of genes (alr2535 to alr2541) putatively encoding the elements of an additional ABC-type transporter for amino acids (Figure 1A). ORF alr2535 would encode a protein of 268 amino acid residues with homology to the periplasmic substrate-binding protein BraC of the branched-chain amino acid transport system of Pseudomonas aeruginosa [42]. ORFs alr2536 and alr2538 would encode 316 and 308 amino acid residue peptides, respectively, with homology to permeases NatC and NatD of the transport system N-I of Anabaena. ORF alr2539 and alr2541 would encode 259 and 264 amino acid residue peptides, respectively, showing homology with ATPases NatA and NatE of the N-I system. Two additional ORFs, alr2537 and asl2540, would encode hypothetical proteins with no homologues in the databases.
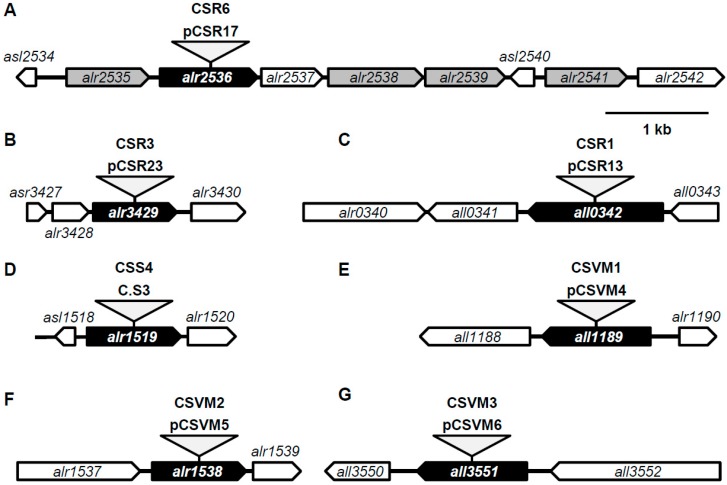
Figure 1.
Insertional inactivation of ORFs alr2536, alr3429, all0342, alr1519, all1189, alr1538, and all3551. Schematic representation of the loci of alr2536 (A); alr3429 (B); all0342 (C); alr1519 (D); all1189 (E); alr1538 (F); and all3551 (G), with indication of the plasmids inserted to produce strains CSR6, CSR3, CSR1, CSS4, CSVM1, CSVM2, and CSVM3, respectively.
To inactivate this putative ABC-type transporter, plasmid pCSR17, based on pRL424, which carries the npt gene with no transcriptional terminators [36], was inserted through a single cross-over event into alr2536, producing the Anabaena mutant strain CSR6 (Figure 1A). Strain CSR6 grew well on solid medium with or without combined nitrogen (not shown). The uptake of some amino acids was studied in nitrate-grown filaments of strain CSR6 in comparison to the wild type. The mutant was mainly impaired in the transport of hydrophobic amino acids (54%–72% of wild-type activity), especially glycine, and impairment in the transport of glutamate and glutamine (63% and 77% of wild-type activity, respectively) was also observed (Table 3). These results suggest that some of the genes in the alr2535 to alr2541 gene cluster encode components of a transport system that can mediate the uptake of some amino acids in Anabaena, mainly hydrophobic amino acids. We have named this system N-III, and the corresponding genes natI to natM (Table 1).
Table 3.
Amino acid transport activities in Anabaena sp. strain PCC 7120 and mutant strains. BG11-grown cells were used in 10-min transport assays with 10 μM [14C]amino acid substrate as described in the Experimental Section. Activities are given in nmol (mg Chl)−1 per 10 min; the mean ± standard deviation of the mean (SE) from the number of measurements indicated in parenthesis is presented. The percentage of the wild-type activity is also given for the mutants. Differences between mutant and wild-type activities were assessed by the Student’s t test (P indicated in each case).
| Substrate | Transport activity (nmol [mg Chl]−1) | ||||||
|---|---|---|---|---|---|---|---|
| PCC 7120 | Mutant strain (inactivated ORF) | ||||||
| CSR6 (alr2536) | CSR3 (alr3429) | CSR1 (all0342) | |||||
| Mean ± SE (n) | Mean ± SE (n) | % (P) | Mean ± SE (n) | % (P) | Mean ± SE (n) | % (P) | |
| Basic | |||||||
| l-Arg | 125 ± 1.59 (25) | 115 ± 16.4 (3) | 92% (0.077) | 148 ± 6.60 (4) | 119% (0.187) | 195 ± 12.5 (3) | 156% (0.068) |
| l-Lys | 138 ± 1.55 (16) | 151 ± 3.92 (2) | 110% (0.167) | 163 ± 6.15 (3) | 118% (0.119) | 140 ± 8.40 (2) | 102% (0.882) |
| l-His | 88.0 ± 1.10 (16) | 71.7 ± 4.80 (3) | 81% (0.174) | 90.0 ± 4.94 (4) | 102% (0.863) | 132 ± 5.28 (3) | 150% (0.023) |
| Acidic | |||||||
| l-Asp | 37.3 ± 0.39 (27) | 33.5 ± 2.18 (3) | 90% (0.440) | 31.1 ± 1.67 (4) | 83% (0.170) | 38.8 ± 2.19 (4) | 104% (0.768) |
| l-Glu | 10.5 ± 0.12 (25) | 6.62 ± 0.70 (3) | 63% (0.061) | 10.2 ± 0.26 (4) | 97% (0.721) | 16.9 ± 1.56 (4) | 161% (0.130) |
| Neutral polar | |||||||
| l-Gln | 99.4 ± 0.90 (30) | 77.0 ± 6.89 (3) | 77% (0.189) | 99.3 ± 6.31 (4) | 100% (0.994) | 158 ± 6.48 (4) | 158% (0.014) |
| l-Ser | 218 ± 2.50 (10) | 195 ± 2.05 (2) | 90% (0.022) | 191 ± 9.37 (3) | 88% (0.237) | 239 ± 34.3 (3) | 110% (0.753) |
| Hydrophobic | |||||||
| l-Ala | 192 ± 1.09 (20) | 139 ± 13.4 (3) | 72% (0.145) | 203 ± 16.3 (4) | 105% (0.772) | 286 ± 6.03 (3) | 149% (0.004) |
