Abstract
Acute and chronic pain control is a significant clinical challenge that has been largely unmet. Local anesthetics are widely used for the control of post-operative pain and in the therapy of acute and chronic pain. While a variety of approaches are currently used to prolong the duration of action of local anesthetics, an optimal strategy to achieve neural blockage for several hours to days with minimal toxicity has yet to be identified. Several drug delivery systems such as liposomes, microparticles and nanoparticles have been investigated as local anesthetic delivery vehicles to achieve prolonged anesthesia. Recently, injectable responsive hydrogels raise significant interest for the localized delivery of anesthetic molecules. This paper discusses the potential of injectable hydrogels to prolong the action of local anesthetics.
Pain is the most common presenting complaint to physicians as a result of accidental or sports related injuries and various diseases. A number of factors influence an individual’s reaction to pain including age, cultural background, perception, past experiences, social expectations, physical and mental health, parental attitudes concerning pain, fear, anxiety, fatigue and the setting in which pain occurs [1,2]. The experience of pain can be broadly classified into acute and chronic pain [3]. Acute pain can be modulated and removed by treating its cause and is usually a sequel to surgery or trauma. Chronic pain is more complex and the source of pain may be difficult to eliminate. Relieving pain has been shown to result in improved healing, faster recovery and an earlier return to former activities and lifestyle [4].
The two major classes of analgesics used for treating pain are opioids and nonsteroidal anti-inflammatory drugs (NSAIDs). The primary mode of action of these analgesics differs; some act centrally, some peripherally and others exert their effect at multiple locations. First-line treatments for pain usually involve nonopioid analgesic agents including aspirin, paracetamol and NSAIDs. These nonopioid analgesics have advantageous in relieving acute pain because they are readily available without a prescription, relatively inexpensive and easy to use. However, many of these nonopioid drugs may only be effective for mild to moderate pain. For patients with moderate to severe pain, opioid analgesics or nonopioid analgesics combined with other analgesic agents are considered. Opioids such as morphine are considered as the gold standard for the management of pain [5]. However, opioid treatments are often associated with a wide range of side effects such as splinting, sedation, nausea, vomiting, impaired bowel motility and development of tolerance (particularly in the case of chronic pain management) [6]. Even though opioids are known to produce analgesia primarily through their actions in the central nervous system, studies have shown the presence and activation of peripheral opioid receptors in painful inflammatory conditions [7]. These drugs provide a critical armamentarium of pain management options and continued research toward development of more potent, more site specific, less toxic and less abusable molecules will further enhance their utility.
The use of local anesthetics to treat pain has many potential advantages compared with the systemic administration of opioid analgesics, in situations where the cause and source of the pain is limited to a particular site or region [8]. It is often used as an adjunct to systemic analgesia, or to provide epidural or anesthesia. Offsetting the use of opioids and NSAIDS with local blockade permits the use of smaller amounts of systemic drugs, thus lowering risks of side effects and potential toxicity. Natural-derived and synthetic local anesthetic molecules, specifically amino amides and amino esters, are currently under investigation (Table 1) [9]. Common local anesthetics include bupivicaine, chloroprocaine, lidocaine, procaine and tetracaine. They work by numbing the target area without causing unconsciousness. The underlying molecular mechanism of local anesthetics is mainly the inhibition of sodium influx through voltage-gated sodium-specific ion channels in the neuronal cell membrane, which prevents transmission of nerve impulses where local anesthetics are applied [10]. Recent research efforts have been focused on establishing appropriate formulations in order to optimize therapeutic efficacy [11]. For instance, eutectic mixture of local anesthetics, known as EMLA, is a mixture of equal amounts of two well-known local anesthetics, prilocaine and lidocaine. EMLA is designed for topical anesthesia during blood sampling, placement of intravenous cannulae and minor superficial procedures [12,13]. Similarly, a eutectic mixture of lidocaine and tetracaine is used to numb the skin before certain minor procedures [12] and tetracaine-epinephrine-cocaine (TAC) solution is indicated as a local dermal anesthetic for the emergency treatment of uncomplicated lacerations [14]. While promising, utilizing synthetic local anesthetic formulations has the potential to cause significant health issues, such as systemic toxicity and adverse local tissue reaction [9,11,15,16].
Table 1.
Classification of nonopioid local anesthetics.
| Amino amides | Amino esters | Naturally derived local anesthetics |
|---|---|---|
| Articaine | Amethocaine | Cabratoxin |
| Bupivacaine | Benzocaine | Tetrodotoxin |
| Dibucaine | Chloroprocaine | Menthol |
| Etidocaine | Cocaine | Neosaxitoxin |
| Levobupivacaine | Cyclomethycaine | Saxitoxin |
| Lignocaine | Dimethocaine | |
| Mepivacaine | Lidocaine | |
| Prilocaine | Piperocaine | |
| Ropivacaine | Propoxycaine | |
| Procaine | ||
| Proparacaine | ||
| Tetracaine |
Current research, therefore, has been focused on the identification of novel local anesthetics with protracted analgesic effect and minimal toxicity. Pharmacological studies have revealed the potent analgesic activity of several neurotoxins isolated from animals, plants and marine organisms. These naturally derived molecules are of particular interest because of their strong pharmacological activity, selectivity, low cytotoxicity and specificity for the site of action. For instance, α-cobratoxin (α-CTx), isolated from the Thailand Cobra, has strong affinity for the α7 subunit of the nAChR (α7nAChR) neuronal receptor [17], which is predominantly located in the peripheral nervous system. It is believed that activation of α7nAChR by αCTx leads to the depolarization of postsynaptic membranes and the prevention of neurotransmitter release [18]. Thus, αCTx has significant potential to serve as a new regimen to combat localized pain with long-lasting analgesic activity, nondependence, relatively low cost and commercial availability [19]. More well-controlled clinical studies are needed to establish the benefits of utilizing naturally derived local anesthetics on patients. In addition, future research will be able to identify additional analgesic molecules for localized pain relief that are more affordable, safer and have longer lasting effects compared with the presently available medications.
Several biomaterial based controlled delivery systems are currently been investigated as an alternative approach to extend the activity of fast-acting local anesthetic molecules. Controlled delivery local anesthetic formulations have the potential to serve as a safe, localized, long-acting postoperative pain management system. Several carriers including liposomes, microparticles and nanoparticles have been investigated with some success; however, they can freely diffuse from the injection site presenting limitations for long-term localized anesthesia. Recently, research has been focused on developing responsive hydrogel carriers as unique delivery vehicles for local anesthetic molecules. The review focuses on the recent advances in the area of hydrogel based carriers for prolonged delivery of local anesthetic molecules.
Long-acting local anesthetics for orthopedic applications
The usage of long-acting local anesthetics in the management of chronic pain in orthopaedics is twofold: to enable diagnosis of some chronic pain conditions and to provide relief for some nonsurgical pain conditions [20,21]. Table 2 shows some chronic pain diagnoses in which local anesthetics play a role in treatment and management:
Table 2.
