Abstract
Brain activation and functional connectivity were investigated in high functioning autism using functional magnetic resonance imaging in an n-back working memory task involving photographic face stimuli. The autism group showed reliably lower activation compared with controls in the inferior left prefrontal area (involved in verbal processing and working memory maintenance) and the right posterior temporal area (associated with theory of mind processing). The participants with autism also showed activation in a somewhat different location in the fusiform area than the control participants. These results suggest that the neural circuitry of the brain for face processing in autism may be analyzing the features of the face more as objects and less in terms of their human significance. The functional connectivity results revealed that the abnormal fusiform activation was embedded in a larger context of smaller and less synchronized networks, particularly indicating lower functional connectivity with frontal areas. In contrast to the underconnectivity with frontal areas, the autism group showed no underconnectivity among posterior cortical regions. These results extend previous findings of abnormal face perception in autism by demonstrating that the abnormalities are embedded in an abnormal cortical network that manages to perform the working memory task proficiently, using a visually oriented, asocial processing style that minimizes reliance on prefrontal areas.
Keywords: autism, face processing, functional connectivity, functional MRI, working memory
Introduction
One of the fundamental scientific issues in autism research concerns the root causes of the psychological impairments. Several cognitively oriented theories, such as executive dysfunction theory (e.g., Hughes et al. 1994; Pennington and Ozonoff 1996; Hill 2004) and weak central coherence theory (Frith and Happé 1994), postulate that cognitive impairments are the underlying cause of autistic symptomatology. On the other hand, socially oriented theories propose that abnormalities in social perception, social cognition, and social motivation cause subsequent failures of language and cognitive development in autism (e.g., Dawson et al. 1998; Mundy and Neal 2001; Klin et al. 2002). Both of these theoretical positions are plausible and have considerable supporting evidence. However, there is a growing body of evidence supporting a third type of theory that characterizes autism as a neural systems disorder with abnormal interregional brain connectivity, affecting cognitive, social, and other types of deficits. This article provides evidence that abnormalities of face processing in autism can be viewed as part of an underconnectivity syndrome.
The evidence for disordered connectivity in autism comes from two main sources: functional connectivity disturbances and white matter disturbances. Functional connectivity, computed in functional magnetic resonance imaging (fMRI) studies as a correlation between the activation in 2 brain regions across time (say once per second), is a measure of how well the cortical areas are synchronized. The underconnectivity theory proposed by Just et al. (2004) is based in part on the observation that in fMRI studies, individuals with autism display lower functional connectivity than controls, particularly between frontal and posterior areas, in a number of tasks. Functional under-connectivity in an fMRI study was first reported in autism in a sentence comprehension task (Just et al. 2004), then in a letter n-back working memory task (Koshino et al. 2005), a visuomotor task (Villalobos et al. 2005), an executive functioning task (Just et al. 2007), an imagery task (Kana et al. 2006), an inhibition task (Kana et al. in press), and in a fixation resting state (Cherkassky et al. 2006). The fMRI findings are consistent with, but much finer-grained than earlier positron emission tomography (PET)–based evidence of lower functional connectivity in autism, where functional connectivity was measured as the correlation across participants between their activation levels (averaged over minutes of activation) in 2 brain areas. Using PET measurements, Horwitz et al. (1988) found a lower level of correlation in regional activation during a resting state between frontal, parietal, and other regions in autism, indicating a form of functional underconnectivity. Castelli et al. (2002) reported a similar measure of functional underconnectivity in a PET study, but during performance of a theory of mind task rather than in a resting state. Thus, a number of functional neuroimaging studies suggest that there is a lower level of coordination among brain areas in autism, and a number of articles have commented on the viability of some form of connectivity account of autism (e.g., Brock et al. 2002; Frith 2003; Belmonte et al. 2004; Just et al. 2004; Courchesne and Pierce 2005; Rippon et al. 2006).