| Gly | 200 ± 3.30 (15) | 107 ± 14.0 (3) | 54% (0.039) | 222 ± 8.84 (4) | 111% (0.337) | 252 ± 5.50 (3) | 126% (0.007) |
| l-Leu | 103 ± 0.99 (17) | 70.4 ± 5.34 (3) | 68% (0.051) | 117 ± 5.33 (4) | 113% (0.315) | 177 ± 12.0 (3) | 171% (0.067) |
| l-Pro | 135 ± 1.99 (16) | 82.8 ± 14.9 (3) | 61% (0.174) | 151 ± 10.0 (3) | 112% (0.449) | 198 ± 13.1 (4) | 147% (0.089) |
| l-Phe | 118 ± 1.87 (16) | 79.4 ± 1.78 (2) | 67% (<0.001) | 91.6 ± 7.79 (4) | 78% (0.197) | 109 ± 8.32 (3) | 92% (0.607) |
The search for genes encoding other putative components of ABC-type amino acid transport systems identified ORF alr3429, which is annotated as GlnH, a periplasmic glutamine-binding protein [43], but is most similar to Synechocystis GtrC, a periplasmic glutamate-binding protein [44]. Although genes encoding an ABC transporter are frequently clustered together, alr3429 upstream and downstream genes have no obvious relation to transport functions. ORF alr3429 was inactivated by insertion of plasmid pCSR23 (based on pRL424) through a single cross-over event producing the Anabaena mutant strain CSR3 (Figure 1B). Strain CSR3 grew well on a solid medium with or without combined nitrogen (not shown). The uptake rate of representative amino acids in this mutant was analyzed, in comparison with the wild type, in filaments that had been grown with nitrate as the nitrogen source. For the tested amino acids, the activity was within a range of about ±20% the wild-type activity (Table 3), giving no support for a specific role of Alr3429 in amino acid transport.
3.2. Predicted Amino Acid Transporters from Other Transporter Families
In the genome of Anabaena, ORF all0342 encodes a predicted 437 amino acid peptide, annotated as H+/Na+-dependent glutamate and aspartate symporter [45], which belongs to the dicarboxylate/amino acid:cation symporter (DAACS) transporter family (TCDB family 2.A.23 [30]). ORF all0342 was inactivated by insertion through a single cross-over event of pCSR13 (based on pRL424), producing Anabaena mutant strain CSR1 (Figure 1C). Strain CSR1 grew well on a solid medium with or without combined nitrogen (not shown). Regarding amino acid uptake, strain CSR1 showed about a 50% increase compared to the wild type in the uptake of some amino acids from all the chemical groups tested (Table 3). This increase was statistically significant for histidine, glutamine, alanine, and glycine (P ≤ 0.05). These results suggest either that All0342 participates in amino acid export, its mutation resulting in retention of some amino acids in the cells, or that lack of All0342 facilitates uptake mediated by other transporters.
ORF alr1519 encodes a predicted 456 amino acid peptide showing homology to transporters of the amino acid-polyamine-organocation (APC) family (TCDB 2.A.3 [30]) that includes solute:cation symporters and solute:solute antiporters [46,47]. ORF alr1519 was inactivated by insertion of the C.S3 cassette (Smr Spr [36]), producing the mutant strain CSS4 (Figure 1D). The growth rate of CSS4 mutant in liquid BG11 medium was similar to that of the wild type (µ, 0.65 day−1 for both strains). However, under diazotrophic conditions the growth rate of the mutant was about 75% that of the wild type (0.36 ± 0.04 day−1 for the CSS4 mutant versus 0.43 ± 0.04 day−1 for the wild type; n = 3). Amino acid uptake was then tested in filaments of strain CSS4 and wild-type Anabaena grown in the presence and absence of combined nitrogen (Table 4). We found only a slight increase in the transport of aspartate, but this alteration was hardly statistically significant. These results give no support for a specific role of Alr1519 in amino acid uptake.
Table 4.
Amino acid transport activities in Anabaena sp. strains PCC 7120 and CSS4. BG11C and BG110C-grown filaments were used in 10-min transport assays with 10 μM [14C]amino acid substrate as described in the Experimental Section. Activities are presented in nmol (mg Chl)-1 per 10 min; the mean ± standard deviation of the mean (SE) from the number of measurements indicated in parenthesis is presented. The percentage of the wild-type activities is also given for the mutant. Differences between the mutant and wild-type activities were assessed by the Student’s t test (P indicated in each case).