Local anesthetics in treatment and diagnoses.
| Local anesthetics assist with the diagnosis (and treatment) of: | Local anesthetics assist with the relief of nonsurgical chronic pain in: |
|---|---|
| Facet joint mediated pain (Medial Branch Blocks) | Sympathetically-mediated pain syndrome such as Complex Regional Pain Syndromes (CRPS) |
| Sacroiliac (SI) joint mediated pain | Meralgia paresthetica |
| Localized myofacial pain syndromes- myofacial trigger points, piriformis syndrome | Occipital neuralgia |
| Diffuse myofacial pain syndrome- fibromyalgia tender points | Post-herpetic neuralgia |
| Scar pain - post-epesiotomy, post-hernia repair, post- surgery |
Clinically, local anesthetics cause a reversible loss of nociception and local anesthesia. In the treatment of chronic pain, local anesthetics can be applied to a number of tissues, including nerve, joint and muscle. When used on nerve structures (nerve block), specific local anesthetic effects include analgesia (loss of pain sensation) and paralysis (loss of muscle power). A delicate balance between these two effects, analgesia and paralysis, must be obtained to optimally treat patients clinically. For the treatment of chronic pain, which is typically an outpatient service, analgesia rather than paralysis is the desired effect by a number order of magnitude. Simply put, the clinician wants the patient to have reduced pain but to be able to walk out of the office and go back home. Thus, a local anesthetic with a longer duration of action and a potency that gives a sensory block with minor motor block would be very valuable for the practicing clinician.
Spinal facet joint mediated pain is diagnosed with either an intra-articular joint injection or a medial branch nerve block. A longer acting injectable local anesthetic, one that lasts 7–20 days, can give the patient longer pain relief so as to complete a course of physical therapy or lend to specific diagnosis. This may also be true with the diagnosis of SI joint mediated pain, which is diagnosed similarly to facet joint mediated pain. In myofacial pain syndromes, such as trigger points and fibromyalgia, physical therapy is the mainstay of treatment. Local anesthetic injections are used to cause analgesia in the painful muscles while a patient completes physical therapy. The longer acting the medication is (e.g., 7–20 days), the fewer injections the patient will need during an 8 to 12 week course of physical therapy. A longer acting local anesthetic can also assist with long-term treatment and diagnosis of sympathetically mediated pain syndromes such as complex regional pain syndromes of the extremities. Lastly, localized peripheral neuropathies, such as meralgia paresthetica and occipital neuralgia, are typically treated with long-acting local anesthetics.
Clinically the duration of action, toxicities and patient-specific factors (such as allergies) largely determine the choice of anesthetics used. The currently used depo-long acting versions of the injectable local anesthetics provide prolonged anesthesia due to the inherent structure of the anesthetic rather than from the delivery mechanism. Examples include lidocaine (moderate potency and duration), procaine (low potency and duration) and bupivacaine (high potency and duration). Increasing the drug concentration (e.g., 1% lidocaine vs 2% lidocaine), can increase the duration but can also increase the medication’s potency this in turn can increase the risk of toxicity and motor nerve involvement (loss of muscle power) [22]. Transdermal lidocaine, via either ointment or patch, has a long duration of action but also has some negative factors. The disadvantages are the low depth of skin penetration limits medication usage to topical surface structures such as the knee joint, and the gel or patch occasionally does not fit the treated location nor stay in place.
Systemic anesthetic usage has very few indications in the treatment of chronic pain. One such group of pain diagnoses is chronic neuropathic pain. Broader indications for their use are with cardiac arrhythmic care. However, the window between toxicity and clinically useful dosing for systemic anesthetics is very narrow. Local anesthetics are safer than general or systemic anesthetics; therefore, they are used whenever possible. In addition, local anesthetics are relatively easy to administer and readily available. However, high doses of local anesthetics also have significant side effects as discussed before. One such anesthetic with some limitations due to toxicity is bupivacaine and its application in joint injections [23]. This has not been determined for muscle or nerve tissues and as such bupivacaine is used for diagnosis and treatment for some painful conditions associated with these tissues. Bupivacaine is long acting so it does have value, but a long acting 1 or 2% lidocaine could be used in its place when toxicities or allergies are a concern.
In summary, the use of long-acting local anesthetics that elicit complete neural blockage for several hours to days is highly desirable in the management of acute and chronic pain [24]. Current research is focused on developing controlled release strategies to prolong the action of the existing anesthetic molecules over a period of days rather than hours, with reduced side effects, to significantly enhance their utility in pain management.
Responsive hydrogels
It has been widely acknowledged that responsive hydrogels may have potential in the biomedical and pharmaceutical fields as a minimally invasive delivery system with controlled release properties [25–27]. Hydrogels are cross-linked networks of hydrophilic polymers with very high water content [28]. The tissue-mimic properties of hydrogel matrices have been shown to be effective in the controlled release of drugs as well as scaffolds for tissue engineering.
Recently, research has focused on developing ‘smart’ or ‘intelligent’ hydrogels that have the ability to respond to subtle changes in the surrounding environment [29–32]. The potential of ‘smart’ hydrogels stems from their ability to make rapid structural changes, volume-phase transitions or sol-gel transitions in response to certain stimuli [29,30,33]. Reactions may be triggered by a number of environmental changes, with the most extensively studied being thermo-responsive and pH-sensitive hydrogels [34]. Hydrogels have also been developed to respond to other external triggers such as light, ions, protein, DNA and electromagnetic radiation [28–34]. Recently, hydrogels have been developed that can respond simultaneously to multiple triggers, such as pH and temperature [35].
Thermo-sensitive hydrogels
Thermo-sensitive hydrogels are among the most heavily investigated type of stimuli-responsive hydrogels due to their practical applications in vitro and in vivo [36–38]. Polymers with critical solution temperatures are of interest in drug delivery, particularly those with a lower critical solution temperature (LCST) at or near physiological temperature. These polymers near LCST exhibit a decrease in solubility resulting in sol-gel transition, and an LCST near 37°C allowing them to be injected and undergo gelation within the body.
One of the most widely studied LCST polymers is poly(N-isopropylacrylamide), (PNIPAAM) [39,40]. PNIPAAM is a synthetic water soluble polymer with an LCST of around 32°C in distilled water [41–43]. Studies have demonstrated the feasibility to modulate the chemistry of the polymer to adjust the LCST closer to the body temperature of 37°C. The addition of hydrophilic co-monomers has been shown to increase the LCST of PNIPAAM whereas the addition of hydrophobic comonomers has been shown to decrease the LCST [44,45]. In spite of its unique properties, PNIPAAM has a number of limitations such as nondegradability and potential toxicity to serve as a suitable candidate for drug delivery [46].
Poly(ethylene glycol) (PEG) is another synthetic polymer extensively investigated to develop thermoresponsive hydrogels [47–51]. PEG is an ideal candidate to serve as the hydrophilic block in these hydrogel systems due to its biocompatibility, FDA approval status and its availability in a variety of telechelic end groups. One of the most commonly investigated thermoresponsive PEG-based injectable hydrogel is poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) copolymers (Pluronic) [52]. However, the nondegradability and poor in vivo stability of the Pluronics are considered limitations for clinical applications [53]. Recent effort has therefore, been focused on developing biodegradable PEG-based injectable hydrogels with degradable hydrophobic blocks such as poly(ε-caprolactone), polyesters and polyurethanes [54–56].