In addition to functional imaging studies, anatomical studies also provide evidence for abnormal connectivity in autism. Findings, such as increased cerebral and cerebellar white matter volume in 2- to 3-year-old boys with autism (Courchesne et al. 2001), localized white matter enlargement in the outer radiate compartment of white matter in children with autism (Herbert et al. 2004), and other white matter abnormalities (see Carper et al. 2002; Herbert et al. 2003) suggest disordered cortico-cortical connections in the autistic brain. Recent DTI (diffusion tensor imaging) studies also provide evidence of white matter abnormalities in autism. Findings of reduced fractional anisotropy in autism (Barnea-Goraly et al. 2004) may reflect decreased fiber density, reduced myelination of fiber tracts, or less directionally coherent organization of fibers within a voxel (Basser 1995; Beaulieu 2002), all of which could reflect decreased anatomical connectivity in autism. A recent DTI study of autism involving a large sample size and a large age range (Keller et al. 2007) demonstrated reductions in the structural integrity of white matter (measured by fractional anisotropy) in child and adult participants with autism, in the corpus callosum area, and in frontal lobe areas near the corpus callosum. The abnormalities in white matter in autism together with the abnormalities in functional connectivity converge on a plausible neural basis for many kinds of behavioral deficits as well as some advantages (Mottron et al. 2006) in autism.
An atypical biological substrate like abnormal white matter could account for disturbances of behavior in a wide variety of domains, including cognitive and social behavior. Cognitive and social processes are both complex, nonunitary brain processes that are not always separable from each other. Complex cognitive functions require the integration of several processes, such as reasoning, planning, language, perception, and working memory. Such functions also play a central role in social cognition and interpersonal interactions. At the same time, social processes enter into cognitive functions, particularly during learning, when motivation plays a critical role. It is increasingly clear that autism entails deficits in both the social and cognitive domains, and extends beyond these, to even postural control (Minshew et al. 2004). Investigating the brain activity in a task with both a cognitive and social component provides an opportunity to compare any observed abnormalities in different types of cortical networks, as well as the interplay between them.
Working memory and face processing are 2 complex information processing functions that have each been found to be disordered in individuals with autism. The rationale for studying face processing in a working memory task is that it requires cognitive processing (the working memory task) of social stimuli (the faces). Baddeley’s (1986) construal of working memory as a system for information storage and manipulation includes executive functioning that is associated with prefrontal cortex. Although several behavioral studies have found deficits in this working memory system in autism (e.g., Minshew et al. 1992; Bennetto et al. 1996; Minshew et al. 1997; Minshew et al. 1999; Luna et al. 2002; Goldberg et al. 2005; Landa and Goldberg 2005; Williams et al. 2005), some others have not (e.g., Russell et al. 1996; Griffith et al. 1999; Ozonoff and Strayer 2001). At a neural level, using fMRI, Luna et al. (2002) found lower activation in autism in dorsolateral prefrontal cortex and posterior cingulate regions in a spatial working memory task. In another fMRI study of working memory using an n-back letter task, Koshino et al. (2005) found that in participants with autism compared with controls, there was lower left frontal activation (a common finding in autism), higher activation in posterior brain areas (more right than left), and lower synchronization among brain regions. Individuals with autism tend to show deficits in tasks that require complex integration of information and heavy reliance on executive functions of the prefrontal cortex. In the current study, we investigate whether face processing in a working memory task might provide evidence of an atypical pattern of brain activity, within a larger theoretical framework that proposes cortical underconnectivity among some brain regions in autism.
More social tasks, such as the processing of faces, have also shown evidence of disordered processing in autism, manifested as lower activation in key regions. Several neuroimaging studies have found lower levels of brain activation in the fusiform face area in autism in face processing tasks (Critchley et al. 2000; Schultz et al. 2000; Pierce et al. 2001; Hall et al. 2003; Hubl et al. 2003; Piggot et al. 2004; Wang et al. 2004; and see Schultz 2005 for review). Faces represent not only a more complex class of visual patterns than most objects, but they also have social meaning. The social significance of facial and nonfacial stimuli has been shown to evoke abnormal activation in autism (e.g., Castelli et al. 2002; Pelphrey et al. 2004), a result that is often associated with theory of mind deficits in autism.