| Substrate | Transport activity (nmol [mg Chl]−1) | |||||
|---|---|---|---|---|---|---|
| BG11C | BG110C | |||||
| PCC 7120 | CSS4 (alr1519) | PCC 7120 | CSS4 (alr1519) | |||
| Mean ± SE (n) | Mean ± SE (n) | % (P) | Mean ± SE (n) | Mean ± SE (n) | % (P) | |
| Basic | ||||||
| l-Arg | 116 ± 7.64 (3) | 113 ± 3.16 (2) | 98% (0.864) | 187 ± 8.96 (3) | 167 ± 3.58 (2) | 89% (0.327) |
| Acidic | ||||||
| l-Asp | 30.3 ± 1.03 (4) | 42.0 ± 3.85 (2) | 139% (0.214) | 62.1 ± 0.98 (4) | 77.6 ± 3.57 (3) | 125% (0.119) |
| l-Glu | 10.4 ± 1.48 (4) | 10.3 ± 0.64 (3) | 99% (0.970) | 34.7 ± 1.63 (4) | 32.2 ± 1.90 (3) | 93% (0.619) |
| Neutral polar | ||||||
| l-Gln | 118 ± 4.46 (3) | 125 ± 2.15 (2) | 106% (0.487) | 216 ± 5.30 (3) | 214 ± 2.06 (2) | 99% (0.854) |
| Hydrophobic | ||||||
| l-Ala | 224 ± 12.6 (3) | 215 ± 17.8 (2) | 96% (0.826) | 309 ± 11.7 (3) | 299 ± 14.0 (2) | 108% (0.748) |
3.3. Release of Amino Acids from Vegetative Cells
Anabaena mutants impaired in transport systems N-I and N-II release hydrophobic amino acids, especially alanine, to the culture medium [27,28]. To gain a better understanding of the kinetics of alanine release, the alanine analogue α-aminoisobutyric acid (AIB), which cannot generally be metabolized, was used. In the N-I mutant strain CSR11 (natD) [27], the rate of [14C]AIB uptake was 2.43 ± 0.48% (n = 3) that of the wild type, indicating that the N-I system is responsible for about 98% of the uptake of this compound in Anabaena.
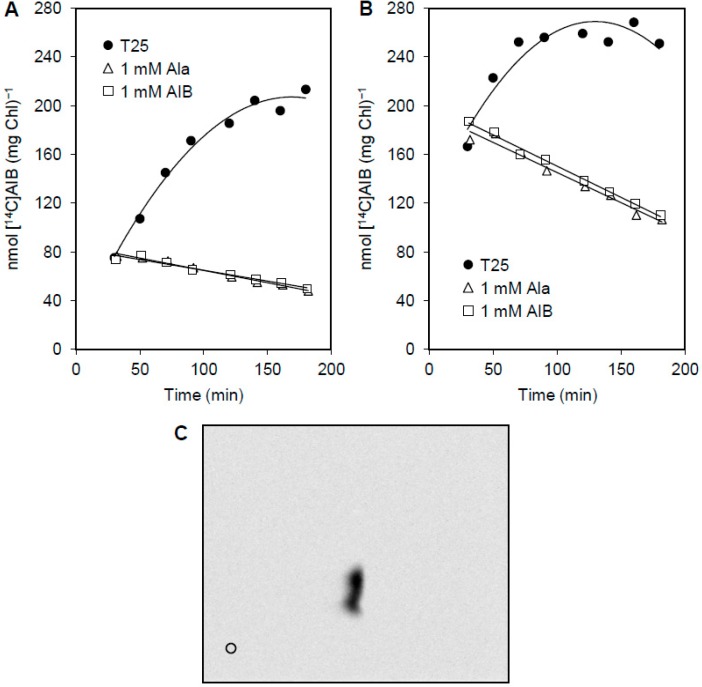
To assay the release of AIB in Anabaena, the following experiment was set up. Filaments grown in the presence of nitrate were incubated with 3 μM [14C]AIB for 40 to 90 min under transport assay conditions. Then, an unlabeled substrate was added at 1 mM concentration and the amount of [14C]AIB remaining in the cells was determined at different times. The release of [14C]AIB in response to the addition of unlabeled substrates could thus be quantified as the decrease in the amount of radioactivity remaining in the cells. In wild-type Anabaena grown with combined nitrogen and incubated with or without combined nitrogen for 24 h, significant release of [14C]AIB was observed in samples supplemented with unlabeled AIB or alanine, but not with a buffer (Figure 2A, B). Note that, at the concentrations used, [14C]AIB uptake likely became negligible when a competitor such as unlabeled AIB or alanine was added, but evidently not when only buffer (T25) was added. The 14C-labeled compound(s) released to the medium were analyzed by TLC and only one spot corresponding to AIB was detected (Figure 2C), consistent with the idea that AIB is not metabolized.
Figure 2.
AIB release in Anabaena. Filaments from BG11 medium (A) or incubated in BG110 medium for 24 h (B) were incubated in Tricine buffer with 3 μM [14C]AIB. After 40 min the filament suspensions were supplemented with 1 mM AIB or alanine in a small volume of buffer or, as a control, with the same volume of buffer (T25). Cell-associated 14C was measured in samples taken from the filament suspensions at the times indicated. (C) A sample of the medium from the end of the assay with BG11-grown cells incubated with [14C]AIB and supplemented with unlabeled AIB was subjected to TLC analysis; o: origin of the chromatography. Only one radioactive spot co-migrating with AIB was detected.