Intra-molecular hydrogen bonding and hydrophobic interactions in natural biodegradable and biocompatible polymers, such as chitosan, have also been extensively investigated to develop thermo-sensitive polymers. For instance, acidic chitosan solutions, when neutralized at low temperature using β-glycerophosphate or ammonium hydrogen phosphate, remain in solution at room temperature and can undergo gelation at or near 37°C [57,58].
Photo responsive hydrogels
In photo responsive hydrogels, light serves as the stimuli to induce a sol-gel phase transition. Both ultraviolet (UV) and visible light have been extensively investigated. Photo responsive hydrogels that are stimulated by visible light provide advantages over UV induced hydrogels since they are readily available, safe and cost effective [29,46].
Visible light responsive hydrogels have been developed as a potential biomaterial with stimuli sensitive properties [59]. PNIPAAM hydrogels modified with chromophore, such as trisodium salt of copper chlorophyllin, have been shown to undergo differential swelling when irradiated with visible light. In the presence of visible light, the chromophore releases heat, thereby increasing the temperature of the polymer network. Since PNIPAAM hydrogel is thermo sensitive as discussed earlier, the increase in temperature impacts the swelling of the hydrogel and thereby affecting drug diffusion.
In addition to photo-responsive hydrogels, photo-cross-linked hydrogels are raising significant attention as drug delivery vehicles. Biodegradable, photo-curable polymers form a versatile class of injectable biomaterials as the aqueous polymer solution can be injected followed by photo-curing in situ using fiber optic cables. Several photo-curable biodegradable gel systems have been developed [60]. One of the limitations of photo-cross-linkable systems is the light attenuation by the initiators, restricting the maximum attainable cure depth to only a few millimeters [61]. To circumvent the limitations of photo polymerized systems, several chemically cross-linked polymeric systems have been developed using functionalized polymers with reactive groups such as thiols, phenols and aldehydes [62–66].
pH-sensitive hydrogels
Changes in pH occur at a number of sites within the body including the stomach, intestine, lysosome, endosome and extracellular tumor sites [67–69]. These pH variations could therefore be used to trigger a site specific drug release from a pH responsive hydrogel. All pH-responsive hydrogels contain an ionizable acidic or alkaline group, which is able to respond to variations in pH or target specific tissue based on physiological pH [69]. When ionized, these functional groups create a repulsion that results in the swelling of the hydrogel. The extent of the swelling of the hydrogel is determined by the degree to which the functional group in the polymer can be ionized [67]. Swelling will occur at the pKa of the functional group and has been found effective with groups with pKa’s ranging from 3 to 10 [69]. Functional groups often found in pH sensitive hydrogels are weak acids or bases such as carboxylic acid, phosphoric acid, sulfonic acid and amines [30]. The most frequently studied pH responsive hydrogels are poly(acrylic acid) [70], poly(methacrylic acid) [71], poly(ethylene imine) [72], chitosan [73], poly(L–lysine) [74] and poly(L–histidine) due to the presence of carboxylic or amino groups in these polymers [75]. One of the limitations inherent in synthetic pH-sensitive hydrogels is that they are not biodegradable and therefore must be removed, thereby limiting their in vivo applications [46].
Hydrogels as localized anesthetic delivery systems
As discussed before, prolonged duration of local anesthetics is a significant current clinical need. Hydrogel systems have been extensively investigated for the sustained localized delivery of small molecules and macromolecules for various indications. These include growth factor delivery for tissue engineering, anti-cancer agents for localized chemotherapy and insulin delivery for diabetes [76]. Recently, significant interest has gone toward developing hydrogels as local anesthetic delivery vehicles due to the minimally invasive application possible with many injectable hydrogel systems, and the ability to localize the drug at the intended site [11] (Table 3).
Table 3.
Hydrogels for prolonged local anesthetic delivery.
| Name | Type | Ref. |
|---|---|---|
| Pluronics F127/hyaluronic acid | Thermogel | [77] |
| Poly(acrylamide-co-monomethyl itaconate) | pH and thermosensitive gel | [78] |
| PCL-PEG-PCL nanoparticles in Pluronics | Thermogel | [79] |
| Poly(Lactic-co-glycolic acid) microparticles in Poloxamer 407 | Thermogel | [80] |
| Poly(acrylic acid) | Polyelectrolyte complex | [81] |
| Poly(N-isopropyl acrylamide) | Thermoresponsive nanogels | [83] |
| Hyaluronic acid | Chemical crosslinking | [84] |
| Hyaluronic acid and hydroxypropylmethyl cellulose | Rheological blend | [85] |
| Chitosan-ammonium hydrogen phosphate | Thermogel | [11] |
Seol et al. developed a Pluronic F127/hyaluronic acid (HA) based gel with thermosensitive properties, which gels quickly upon exposure to body temperature, as a sustained delivery vehicle for a nonopioid local anesthetic, bupivacaine hydrochloride (BH) [77]. The authors demonstrated the feasibility of injecting and localizing the gel in situ, as well as the sustained release of the drug from the gel over a period of several days in vitro. The BH released from the gel was found to be less cytotoxic to L929 fibroblastic cells compared with adding BH directly to the culture media, presumably due to the slow release of BH from the gel. Similarly, Bernardo et al., developed a dual pH- and thermo-sensitive hydrogel system for the sustained delivery of bupivacaine [78]. The polymeric system used included copolymers of poly(acrylamide-co-monomethyl itaconate; A/MMI) cross-linked with N,N′-methylene bisacrylamide (NBA). The swelling behavior of the hydrogel depended on the pH of the medium. Also during the initial stages both swelling and drug release kinetics were in accordance with the second Fick’s law. The system showed the feasibility to modulate the release profile of bupivacaine by changing the pH; a 60% release was observed at pH 7.5, whereas approximately 80% release was observed at pH 1.5. These pH sensitive hydrogels have the potential to serve as efficient delivery system to deliver analgesics to organs such as stomach that involves significant changes in pH.
As indicated before, long-lasting anesthetic is helpful during the post-operative period. Yin et al., investigated a composite hydrogel-nanoparticle system for the extended release of hydrophobic drugs, such as lidocaine [79]. Lidocaine was loaded into biodegradable PCL-PEG-PCL (PCEC) nanoparticles, and the nanoparticles were then suspended in a thermogelling Pluronic solution. The thermogelling solution can be injected into the body, where it gels upon exposure to the physiological temperature thereby localizing the nanoparticles to the point of application. A sustained drug release profile was observed due to the diffusion of the drug from the nanoparticles. The in vivo efficacy of the system was evaluated in subcutaneous rat model and measured by tail flick latency tests. The Lidocane-nanogel produced effective anesthesia for 360 ± 13 min compared with lidocaine delivered directly from the gel (150 ± 33 min) and lidocaine solution (110 ± 45 min). Nano and microparticles have been previously explored as drug delivery vehicles for local anesthetics. The study demonstrated the potential of further improving the efficacy and localization of these carriers by combining them with hydrogel systems.