Investigating working memory (a cognitive function) and face processing (a social and visual function) simultaneously is particularly apt in the context of autism, because the disorder is both social and cognitive. The study reported here used an n-back working memory task involving faces, combining the cognitive and social domains. The emphasis in this task is on working memory, but what the participants have to maintain and update in working memory is faces. There are several approaches or processing styles that can be used to perform the task. One possibility is to construct a verbal code for each stimulus face (e.g., elderly, angular, balding) and then perform the task on the basis of the codes. The use of such verbal codes in a face working memory task may evoke more left-lateralized activation, as suggested by Haxby et al. (1995), so it may be possible to determine if the autism group uses such codes to a lesser degree than the control group, possibly because of a preference for visual thinking in autism (Kana et al. 2006). A second possibility is to construct a verbal code for the social aspects of the face (e.g., hostile, sincere, leering), and then perform the task using these codes. Yet a third possibility is to form a visual representation of the face or its parts. Of course, any mixture of such codes could be used.
Based on findings from previous studies, we hypothesized that participants with autism would be more likely to rely on a visual strategy than on a verbal or social coding strategy. The use of a verbal coding strategy in autism (which would entail activation in left hemisphere language regions, such as inferior frontal gyrus [IFG]) seems unlikely because many previous studies have found lower or abnormal activation in the frontal regions in autism (e.g., Happé al. 1996; Ring et al. 1999; Castelli et al. 2002; Luna et al. 2002; Belmonte and Yurgelun-Todd 2003; Just et al. 2004; Koshino et al. 2005). It has also been shown that people with autism make limited use of inner speech in executive function tasks (Whitehouse et al. 2006). Also, there have been numerous studies that used tasks that are usually associated with left hemisphere functions such as language, sequential processing and symbol use, and found behavioral performance impairments in autism (e.g., Blackstock 1978; Prior and Bradshaw 1979; Dawson 1983; Jolliffe and Baron-Cohen 1997; Rinehart et al. 2002). These findings suggest that a verbal coding strategy may be a less readily available tool for participants with autism. The use of a social coding strategy also seems unlikely in autism, given that people with autism have difficulty in theory of mind processing (Baron-Cohen et al. 1999; Castelli et al. 2002; Pelphrey et al. 2004) and in face processing (Critchley et al. 2000; Schultz et al. 2000; Pierce et al. 2001; Hall et al. 2003; Hubl et al. 2003; Piggot et al. 2004; Wang et al. 2004).
That leaves the third approach, forming visual representations of the faces or their parts, which we hypothesized would be a likely approach for people with autism. Individuals with autism have been reported to use information processing approaches that focus on perceptual detail processing. Mottron and Burack’s (2001; Mottron et al. 2006) Enhanced Perceptual Functioning (EPF) model for how people with autism deal with complex tasks characterizes this profile as locally oriented visual and auditory perception, enhanced low-level discrimination, and increased reliance on more posterior brain networks. This type of processing has been reported in an n-back working memory task with letters (Koshino et al. 2005), where the participants with autism showed more visual coding and posterior brain activation than the control participants. Visual representations are also more likely to be used by people with autism in comprehending abstract sentences (Kana et al. 2006).