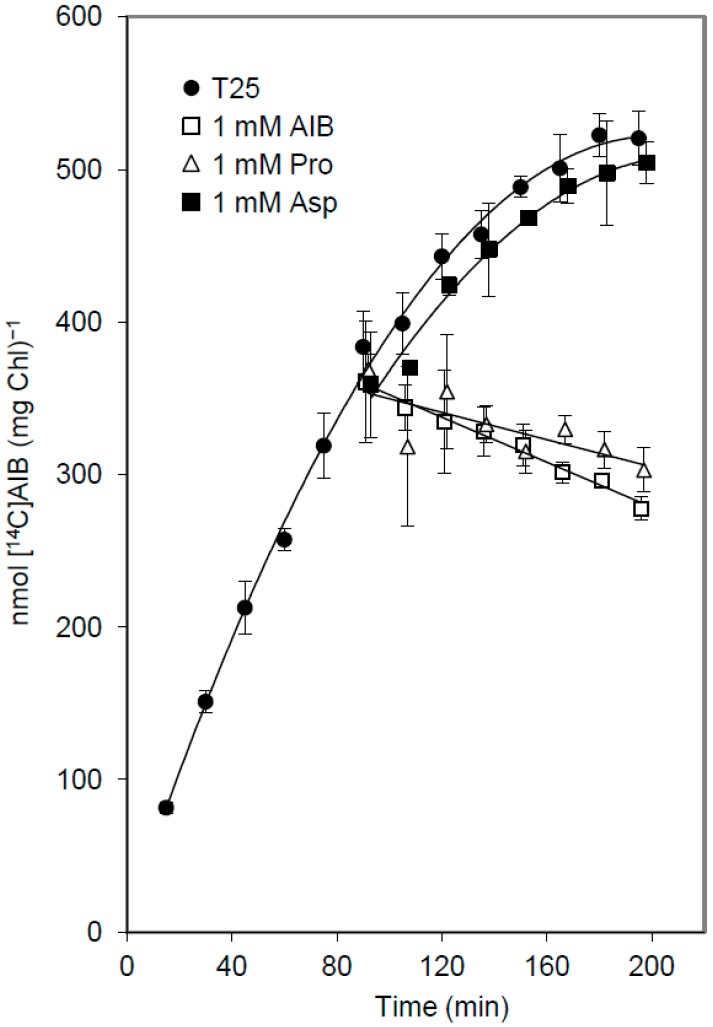
The release of [14C]AIB in response to the addition of different concentrations of AIB ranging from 0.1 to 3 mM was assayed with no significant differences in the rate of release (not shown). To test whether the transport system N-I or N-II is involved in the release of [14C]AIB, assays were performed with specific substrates of these transporters. Filaments were incubated in the presence of 3 μM [14C]AIB for 90 min, and the release of [14C]AIB in response to the addition of an excess of unlabeled AIB, proline (a specific substrate of system N-I), or aspartate (a specific substrate of system N-II) was determined (Figure 3). Whereas the addition of proline produced the release of [14C]AIB in a similar way to AIB, the addition of aspartate did not affect [14C]AIB uptake, which continued in the presence of aspartate. These results indicate that net [14C]AIB release is dependent on the addition of a substrate of transport system N-I. We suggest that [14C]AIB, accumulated within the cells, is released to some extent and then re-incorporated through the N-I transporter, and that saturation of this transporter by unlabeled AIB, alanine (Figure 2), or proline (Figure 3) impedes re-incorporation, permitting us to observe net release.
Figure 3.
Specificity of AIB release in Anabaena. Filaments grown in BG11 medium were incubated in Tricine buffer with 3 μM [14C]AIB. After 90 min the filament suspensions were supplemented with 1 mM AIB, proline, or aspartate in a small volume of buffer or, as a control, with the same volume of buffer (T25). Cell-associated 14C was measured in samples taken from the filament suspensions at the times indicated.
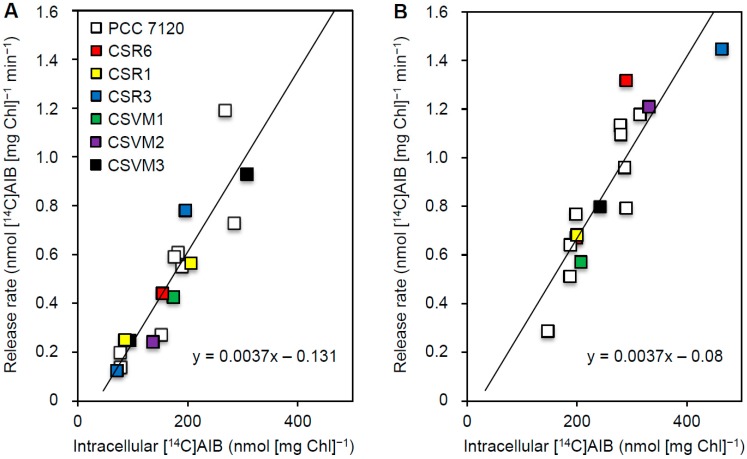
In the experiments of AIB release, we observed that the rate of release depended on the intracellular concentration of [14C]AIB reached in each particular cell suspension, which depended on the time at which unlabeled AIB was added (cell suspensions were assayed at the same growth stage in all experiments). Because the level of accumulated [14C]AIB was known for each assay, we could represent the rate of release as a function of the intracellular [14C]AIB concentration (see data for the wild type, PCC 7120, in Figure 4). A linear relation, y = 0.004x + b, where y is the release rate and x the intracellular concentration of [14C]AIB, could be established, with r2 coefficients of 0.779 for BG11-grown filaments and 0.913 for filaments that had been incubated in the absence of combined nitrogen (BG110 medium).
Figure 4.
Kinetics of AIB release. The rate of [14C]AIB release in response to the addition of 1 mM unlabeled AIB to filaments incubated in Tricine buffer with 3 μM [14C]AIB was measured. Unlabeled AIB was added after 40 to 90 min of incubation with [14C]AIB. (A) Nitrate-grown filaments. (B) Filaments incubated in BG110 medium for 24 h. Assuming an intracellular volume of 125 µL (mg Chl)−1, the range of intracellular [14C]AIB concentrations reached in the assays was from about 0.5 to 4 mM.