Similarly, Chen et al., developed an injectable microparticle-gel system for prolonged and localized release of lidocaine [80]. In this study, lidocaine-loaded degradable poly(lactic-co-glycolic acid) microparticles were suspended in thermosensitive poloxamer 407 solution. The in vivo efficacy of the system was evaluated using a rat sciatic nerve blockade model by monitoring the sensory and motor functions. The study demonstrated that microsphere-poloxamer formulation yielded the longest duration of sensory and motor block for a period of approximately 8.5 h compared with 5 h in the case of microspheres in saline, 5 h in the case of lidocaine in gel and 2 h by lidocaine in saline. The study corroborated the potential of multifaceted hydrogel system in developing long-lasting local analgesic formulations.
In another study, Jimenez-Kairuz et al., developed a sustained delivery lidoaine system using a carbomer (polyacrylic acid) based hydrogel [81]. The carbomerlidocaine system served as a reservoir of lidocaine wherein a high proportion of the drug existed in the form of R-COO-LH+. An in vitro release study demonstrated that the dissociation of ion pairs controlled the release rate of lidocaine. Moreover, the release rate can be increased by the addition of a second counter-ion or through the diffusion of neutral salts such as sodium chloride into the gel matrix. The study demonstrated that better understanding of the ion paring affinity between charged drug molecules and polyelectrolyte carriers would help to predict the release kinetics under different conditions. Loughlin et al., studied the role of polyol sugars in modulating the properties of tetra-hydroxyborate cross-linked poly(vinyl alcohol) gels as lidocaine delivery system [82]. Addition of the hydrochloride salt of lidocaine can lead to network constriction of poly(vinyl alcohol) induced by ionic and pH effects making it unsuitable for topical applications. The study demonstrated that the addition of D-mannitol can circumvent the problem. A formulation with 2% mannitol showed an initial burst release of lidocaine and a drug release mechanism dependent on temperature with a diffusion controlled release profile.
In addition to nanospheres, thermo-responsive nanogel systems have also been developed to achieve prolonged duration of local anesthesia [83]. The thermo-responsive nanogels were developed from PNIPAAM and their tunable size, number of functional groups, thermo-responsiveness and anionic charge make them attractive candidates for prolonged drug release. In vivo studies using a rat sciatic nerve blockade model demonstrated nerve block durations of up to 9 h using acrylic acid conjugated nanogels loaded with bupivacaine. The study also demonstrated the feasibility to modulate the release by changing the composition and size of the nanogels. Increasing the anionic charge density of the nanogels and decreasing the nanogel size facilitated longer duration of anesthetic release.
Apart from these nondegradable polymer systems, several injectable and degradable hydrogel systems have been developed for local anesthetic delivery. Jia et al., investigated the feasibility of using hydrazide and aldehyde modified HA as an injectable delivery vehicle for bupivacaine [84]. The efficacy of the system was evaluated in a rat sciatic nerve blockade model. It was reported that a 2% cross-linked HA doubled the duration of block of 0.1–0.5% bupivacaine, without a statistically significant increase in myotoxicity. The 1% cross-linked HA also prolonged the nerve block compared with uncross-linked HA demonstrating the role of polymer cross-linking in prolonging the effect of local anesthetics.
Hoare et al., recently developed rheological polymer blends of HA and hydroxypropylmethyl cellulose (HPMC) as another approach to achieve sustained delivery of local anesthetics [85]. In a rat sciatic nerve blockade model, HA-HPMC loaded with bupivacaine prolonged the sensory block approximately threefold compared with bupivacaine solution. Incorporation of HPMC modulated the rheological property of the formulation, thereby allowing better polymer injection and reduced polymer hydration, leading to sustained drug release. Moreover, the blends exhibited no cytotoxicity in vivo with a mild short-term inflammatory reaction at the site of injection and were largely resorbed by four days post injection.
Pignatello et al., investigated the potential of chitosan glutamate hydrogels as localized anesthetic delivery vehicles for buccal application [86]. Chitosan hydrogels are known to prolong both the retention times on the oral mucosa as well as drug release. The anesthetic activity of mucoadhesive lidocaine-loaded chitosan gels was accessed in healthy volunteers and demonstrated its potential to reduce pain symptoms that characterize aphthosis and other mouth diseases.
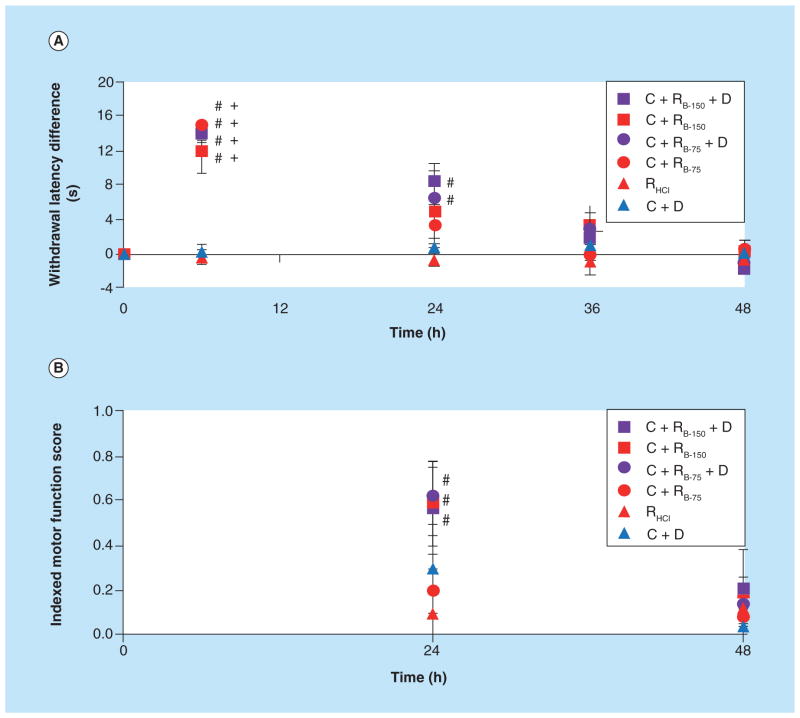
We have investigated the feasibility of using thermogelling chitosan formulation as an injectable localized and sustained delivery vehicle for ropivacaine [11]. Ropivacaine (N-n-propyl 2′,6′-pipecoloxylidide) is an amino-amide local anesthetic and is the propyl analogue of bupivacaine [87]. Numerous preclinical and clinical studies have demonstrated lower cardio- and neurotoxicity associated with ropivacaine compared with bupivacaine [88]. Ropivacaine is a pure S(-) enantiomer, while bupivacaine is a racemic mixture. Studies have shown that enantiomerically pure drugs are less toxic when compared with racemates [89]. The lipid solubility of ropivacaine is lower than bupivacaine, which should increase tissue retention. Ropivacaine also shows less vasodilation compared with bupivacaine and is capable of producing mild vasoconstriction, thus further reducing the extent of systemic plasma absorption. Another significant advantage of ropivacaine is its greater degree of separation between sensory and motor blockade resulting in less motor block compared with bupivacaine [90]. These attributes make ropivacaine an ideal molecule for localized anesthesia. The chitosan thermogels were prepared by neutralizing acidic chitosan solution with ammonium hydrogen phosphate at low temperature. At higher temperatures (approximately 37°C), the thermogelation of the neutral solution is promoted by the combined effect of electrostatic attraction and increased hydrophobic interactions between polymer chains. We have demonstrated that the use of ropivacaine nanoparticles with lower solubility, a drug action enhancer (dexamethasone) and a thermogel matrix together could lead to a multifaceted delivery system capable of providing moderate term pain management [11]. Ropivacaine-based nanoparticles were prepared as described [91]. The controlled in vitro release of ropivacaine from the chitosan thermogel resulted in less than 50% of the drug being released after 7 days (Figure 1). Sensory blockade for animals given two doses of ropivacaine (75 and 150 mg/kg) along with dexamethasone in thermogel was found to be similar to animals given ropivacaine alone (5 and 150 mg/kg doses) in thermogel at 6 h; however, the blockade persisted much longer with slow return of sensory function over 24–48 h in the case of (ropivacaine + dexamethasone) in the thermogel group (Figure 2). Thus, the delivery system demonstrated efficacy for more than 30 h in vivo, providing significant potential for clinical applications. Future studies will be focused on modulating the degradation kinetics of the injectable formulation to further control the drug release as well as the complete removal of the biomaterial after the intended application.