For a particular “processing style,” such as the use of visual representations, to have arisen among people with autism, there must be some common determinant that makes the approach an appropriate adaptation for the disorder. The emergence of a common processing approach might be the result of abnormal connectivity among cortical regions. According to the under-connectivity theory of autism (Just et al. 2004), the cognitive deficit in autism is most likely to arise when the task requires integrative processing at a higher cognitive level requiring sustained involvement of frontal areas. The theory predicts that psychological and neurological functioning that is dependent on the coordination of brain networks that require frontal participation is likely to exhibit underfunctioning (underactivation in frontal areas and functional underconnectivity between frontal and posterior areas). In the study reported below, we investigated the brain activity during a task requiring working memory for faces, focusing on frontal, parietal, and fusiform activation, expecting lower functional connectivity between frontal and fusiform areas in the autism group. We hypothesized that the participants with autism would show underconnectivity between the fusiform gyrus (face processing area) and frontal regions. The functional connectivity analyses should indicate whether any abnormality in fusiform face area activation is embedded in a cortical network abnormality.
There are several ways that an abnormality in a cortical network can be demonstrated. One common way is to apply a clustering or factoring algorithm such as factor analysis to the pairwise functional connectivities between pairs of brain areas (McLaughlin et al. 1992; Peterson et al. 1999). This clustering of all of the activated areas into groupings that are internally synchronized identifies the cortical subnetworks that are functioning in a task. A relevant previous outcome of this type of analysis showed that the subnetworks in the autism group were smaller and more fragile, providing an additional perspective on underconnectivity (e.g., Koshino et al. 2005; Kana et al. 2006; Just et al. 2007). In the current study, we hypothesized that in addition to the autism group having smaller and more fragile networks, the subnetwork including the fusiform gyrus would differ between the two groups, and that the subnetworks that included both frontal and parietal areas would differ between the two groups. Because it is unlikely that autism can be localized to a particular brain area, it is especially important to understand abnormalities in a given area (such as the fusiform gyrus) with respect to their cortical network context.
Methods
Participants
Eleven high functioning individuals with autism (all males) and 11 healthy normal control participants (10 males and one female) were included in the study (full scale and verbal IQ scores of 80 or above). The diagnosis of autism was established using the Autism Diagnostic Interview-Revised (Lord et al. 1994), and the Autism Diagnostic Observation Schedule (Lord et al. 2000: mean communication total = 4.6, standard deviation [SD] = 0.9, mean social total = 8.7, SD = 2.6). The diagnosis of autism provided by the two structured instruments was confirmed by expert clinical opinion. The Benton Face Recognition Test (Benton et al. 1994) was administered to participants to obtain a behavioral measure of face-recognition ability. All participants were required to be in good medical health. Potential participants with autism were excluded on the basis of an associated genetic or metabolic disorder, such as fragile-X syndrome or tuberous sclerosis. Potential control and autistic participants were also excluded if they had evidence of birth asphyxia, head injury, or a seizure disorder. Exclusions were based on neurological history, physical examination, and chromosomal analysis. Written informed consent was obtained from participants or their guardians, using procedures approved by the University of Pittsburgh Medical Center Institutional Review Board and the Carnegie Mellon University Institutional Review Board.
The control participants were community volunteers recruited to match the participants with autism on age, Full Scale IQ, gender, race, and family of origin socioeconomic status, as measured by the Hollingshead method (Hollingshead, unpublished data). Potential control participants were screened by questionnaire, telephone, face-to-face interview, and observation during screening psychometric tests. Exclusionary criteria, evaluated through these procedures, included current or past history of psychiatric and neurological disorders, birth injury, developmental delay, school problems, acquired brain injury, learning disabilities, and medical disorders with implications for the central nervous system or requiring regular medication usage. Potential control participants were also screened to exclude a family history of autism, developmental cognitive disorder, learning disability, affective disorder, anxiety disorder, schizophrenia, obsessive compulsive disorder, or other neurological or psychiatric disorder thought to have a genetic component. There were no statistically reliable differences between the autism and control participants in age or IQ (see Table 1). All autism participants were Caucasian, whereas the control group included one African American and one Hispanic. Nine members of the autism group and 10 members of the control group were right-handed. Four participants in the autism group were taking the following medications, but not on the day of the scan: Albuterol (bronchodilator), Targetol (anticonvulsant), Luvox (antidepressant), and Beconase (for hayfever).
Table 1.