Blast searches of the Anabaena genome for genes homologous to exporters from the EamA (amino acid, metabolite efflux pumps) family (TCDB 2.A.7.3.2, which belongs to the Drug and Metabolite Exporter [DME] family, TCDB 2.A.7.3 [30]) identified three open reading frames: all1189, alr1538, and all3551. Each of these ORFs was inactivated by insertion through single cross-over of a plasmid based on pCSV3 (Smr Spr). Insertion of pCSVM4, pCSVM5, and pCSVM6 produced mutant strains CSVM1, CSVM2, and CSVM3, respectively (Figure 1). The three mutants grew similarly to the wild type both in the presence and in the absence of combined nitrogen (not shown). Activity of [14C]AIB release in response to the addition of unlabeled AIB was tested in mutant strains CSVM1, CSVM2, and CSVM3, as well as in CSR1, CSR6, and CSR3 described above. Significant export was observed in every case, providing no indication of the involvement in AIB release of the protein encoded by any of the mutated genes. Instead, we observed that the rate of [14C]AIB release in the mutants fitted the relation to intracellular [14C]AIB concentration previously established for the wild type. Adding the data from the mutants to those of the wild type, the same linear relation was observed with r2 coefficients of 0.818 for BG11-grown filaments and 0.764 for filaments that had been incubated in BG110 medium. The linear dependence of the rate of AIB release on the intracellular concentration of AIB suggests that the release takes place by diffusion. Additionally, the fact that b takes a negative value (−0.131 for BG11-grown cells and −0.080 for BG110-incubated cells) implies that there was an intracellular concentration threshold for the observation of [14C]AIB release. Assuming an intracellular volume of 125 µL (mg Chl)−1 [48,49], this threshold was in the range 0.17–0.28 mM. This concentration might correspond to AIB immobilized through its interaction with cellular components.
4. Discussion
In this work we have investigated new possible amino acid transporters encoded in the genome of the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. The amino acid transport systems previously known in this cyanobacterium are the ABC-type transporters N-I, N-II, and Bgt [23,27,28]. The genes encoding the elements of each of these transporters are summarized in Table 1. The N-I system is encoded by five genes, which are spread in the genome: natA (all1046) and natE (all2912) encode ATPases, natC (all1047) and natD (all1284) encode transmembrane (permease) proteins, and natB (alr1834) encodes a periplasmic substrate-binding protein [27]. The system N-II is encoded by a cluster of four genes: natF (alr4164) encodes a periplasmic substrate-binding protein, natG (alr4165) and natH (alr4166) encode transmembrane (permease) proteins, and bgtA (alr4167) encodes an ATPase [28]. The third one, the system Bgt, is the product of bgtB (alr3187), which encodes a permease protein with an extended periplasmic domain with homology to substrate binding proteins, and bgtA (alr4167), which encodes an ATPase that is shared with the system N-II [28].
Seven loci have been investigated here, but evidence for the involvement in amino acid transport was only found for two of them (see Results). A cluster of genes encoding the elements of a new ABC-type amino acid uptake transporter has been identified in this work: alr2535 encodes a predicted periplasmic binding protein, alr2536 and alr2538 encode predicted transmembrane (permease) proteins, and alr2539 and alr2541 encode predicted ATPases. As judged from the transport activities displayed by an alr2536 insertional mutant, this putative transporter can mediate uptake of glycine and other hydrophobic amino acids (Table 1). We have termed this amino acid transporter system N-III. Similarly to the previously characterized amino acid transport systems N-I and N-II of Anabaena, system N-III mainly transports one group of amino acids (hydrophobic amino acids), but has a relatively low specificity, being able to mediate as well the uptake of some other amino acids such as glutamate and glutamine. Nonetheless, system N-III is phylogenetically most similar to transporters in the Hydrophobic Amino Acid Uptake Transporter (HAAT) family (TCDB 3.A.1.4 [30]), as the N-I system is.
Members of the DAACS family of transporters (TCDB 2.A.23 [30]) are involved in the uptake of different substrates, including amino acids, mediated by a solute:Na+ symport mechanism [45]. Anabaena ORF all0342 encodes a protein that is homologous to members of this family. The analysis of a mutant of this gene showed increased uptake of a number of amino acids. These results could be interpreted assuming that All0342 participates in amino acid export with a low specificity, since basic, neutral, and hydrophobic amino acids were affected. An effect on amino acid export implies that blocking export would result in increased retention within the cells of amino acids taken up by different transporters. Alternatively, All0342 might work in amino acid uptake competing with other transporters, so that the lack of All0342 would facilitate uptake mediated by those transporters.
Release of amino acids to the culture medium is a characteristic feature of some amino acid transport mutants. In the Anabaena nat mutants the set of released amino acids includes alanine, glycine, isoleucine, leucine, phenylalanine, proline, tyrosine, and valine, which are transported mainly by system N-I [23,27]. Because the released amino acids are all hydrophobic, they may leak out of the cells by diffusion, and a function of the N-I system may be the recapture of these leaked amino acids [23,31,32]. In order to test this further, a method to study kinetically the release of AIB from vegetative cells has been set up. Release of AIB is observed only when the N-I system is engaged in the uptake of specific substrates of this transporter, but not when a specific substrate of the N-II system is added. Because the N-I system is responsible for 98% of the uptake of AIB, these data support a function of the N-I system in recapturing the leaked amino acids. With the aim of identifying possible molecular actors of the release of AIB from Anabaena cells, the kinetics of AIB release was studied in wild-type Anabaena and some of the mutants generated in this work. The rate of release of AIB directly depends on the intracellular concentration of the substrate, following a first-order equation, which is consistent with diffusion. This could be simple diffusion through the lipid bilayer [50,51] or facilitated diffusion [52]. None of the investigated mutants was, however, impaired in release of AIB and, indeed, all of them showed the same trend as the wild type. Thus, if release were facilitated, proteins encoded by genes other than those inactivated in this work would be involved. We suggest that hydrophobic amino acids can leak out from the Anabaena filaments to the culture medium and that the ABC-type transporter N-I is important for recapturing the amino acids that have been lost from the cells. This function could be especially relevant under diazotrophic conditions, in which nitrogen is at a premium.