Figure 1. Ropivacaine base released from chitosan thermogel as determined by high-performance liquid chromatography.
The thermogel mediated a gradual release of the drug nanoparticles over the course of 7 days. Data are presented as mean ± standard deviation (N = 3).
Figure 2. In vivo efficacy of the delivery system.
(A) Thermal paw withdrawal latency difference and (B) composite motor function score for animals rats given different anesthetic regimens (N ≥ 3). Chitosan thermogel delivery of ropivacaine base nanoparticles (C + RB-75 and C + RB-150) enhanced both in vivo sensory and motor blockade over the clinically utilized treatment (RHCl). Sensory blockade was extended and motor blockade was enhanced by the addition of dexamethasone to the localized anesthetic delivery system (C + RB-75 + D and C + RB-150 + D). p < 0.05 over RHCl (#) and C + D (+). Data are presented as mean ± standard error (N ≥ 3).
In spite of the unique properties of photoresponsive/cross-linked hydrogel delivery systems, they have not been extensively investigated for prolonged local anesthetic delivery. Literature search showed only one study evaluating the potential of a photocross-linked semi-interpenetrating network composed of poly(ethylene glycol) and gelatin to deliver bupivacaine and silver sulfadiazine for treating wound in a swine model [92]. The study demonstrated the feasibility to deliver these drugs from the hydrogel wound dressing and concluded that further optimization is required to determine the drug and carrier effectiveness.
Future perspective
Extended duration local anesthetic formulations are highly desirable for clinical use. The development of effective delivery systems capable of modulating the release rate of local anesthetic molecules and which enhances their localization could significantly increase the prolonged duration of activity and reduce systemic toxicity. Recent studies demonstrated the significant advantages in using injectable hydrogel systems for the localized prolonged delivery of local anesthetics. In spite of its unique properties, the current hydrogel systems can prolong the effectiveness of the drug only for few hours. The ultimate goal will be to achieve prolonged anesthesia for 7–30 days without cytotoxicity. Combination therapy involving local anesthetics/vasoconstrictors/NSAIDs along with injectable multifaceted delivery systems may in the near future provide the clinicians with a broader spectrum of formulations with varying degree of prolongation of anesthesia.
Executive summary.
Introduction
Reducing the acute and chronic pain commonly associated with injuries and diseases using analgesics has been shown to improve healing and is, therefore, a topic of great interest.
Local application of analgesics has potential advantages compared to systemic administration, including the use of smaller dosages to reduce side effects and potential toxicity.
Biomaterial-based controlled delivery systems have the potential to extend the activity of fast-acting local analgesics, in order to develop a safe, localized, long-acting pain management system.
Long acting local anesthetics for orthopaedic applications
A long-lasting local anesthetic (relief for 7–20 days) is desirable for the treatment of chronic local pain.
Current research is focused on developing controlled local release strategies to maintain an optimal therapeutic level of anesthetic over a period of days, to produce effective analgesia localized to the site of interest, and to restrict negative side effects.
Responsive hydrogels
Hydrogels (cross-linked networks of hydrophilic polymers with high water content) have potential as local, minimally invasive, controlled release delivery systems due to their structure.
Responsive hydrogels are those able to change properties in response to environmental stimuli such as temperature or pH.
Thermo-sensitive hydrogels transition from a liquid to gel state as the temperature increases over a critical solution temperature specific to the polymer.
Hydrogels can be modified to be light-sensitive or photo-crosslinked to allow for stimulation of gelation via exposure to visible/UV light or photo-curing, respectively.
pH-responsive hydrogels contain ionizable functional groups that can respond to physiological variations in pH, promoting swelling of the hydrogel correlating to the degree of ionization.
Responsiveness can be altered by altering the chemical structure of the polymers, potentially allowing engineered polymers to achieve a specific response desired for a given application.
Hydrogels as localized anesthetic delivery systems
The ability to localize drug delivery through minimally invasive administrations of hydrogels makes injectable hydrogel systems promising candidates for sustained, localized anesthetic delivery.
A variety of responsive hydrogel systems for administration of bupivacaine, ropivacaine, and lidocaine have been investigated through in vitro and in vivo models, and have shown indications of controlled release, prolonged analgesic effects, and low cytotoxicity.
Future perspective
There exists the potential for acute and chronic pain control using injectable hydrogel systems with a localized prolonged delivery of anesthetics, without systemic toxicity.
The goal of current research is to modify the hydrogel systems in order to achieve a longer duration of anesthesia (7–30 days) without cytotoxicity.
Composite hydrogel-nano/microparticle systems may continue to be studied in order to enhance efficacy, drug release kinetics, and localization capabilities of hydrogel therapies during post-operative periods.
Future research may also explore novel therapies that combine local anesthetics, vasoconstrictors, NSAIDs, and injectable multi-faceted delivery systems to provide clinicians with a variety of approaches to prolong anesthesia for the duration needed.