The genome of Anabaena is predicted to contain 6223 genes [53], of which 481 are predicted to encode transport proteins [54]. ABC-type transporters are particularly abundant in cyanobacteria as compared to other bacteria [55], and proteins constituting ABC-type transporters represent in Anabaena about 61% of the total number of membrane transporter proteins [54]. The ample use of ABC-type transporters in cyanobacteria may be related to the fact that in these organisms the bulk of energy conservation takes place in intracellular membranes, the photosynthetic thylakoids, which are separated from the cytoplasmic membrane. ATP generated in the thylakoids can then be used for cytoplasmic membrane transport directly by ABC-type transporters. Whereas many transporters in Anabaena, including ABC-type and other transporters, are involved in the uptake of inorganic nutrients [56], there are also many transporters that mediate the uptake of organic substances including, for instance, sugars [22,57] and oxo-acids [58]. The mainly photoautotrophic lifestyle of organisms such as Anabaena and most cyanobacteria may be therefore complemented by the ability to utilize organic compounds that could be found in their natural habitats.
5. Conclusions
The genome of the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120 contains a large number of genes encoding membrane transport proteins, many of which are elements of ABC-type transporters. Here we identified a new ABC-type uptake transporter, system N-III, mainly involved in the uptake of hydrophobic amino acids. We also identified a membrane protein, All0342, homologous to transporters of the dicarboxylate/amino acid:cation symporter (DAACS) family, whose mutation results in an increased accumulation of a wide range of amino acids in uptake assays. Finally, studying α-aminoisobutyric acid (AIB) export, we found that release of hydrophobic amino acids from cyanobacterial cells likely takes place by means of diffusion.
Acknowledgments
This work was supported by the Plan Nacional de Investigación, Spain, co-financed by the European Regional Development Fund (grants BFU2005-07672, BFU2008-03811, and BFU2011-22762).
Author Contributions
All authors designed and interpreted experimental work; Rafael Pernil, Silvia Picossi and Vicente Mariscal performed experiments; Rafael Pernil, Antonia Herrero, Enrique Flores and Vicente Mariscal drafted the manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Flores E., Herrero A. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 2010;8:39–50. doi: 10.1038/nrmicro2242. [DOI] [PubMed] [Google Scholar]
- 2.Thomas J., Meeks J.C., Wolk C.P., Shaffer P.W., Austin S.M. Formation of glutamine from [13N]ammonia, [13N]dinitrogen, and [14C]glutamate by heterocysts isolated from Anabaena cylindrica. J. Bacteriol. 1977;129:1545–1555. doi: 10.1128/jb.129.3.1545-1555.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnat M., Herrero A., Flores E. Compartmentalized cyanophycin metabolism in the diazotrophic filaments of a heterocyst-forming cyanobacterium. Proc. Natl. Acad. Sci. USA. 2014;111:3823–3828. doi: 10.1073/pnas.1318564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jüttner F. 14C-labeled metabolites in heterocysts and vegetative cells of Anabaena cylindrica filaments and their presumptive function as transport vehicles of organic carbon and nitrogen. J. Bacteriol. 1983;155:628–633. doi: 10.1128/jb.155.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-Igual R., Flores E., Herrero A. Inactivation of a heterocyst-specific invertase indicates a principal role of sucrose catabolism in heterocysts of Anabaena sp. J. Bacteriol. 2010;192:5526–5533. doi: 10.1128/JB.00776-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pernil R., Herrero A., Flores E. Catabolic function of compartmentalized alanine dehydrogenase in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 2010;192:5165–5172. doi: 10.1128/JB.00603-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas W.A., Nishi C.N., Giarrocco L.E., Salerno G.L. Differential roles of alkaline/neutral invertases in Nostoc sp. PCC 7120: Inv-B isoform is essential for diazotrophic growth. Planta. 2011;233:153–162. doi: 10.1007/s00425-010-1288-5. [DOI] [PubMed] [Google Scholar]
- 8.Ayre B.G. Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol. Plant. 2011;4:377–394. doi: 10.1093/mp/ssr014. [DOI] [PubMed] [Google Scholar]
- 9.Kolman M.A., Nishi C.N., Perez-Cenci M., Salerno G.L. Sucrose in cyanobacteria: From a salt-response molecule to play a key role in nitrogen fixation. Life. 2015;5:102–126. doi: 10.3390/life5010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martín-Figueroa E., Navarro F., Florencio F.J. The GS-GOGAT pathway is not operative in the heterocysts. Cloning and expression of glsF gene from the cyanobacterium Anabaena sp. PCC 7120. FEBS Lett. 2000;476:282–286. doi: 10.1016/S0014-5793(00)01722-1. [DOI] [PubMed] [Google Scholar]
- 11.Sherman D.M., Tucker D., Sherman L.A. Heterocyst development and localization of cyanophycin in N2-fixing cultures of Anabaena sp. PCC 7120 (Cyanobacteria) J. Phycol. 2000;36:932–941. doi: 10.1046/j.1529-8817.2000.99132.x. [DOI] [Google Scholar]
- 12.Wolk C.P., Thomas J., Shaffer P.W., Austin S.M., Galonsky A. Pathway of nitrogen metabolism after fixation of 13N-labeled nitrogen gas by the cyanobacterium, Anabaena cylindrica. J. Biol. Chem. 1976;251:5027–5034. [PubMed] [Google Scholar]
- 13.Mariscal V., Herrero A., Flores E. Continuous periplasm in a filamentous, heterocyst-forming cyanobacterium. Mol. Microbiol. 2007;65:1139–1145. doi: 10.1111/j.1365-2958.2007.05856.x. [DOI] [PubMed] [Google Scholar]
- 14.Haselkorn R. Cell-cell communication in filamentous cyanobacteria. Mol. Microbiol. 2008;70:783–785. doi: 10.1111/j.1365-2958.2008.06475.x. [DOI] [PubMed] [Google Scholar]
- 15.Mariscal V., Flores E. Multicellularity in a heterocyst-forming cyanobacterium: Pathways for intercellular communication. Adv. Exp. Med. Biol. 2010;675:123–135. doi: 10.1007/978-1-4419-1528-3_8. [DOI] [PubMed] [Google Scholar]
- 16.Mariscal V. Cell-cell joining proteins in heterocyst-forming cyanobacteria. In: Flores E., Herrero A., editors. The Cell Biology of Cyanobacteria. Caister Academic Press; Norfolk, UK: 2014. pp. 293–304. [Google Scholar]
- 17.Mullineaux C.W., Mariscal V., Nenninger A., Khanum H., Herrero A., Flores E., Adams D.G. Mechanism of intercellular molecular exchange in heterocyst-forming cyanobacteria. EMBO J. 2008;27:1299–1308. doi: 10.1038/emboj.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merino-Puerto V., Schwarz H., Maldener I., Mariscal V., Mullineaux C.W., Herrero A., Flores E. FraC/FraD-dependent intercellular molecular exchange in the filaments of a heterocyst-forming cyanobacterium, Anabaena sp. Mol. Microbiol. 2011;82:87–98. doi: 10.1111/j.1365-2958.2011.07797.x. [DOI] [PubMed] [Google Scholar]
- 19.Flores E., Pernil R., Muro-Pastor A.M., Mariscal V., Maldener I., Lechno-Yossef S., Fan Q., Wolk C.P., Herrero A. Septum-localized protein required for filament integrity and diazotrophy in the heterocyst forming cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 2007;189:3884–3890. doi: 10.1128/JB.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merino-Puerto V., Mariscal V., Mullineaux C.W., Herrero A., Flores E. Fra proteins influencing filament integrity, diazotrophy and localization of septal protein SepJ in the heterocyst-forming cyanobacterium Anabaena sp. Mol. Microbiol. 2010;75:1159–1170. doi: 10.1111/j.1365-2958.2009.07031.x. [DOI] [PubMed] [Google Scholar]
- 21.Flores E., Herrero A., Wolk C.P., Maldener I. Is the periplasm continuous in filamentous multicellular cyanobacteria? Trends Microbiol. 2006;14:439–443. doi: 10.1016/j.tim.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Nicolaisen K., Mariscal V., Bredemeier R., Pernil R., Moslavac S., López-Igual R., Maldener I., Herrero A., Schleiff E., Flores E. The outer membrane of a heterocyst-forming cyanobacterium is a permeability barrier for uptake of metabolites that are exchanged between cells. Mol. Microbiol. 2009;74:58–70. doi: 10.1111/j.1365-2958.2009.06850.x. [DOI] [PubMed] [Google Scholar]
- 23.Montesinos M.L., Herrero A., Flores E. Amino acid transport systems required for diazotrophic growth in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 1995;177:3150–3157. doi: 10.1128/jb.177.11.3150-3157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flores E., Muro-Pastor M.I. Uptake of glutamine and glutamate by the dinitrogen-fixing cyanobacterium Anabaena sp. PCC 7120. FEMS Microbiol. Lett. 1988;56:127–130. doi: 10.1111/j.1574-6968.1988.tb03163.x. [DOI] [Google Scholar]
- 25.Herrero A., Flores E. Transport of basic amino acids by the dinitrogen-fixing cyanobacterium Anabaena PCC 7120. J. Biol. Chem. 1990;265:3931–3935. [PubMed] [Google Scholar]
- 26.Xu P., McAuley P.J. Uptake of amino acids by the cyanobacterium Anabaena ATCC 27893. New Phytol. 1990;115:581–585. doi: 10.1111/j.1469-8137.1990.tb00488.x. [DOI] [Google Scholar]
- 27.Picossi S., Montesinos M.L., Pernil R., Lichtlé C., Herrero A., Flores E. ABC-type neutral amino acid permease N-I is required for optimal diazotrophic growth and is repressed in the heterocysts of Anabaena sp. strain PCC 7120. Mol. Microbiol. 2005;57:1582–1592. doi: 10.1111/j.1365-2958.2005.04791.x. [DOI] [PubMed] [Google Scholar]
- 28.Pernil R., Picossi S., Mariscal V., Herrero A., Flores E. ABC-type amino acid uptake transporters Bgt and N-II of Anabaena sp. strain PCC 7120 share an ATPase subunit and are expressed in vegetative cells and heterocysts. Mol. Microbiol. 2008;67:1067–1080. doi: 10.1111/j.1365-2958.2008.06107.x. [DOI] [PubMed] [Google Scholar]
- 29.Busch W., Saier M.H., Jr. The transporter classification (TC) system. Crit. Rev. Biochem. Mol. Biol. 2002;37:287–337. doi: 10.1080/10409230290771528. [DOI] [PubMed] [Google Scholar]
- 30.Transporter Classification Database. [(accessed on 15 April 2015)]. Available online: http://www.tcdb.org.