Key terms
- Local anesthetics
Anesthetics which cause loss of sensation only to the area to which it is applied
- Controlled delivery
Method to release drug over a period of time in a controlled manner
- Bupivacaine
An aminoamide local anesthetic drug
- Hydrogels
Cross-linked polymer network containing aqueous solution
- Lidocaine
A common local anesthetic drug
- Ropivacaine
A long-acting local anesthetic structurally related to Bupivacaine
- Thermogels
Temperature sensitive hydrogels
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
Funding support from US Army Medical research and Materiel Command, Maryland [W81XWH-10–1–0653]. National Institutes of Health – AR066320. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Bonica J, Loeser JD. Medical evaluation of the patient with pain. In: Bonica J, Chapman C, Fordyce W, editors. The Mangement of Pain. Lea & Febiger; Philadelphia, PA, USA: 1990. pp. 563–579. [Google Scholar]
- 2.Zarbock SF. Technology + teamwork = success. Home Care Provider. 1999;4(2):56–57. doi: 10.1016/s1084-628x(99)90102-8. [DOI] [PubMed] [Google Scholar]
- 3.Woolf CJ, Borsook D, Koltzenburg M. Mechanism-based classifications of pain and analgesic drug delivery. In: Bountra C, Munglani R, Schmidt WK, editors. Pain: current understanding, emerging therapies and novel approaches to drug discovery. Marcel Dekker; New York, NY, USA: 2003. [Google Scholar]
- 4.Lang JD. PAIN: a prelude. In: Lang JD, Mcardle P, editors. Critical Care Clinics. W. B. Saunders Co; Philadelphia, PA, USA: 1999. pp. 1–16. [DOI] [PubMed] [Google Scholar]
- 5.Kotani K. Morphine use for at-home cancer patients in Japan. Tohoku J Exp Med. 2004;204(2):119–123. doi: 10.1620/tjem.204.119. [DOI] [PubMed] [Google Scholar]
- 6.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174(11):1589–1594. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Physician. 2011;14(3):249–258. [PubMed] [Google Scholar]
- 8.White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94(3):577–585. doi: 10.1097/00000539-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012;59(2):90–101. doi: 10.2344/0003-3006-59.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89(1):52–61. doi: 10.1093/bja/aef163. [DOI] [PubMed] [Google Scholar]
- 11.Foley PL, Ulery BD, Kan HM, et al. A chitosan thermogel for delivery of ropivacaine in regional musculoskeletal anesthesia. Biomaterials. 2013;34(10):2539–2546. doi: 10.1016/j.biomaterials.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Kundu S, Achar S. Principles of office anesthesia: part II. Topical anesthesia. Am Fam Physician. 2002;66(1):99–102. [PubMed] [Google Scholar]
- 13.Tadicherla S, Berman B. Percutaneous dermal drug delivery for local pain control. Ther Clin Risk Manag. 2006;2(1):99–113. [PMC free article] [PubMed] [Google Scholar]
- 14.Kravitz ND. The use of compound topical anesthetics: a review. J Am Dent Assoc. 2007;138(10):1333–1339. doi: 10.14219/jada.archive.2007.0048. [DOI] [PubMed] [Google Scholar]
- 15.Cheung HM, Lee SM, Macleod BA, Ries CR, Schwarz SK. A comparison of the systemic toxicity of lidocaine versus its quaternary derivative QX-314 in mice. Can J Anaesth. 2011;58(5):443–450. doi: 10.1007/s12630-011-9479-5. [DOI] [PubMed] [Google Scholar]
- 16.Little C, Kelly OJ, Jenkins MG, Murphy D, Mccarron P. The use of topical anaesthesia during repair of minor lacerations in Departments of Emergency Medicine: a literature review. Int Emerg Nurs. 2009;17(2):99–107. doi: 10.1016/j.ienj.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Antil-Delbeke S, Gaillard C, Tamiya T, et al. Molecular determinants by which a long chain toxin from snake venom interacts with the neuronal alpha 7-nicotinic acetylcholine receptor. J Biol Chem. 2000;275(38):29594–29601. doi: 10.1074/jbc.M909746199. [DOI] [PubMed] [Google Scholar]
- 18.Alkondon M, Albuquerque EX. Initial characterization of the nicotinic acetylcholine receptors in rat hippocampal neurons. J Recept Res. 1991;11(6):1001–1021. doi: 10.3109/10799899109064693. [DOI] [PubMed] [Google Scholar]
- 19.Cheng BC, Zhou XP, Zhu Q, et al. Cobratoxin inhibits pain-evoked discharge of neurons in thalamic parafascicular nucleus in rats: involvement of cholinergic and serotonergic systems. Toxicon. 2009;54(3):224–232. doi: 10.1016/j.toxicon.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Bogduk N. Practice guidelines: spinal diagnostic and treatment procedures. International Spinal Intervention Society. 2004:66–86. [Google Scholar]
- 21.Alanmanou E. Diagnostic neural blocks. In: Sran Rogers., editor. Decision making in pain management. 2. Philadelphia, PA, USA: 2006. pp. 40–41. [Google Scholar]
- 22.Anderson DM, Beyer JA. Local anesthetic choice. In: Sran Rogers., editor. Decision making in pain management. 2. Philadelphia, PA, USA: 2006. pp. 238–241. [Google Scholar]
- 23.Chu CR, Coyle CH, Chu CT, et al. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am. 2010;92(3):599–608. doi: 10.2106/JBJS.I.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilfeld BM, Malhotra N, Furnish TJ, Donohue MC, Madison SJ. Liposomal bupivacaine as a single-injection peripheral nerve block: a dose-response study. Anesth Analg. 2013;117(5):1248–1256. doi: 10.1213/ANE.0b013e31829cc6ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 26.Hennink WE, Van Nostrum CF. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev. 2002;54(1):13–36. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 27.Kost J, Langer R. Responsive polymeric delivery systems. Adv Drug Deliv Rev. 2001;46(1–3):125–148. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 28.Spiller KL, Laurencin SJ, Charlton D, Maher SA, Lowman AM. Superporous hydrogels for cartilage repair. Evaluation of the morphological and mechanical properties. Acta Biomater. 2008;4(1):17–25. doi: 10.1016/j.actbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Bawa P, Pillay V, Choonara YE, Du Toit LC. Stimuli-responsive polymers and their applications in drug delivery. Biomed Mater. 2009;4(2):022001. doi: 10.1088/1748-6041/4/2/022001. [DOI] [PubMed] [Google Scholar]
- 30.He C, Kim SW, Lee DS. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control Release. 2008;127(3):189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Kim B, Soo Lee H, Kim J, Kim SH. Microfluidic fabrication of photo-responsive hydrogel capsules. Chem Comm. 2013;49(18):1865–1867. doi: 10.1039/c3cc37719a. [DOI] [PubMed] [Google Scholar]
- 32.Calejo MT, Sande SA, Nystrom B. Thermoresponsive polymers as gene and drug delivery vectors: architecture and mechanism of action. Exp Opin Drug Deliv. 2013;10(12):1669–1686. doi: 10.1517/17425247.2013.846906. [DOI] [PubMed] [Google Scholar]
- 33.Geever LM, Cooney CC, Lyons JG, et al. Characterisation and controlled drug release from novel drug-loaded hydrogels. Eur J Pharm Biopharm. 2008;69(3):1147–1159. doi: 10.1016/j.ejpb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Prabaharan M, Mano JF. Stimuli-responsive hydrogels based on polysaccharides incorporated with thermo-responsive polymers as novel biomaterials. Macromol Biosci. 2006;6(12):991–1008. doi: 10.1002/mabi.200600164. [DOI] [PubMed] [Google Scholar]
- 35.Garbern JC, Hoffman AS, Stayton PS. Injectable pH- and temperature-responsive poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for delivery of angiogenic growth factors. Biomacromolecules. 2010;11(7):1833–1839. doi: 10.1021/bm100318z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gil ES, Hudson SM. Stimuli-reponsive polymers and their bioconjugates. Prog Polym Sci. 2004;29(12):1173–1222. [Google Scholar]
- 37.Nakayama M, Okano T, Miyazaki T, Kohori F, Sakai K, Yokoyama M. Molecular design of biodegradable polymeric micelles for temperature-responsive drug release. J Control Release. 2006;115(1):46–56. doi: 10.1016/j.jconrel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68(1):34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eeckman F, Moes AJ, Amighi K. Poly(N-isopropylacrylamide) copolymers for constant temperature controlled drug delivery. Int J Pharm. 2004;273(1–2):109–119. doi: 10.1016/j.ijpharm.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Eeckman F, Moës AJ, Amighi K. Synthesis and characterization of thermosensitive copolymers for oral controlled drug delivery. Eur Polym J. 2004;40(4):873–881. [Google Scholar]
- 41.Kim SJ, Lee CK, Lee YM, Kim SI. Preparation and characterization of thermosensitive poly(N-isopropylacrylamide)/poly(ethylene oxide) semi-interpenetrating polymer networks. J Appl Polym Sci. 2003;90(11):3032–3036. [Google Scholar]
- 42.Geever LM, Devine DM, Nugent MJD, Kennedy JE, Lyons JG, Higginbotham CL. The synthesis, characterisation, phase behaviour and swelling of temperature sensitive physically crosslinked poly(1-vinyl-2-pyrrolidinone)/poly(N-isopropylacrylamide) hydrogels. Eur Polym J. 2006;42(1):69–80. [Google Scholar]
- 43.Caykara T, Kiper S, Demirel G. Thermosensitive poly(N-isopropylacrylamide-co-acrylamide) hydrogels: Synthesis, swelling and interaction with ionic surfactants. Eur Polym J. 2006;42(2):348–355. [Google Scholar]
- 44.Eeckman F, Moes AJ, Amighi K. Evaluation of a new controlled-drug delivery concept based on the use of thermoresponsive polymers. Int J Pharm. 2002;241(1):113–125. doi: 10.1016/s0378-5173(02)00198-9. [DOI] [PubMed] [Google Scholar]
- 45.Liu W, Zhang B, Lu WW, et al. A rapid temperature-responsive sol-gel reversible poly(N-isopropylacrylamide)-g-methylcellulose copolymer hydrogel. Biomaterials. 2004;25(15):3005–3012. doi: 10.1016/j.biomaterials.2003.09.077. [DOI] [PubMed] [Google Scholar]
- 46.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53(3):321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 47.Tai H, Tochwin A, Wang W. Thermoresponsive hyperbranched polymers via in situ RAFT copolymerization of peg-based monomethacrylate and dimethacrylate monomers. J Polym Sci Part A Polym Chem. 2013;51(17):3751–3761. [Google Scholar]
- 48.Badi N, Lutz JF. PEG-based thermogels: applicability in physiological media. J Control Release. 2009;140(3):224–229. doi: 10.1016/j.jconrel.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Dong Y, Gunning P, Cao H, et al. Dual stimuli responsive PEG based hyperbranched polymers. Polym Chem. 2010;1(6):827–830. [Google Scholar]
- 50.Censi R, Vermonden T, Deschout H, et al. Photopolymerized thermosensitive poly(HPMAlactate)-PEG-based hydrogels: effect of network design on mechanical properties, degradation, and release behavior. Biomacromolecules. 2010;11(8):2143–2151. doi: 10.1021/bm100514p. [DOI] [PubMed] [Google Scholar]
- 51.Dong Y, Saeed AO, Hassan W, et al. “One-step” preparation of thiolene clickable PEG-based thermoresponsive hyperbranched copolymer for in situ crosslinking hybrid hydrogel. Macromol Rapid Commun. 2011 doi: 10.1002/marc.201100534. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 52.Kabanov AV, Alakhov VY. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst. 2002;19(1):1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 53.Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23(12):2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- 54.Payyappilly S, Dhara S, Chattopadhyay S. Thermoresponsive biodegradable PEG-PCL-PEG based injectable hydrogel for pulsatile insulin delivery. J Biomed Mater Res A. 2014;102(5):1500–1509. doi: 10.1002/jbm.a.34800. [DOI] [PubMed] [Google Scholar]
- 55.Li Z, Zhang Z, Liu KL, Ni X, Li J. Biodegradable hyperbranched amphiphilic polyurethane multiblock copolymers consisting of poly(propylene glycol), poly(ethylene glycol), and polycaprolactone as in situ thermogels. Biomacromolecules. 2012;13(12):3977–3989. doi: 10.1021/bm3012506. [DOI] [PubMed] [Google Scholar]
- 56.Boffito M, Sirianni P, Di Rienzo AM, Chiono V. Thermosensitive block copolymer hydrogels based on poly(varepsilon-caprolactone) and polyethylene glycol for biomedical applications: State of the art and future perspectives. J Biomed Mater Res A. 2014 doi: 10.1002/jbm.a.35253. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 57.Nair LS, Starnes T, Ko JW, Laurencin CT. Development of injectable thermogelling chitosan-inorganic phosphate solutions for biomedical applications. Biomacromolecules. 2007;8(12):3779–3785. doi: 10.1021/bm7006967. [DOI] [PubMed] [Google Scholar]
- 58.Cho J, Heuzey MC, Begin A, Carreau PJ. Physical gelation of chitosan in the presence of beta-glycerophosphate: the effect of temperature. Biomacromolecules. 2005;6(6):3267–3275. doi: 10.1021/bm050313s. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki A, Toyoichi T. Phase transition in polymer gels induced by visible light. Nature. 1990;346(6282):345–347. [Google Scholar]
- 60.Amini AA, Nair LS. Injectable hydrogels for bone and cartilage repair. Biomed Mater. 2012;7(2):024105. doi: 10.1088/1748-6041/7/2/024105. [DOI] [PubMed] [Google Scholar]
- 61.Rydholm AE, Bowman CN, Anseth KS. Degradable thiol-acrylate photopolymers: polymerization and degradation behavior of an in situ forming biomaterial. Biomaterials. 2005;26(22):4495–4506. doi: 10.1016/j.biomaterials.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 62.Vernon B, Tirelli N, Bachi T, Haldimann D, Hubbell JA. Water-borne, in situ crosslinked biomaterials from phase-segregated precursors. J Biomed Mater Res Part A. 2003;64(3):447–456. doi: 10.1002/jbm.a.10369. [DOI] [PubMed] [Google Scholar]