- 31.Labarre J., Thuriaux P., Chauvat F. Genetic analysis of amino acid transport in the facultatively heterotrophic cyanobacterium Synechocystis sp. strain 6803. J. Bacteriol. 1987;169:4668–4673. doi: 10.1128/jb.169.10.4668-4673.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montesinos M.L., Herrero A., Flores E. Amino acid transport in taxonomically diverse cyanobacteria and identification of two genes encoding elements of a neutral amino acid permease putatively involved in recapture of leaked hydrophobic amino acids. J. Bacteriol. 1997;179:853–862. doi: 10.1128/jb.179.3.853-862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rippka R., Deruelles J., Waterbury J.B., Herdman M., Stanier R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979;111:1–61. doi: 10.1099/00221287-111-1-1. [DOI] [Google Scholar]
- 34.Cai Y.P., Wolk C.P. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J. Bacteriol. 1990;172:3138–3145. doi: 10.1128/jb.172.6.3138-3145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K. Current Protocols in Molecular Biology. Greene Publishing and Wiley-Interscience; New York, NY, USA: 2015. [Google Scholar]
- 36.Elhai J., Wolk C.P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 37.Frías J.E., Flores E., Herrero A. Activation of the Anabaena nir operon promoter requires both NtcA (CAP family) and NtcB (LysR family) transcription factors. Mol. Microbiol. 2000;38:613–625. doi: 10.1046/j.1365-2958.2000.02156.x. [DOI] [PubMed] [Google Scholar]
- 38.Black T.A., Cai Y., Wolk C.P. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 1993;9:77–84. doi: 10.1111/j.1365-2958.1993.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 39.Elhai J., Vepritskiy A., Muro-Pastor A.M., Flores E., Wolk C.P. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J. Bacteriol. 1997;179:1998–2005. doi: 10.1128/jb.179.6.1998-2005.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markwell M.A.K., Hass S.M., Bieber L.L., Tolbert N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 41.Mackinney G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941;140:109–112. [Google Scholar]
- 42.Hoshino T., Kose K. Cloning and nucleotide sequence of braC, the structural gene for the leucine-, isoleucine-, and valine-binding protein of Pseudomonas aeruginosa PAO. J. Bacteriol. 1989;171:6300–6306. doi: 10.1128/jb.171.11.6300-6306.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto S., Yamanishi Y., Ehira S., Kawashima S., Tonomura K., Kanehisa M. Prediction of nitrogen metabolism-related genes in Anabaena by kernel-based network analysis. Proteomics. 2007;7:900–909. doi: 10.1002/pmic.200600862. [DOI] [PubMed] [Google Scholar]
- 44.Quintero M.J., Montesinos M.L., Herrero A., Flores E. Identification of genes encoding amino acid permeases by inactivation of selected ORFs from the Synechocystis genomic sequence. Genome Res. 2001;11:2034–2040. doi: 10.1101/gr.196301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slotboom D.J., Konings W.N., Lolkema J.S. Structural features of the glutamate transporter family. Microbiol. Mol. Biol. Rev. 1999;63:293–307. doi: 10.1128/mmbr.63.2.293-307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong F.H., Chen J.S., Reddy V., Day J.L., Shlykov M.A., Wakabayashi S.T., Saier M.H., Jr. The amino acid-polyamine-organocation superfamily. J. Mol. Microbiol. Biotechnol. 2012;22:105–113. doi: 10.1159/000338542. [DOI] [PubMed] [Google Scholar]
- 47.Rudnick G., Krämer R., Blakely R.D., Murphy D.L., Verrey F. The SLC6 transporters: Perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch. 2014;466:25–42. doi: 10.1007/s00424-013-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ihlenfeldt M.J.A., Gibson J. CO2 fixation and its regulation in Anacystis nidulans (Synechococcus) Arch. Microbiol. 1975;102:13–21. doi: 10.1007/BF00428339. [DOI] [PubMed] [Google Scholar]
- 49.Raboy B., Padan E. Active transport of glucose and α-methylglucoside in the cyanobacterium Plectonema boryanum. J. Biol. Chem. 1978;253:3287–3291. [PubMed] [Google Scholar]
- 50.Klein R.A., Moore M.J., Smith M.W. Selective diffusion of neutral amino acids across lipid bilayers. Biochim. Biophys. Acta. 1971;233:420–433. doi: 10.1016/0005-2736(71)90339-7. [DOI] [PubMed] [Google Scholar]
- 51.Chakrabarti A.C. Permeability of membranes to amino acids and modified amino acids: Mechanisms involved in translocation. Amino Acids. 1994;6:213–229. doi: 10.1007/BF00813743. [DOI] [PubMed] [Google Scholar]
- 52.Krämer R. Secretion of amino acids by bacteria: Physiology and mechanism. FEMS Microbiol. Rev. 1994;13:75–93. doi: 10.1111/j.1574-6976.1994.tb00036.x. [DOI] [Google Scholar]
- 53.CyanoBase. [(accessed on 15 April 2015)]. Available online: http://genome.microbedb.jp/cyanobase/Anabaena.
- 54.TransportDB. [(accessed on 15 April 2015)]. Available online: http://www.membranetransport.org/index_v2_rc1.html.
- 55.Paulsen I.T., Nguyen L., Sliwinski M.K., Rabus R., Saier M.H., Jr. Microbial genome analyses: Comparative transport capabilities in eighteen prokaryotes. J. Mol. Biol. 2000;301:75–100. doi: 10.1006/jmbi.2000.3961. [DOI] [PubMed] [Google Scholar]
- 56.Hahn A., Schleiff E. The cell envelope. In: Flores E., Herrero A., editors. The Cell Biology of Cyanobacteria. Caister Academic Press; Norfolk, UK: 2014. pp. 29–87. [Google Scholar]
- 57.Stebegg R., Wurzinger B., Mikulic M., Schmetterer G. Chemoheterotrophic growth of the cyanobacterium Anabaena sp. strain PCC 7120 dependent on a functional cytochrome c oxidase. J. Bacteriol. 2012;194:4601–607. doi: 10.1128/JB.00687-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pernil R., Herrero A., Flores E. A TRAP transporter for pyruvate and other monocarboxylate 2-oxoacids in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 2010;192:6089–6092. doi: 10.1128/JB.00982-10. [DOI] [PMC free article] [PubMed] [Google Scholar]