- 63.Mchale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11(11–12):1768–1779. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 64.Balakrishnan B, Jayakrishnan A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials. 2005;26(18):3941–3951. doi: 10.1016/j.biomaterials.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Amini AA, Nair LS. Recombinant human lactoferrin as a biomaterial for bone tissue engineering: mechanism of antiapoptotic and osteogenic activity. Adv Healthc Mat. 2013 doi: 10.1002/adhm.201300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saeed AO, Newland B, Pandit A, Wang W. The reverse of polymer degradation: in situ crosslinked gel formation through disulfide cleavage. Chem Commun. 2012;48(4):585–587. doi: 10.1039/c1cc16538k. [DOI] [PubMed] [Google Scholar]
- 67.Chaterji S, Kwon IK, Park K. Smart polymeric gels: redefining the limits of biomedical devices. Prog Polym Sci. 2007;32(8–9):1083–1122. doi: 10.1016/j.progpolymsci.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson P, Jones AT, Stephens DJ. Intracellular trafficking pathways and drug delivery: fluorescence imaging of living and fixed cells. Adv Drug Deliv Rev. 2005;57(1):43–61. doi: 10.1016/j.addr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58(15):1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 70.Ramesh Babu V, Krishna Rao KSV, Sairam M, Naidu BVK, Hosamani KM, Aminabhavi TM. pH sensitive interpenetrating network microgels of sodium alginate-acrylic acid for the controlled release of ibuprofen. J Appl Polym Sci. 2006;99(5):2671–2678. [Google Scholar]
- 71.Nakamura K, Murray RJ, Joseph JI, Peppas NA, Morishita M, Lowman AM. Oral insulin delivery using P(MAA-g-EG) hydrogels: effects of network morphology on insulin delivery characteristics. J Control Release. 2004;95(3):589–599. doi: 10.1016/j.jconrel.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 72.Sethuraman VA, Na K, Bae YH. pH-responsive sulfonamide/PEI system for tumor specific gene delivery: an in vitro study. Biomacromolecules. 2006;7(1):64–70. doi: 10.1021/bm0503571. [DOI] [PubMed] [Google Scholar]
- 73.Chen SC, Wu YC, Mi FL, Lin YH, Yu LC, Sung HW. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J Control Release. 2004;96(2):285–300. doi: 10.1016/j.jconrel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 74.Burke SE, Barrett CJ. pH-responsive properties of multilayered poly(L-lysine)/hyaluronic acid surfaces. Biomacromolecules. 2003;4(6):1773–1783. doi: 10.1021/bm034184w. [DOI] [PubMed] [Google Scholar]
- 75.Park JS, Han TH, Lee KY, et al. N-acetyl histidine-conjugated glycol chitosan self-assembled nanoparticles for intracytoplasmic delivery of drugs: endocytosis, exocytosis and drug release. J Control Release. 2006;115(1):37–45. doi: 10.1016/j.jconrel.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 76.Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 77.Seol D, Magnetta MJ, Ramakrishnan PS, et al. Biocompatibility and preclinical feasibility tests of a temperature-sensitive hydrogel for the purpose of surgical wound pain control and cartilage repair. J Biomed Mater Res B Appl Biomater. 2013;101(8):1508–1515. doi: 10.1002/jbm.b.32981. [DOI] [PubMed] [Google Scholar]
- 78.Bernardo MV, Blanco MD, Olmo R, Teijón JM. Delivery of bupivacaine included in poly(acrylamide-co-monomethyl itaconate) hydrogels as a function of the pH swelling medium. J Appl Polym Sci. 2002;86(2):327–334. [Google Scholar]
- 79.Yin QQ, Wu L, Gou ML, Qian ZY, Zhang WS, Liu J. Long-lasting infiltration anaesthesia by lidocaine-loaded biodegradable nanoparticles in hydrogel in rats. Acta Anaesthesiol Scand. 2009;53(9):1207–1213. doi: 10.1111/j.1399-6576.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- 80.Chen PC, Kohane DS, Park YJ, Bartlett RH, Langer R, Yang VC. Injectable microparticle-gel system for prolonged and localized lidocaine release. II In vivo anesthetic effects. J Biomed Mater Res A. 2004;70(3):459–466. doi: 10.1002/jbm.a.30101. [DOI] [PubMed] [Google Scholar]
- 81.Jimenez-Kairuz A, Allemandi D, Manzo RH. Mechanism of lidocaine release from carbomer–lidocaine hydrogels. J Pharm Sci. 2002;91(1):267–272. doi: 10.1002/jps.10036. [DOI] [PubMed] [Google Scholar]
- 82.Loughlin RG, Tunney MM, Donnelly RF, Murphy DJ, Jenkins M, Mccarron PA. Modulation of gel formation and drug-release characteristics of lidocaine-loaded poly(vinyl alcohol)-tetraborate hydrogel systems using scavenger polyol sugars. Eur J Pharm Biopharm. 2008;69(3):1135–1146. doi: 10.1016/j.ejpb.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 83.Hoare T, Young S, Lawlor MW, Kohane DS. Thermoresponsive nanogels for prolonged duration local anesthesia. Acta Biomater. 2012;8(10):3596–3605. doi: 10.1016/j.actbio.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jia X, Colombo G, Padera R, Langer R, Kohane DS. Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid. Biomaterials. 2004;25(19):4797–4804. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 85.Hoare T, Bellas E, Zurakowski D, Kohane DS. Rheological blends for drug delivery. II Prolongation of nerve blockade, biocompatibility, and in vitro-in vivo correlations. J Biomed Mater Res A. 2010;92(2):586–595. doi: 10.1002/jbm.a.32420. [DOI] [PubMed] [Google Scholar]
- 86.Pignatello R, Basile L, Puglisi G. Chitosan glutamate hydrogels with local anesthetic activity for buccal application. Drug deliv. 2009;16(3):176–181. doi: 10.1080/10717540902861267. [DOI] [PubMed] [Google Scholar]
- 87.McClure JH. Ropivacaine. Br J Anaesth. 1996;76(2):300–307. doi: 10.1093/bja/76.2.300. [DOI] [PubMed] [Google Scholar]
- 88.Zink W, Graf BM. Benefit-risk assessment of ropivacaine in the management of postoperative pain. Drug Saf. 2004;27(14):1093–1114. doi: 10.2165/00002018-200427140-00003. [DOI] [PubMed] [Google Scholar]
- 89.Owen MD, Dean LS. Ropivacaine. Expert Opin Pharmacother. 2000;1(2):325–336. doi: 10.1517/14656566.1.2.325. [DOI] [PubMed] [Google Scholar]
- 90.Datta S, Camann W, Bader A, Vanderburgh L. Clinical effects and maternal and fetal plasma concentrations of epidural ropivacaine versus bupivacaine for cesarean section. Anesthesiology. 1995;82(6):1346–1352. doi: 10.1097/00000542-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 91.Ulery BD, Kan HM, Williams BA, et al. Facile fabrication of polyanhydride/anesthetic nanoparticles with tunable release kinetics. Adv Healthc Mater. 2013;3(6):843–847. doi: 10.1002/adhm.201300521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Faucher LD, Kleinbeck KR, Kao WJ. Multifunctional photopolymerized semiinterpenetrating network (sIPN) system containing bupivacaine and silver sulfadiazine is an effective donor site treatment in a swine model. J Burn Care Res. 2010;31(1):137–145. doi: 10.1097/BCR.0b013e3181cb8f27. [DOI] [PMC free article] [PubMed] [Google Scholar]