Demographic information
| Autism | Control | t(20) | P | ||
|---|---|---|---|---|---|
| Age (years) | Mean ± SD | 24.5 ± 10.2 | 28.7 ± 10.9 | 0.93 | 0.36 |
| FSIQ | Mean ± SD | 104.5 ± 13.1 | 108.6 ± 9.1 | 0.87 | 0.39 |
| VIQ | Mean ± SD | 106.1 ± 14.1 | 108.0 ± 8.6 | 0.38 | 0.71 |
| PIQ | Mean ± SD | 102.1 ± 13.8 | 108.6 ± 9.9 | 1.27 | 0.22 |
| Handedness | Right:left | 9:2 | 10:1 | ||
| Gender | Male:female | 11:0 | 10:1 |
Stimulus Materials and Experimental Design
An n-back working memory task involving face recognition had 3 experimental conditions: 0-back, 1-back, and 2-back, with the timing shown in Figure 1. In the 0-back condition, participants were asked to remember a target face that was presented at the beginning of each trial block. A sequence of 20 face stimuli was then presented and participants were instructed to press a response button if the presented face was the same as the target. In the 1-back condition, participants were told to respond if the presented face was the same as the immediately preceding one. In the 2-back condition, they responded if the presented face was the same as the one that had been presented two faces ago. The stimuli were 27 gray-scale pictures of faces (all males) taken from the Recognition Memory Test (Warrington 1984). A common baseline was a fixation condition.
Figure 1.
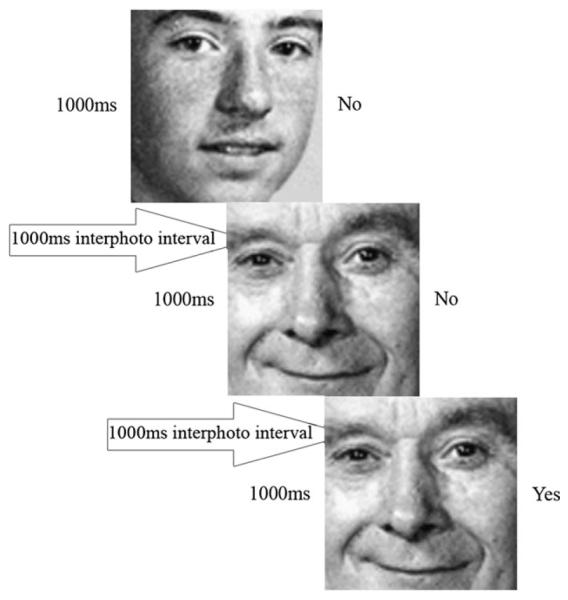
Schematic representation of the 1-back condition. Each stimulus face was presented for 1000 ms then followed by a blank display for 1000 ms. Participants were asked to judge if each face was the same as the one that appeared one face ago.
Stimuli were projected onto a viewing screen attached within the bore of the scanner, and viewed at a distance of approximately 20 cm from the participant’s eyes through 2 mirrors positioned on top of the head coil. Two fiber optic button boxes were used for participants to signal their responses. Stimulus presentation and behavioral data collection were controlled with the CogLab experimental presentation software.
Each participant practiced for the experiment in a separate practice session approximately 2 h before the MRI session. At the beginning of the fMRI scan, 6 additional practice trials were presented followed by a 6-s rest. The experiment consisted of 4 epochs of each of the 3 working memory load conditions presented in a random order, with 20 stimulus faces presented per epoch. At the beginning of each experimental epoch, the instruction for the condition was presented on the screen for 6 s. A 24-s fixation epoch occurred after every 3 experimental epochs. The face stimuli were presented for 1000 ms with an inter-stimulus interval of 1000 ms. The entire fMRI session took approximately 15 min.
fMRI Procedure and Analysis
The single-shot spiral fMRI used blood oxygen level–dependent contrast in a 3.0-T GE Medical Systems scanner, with the following parameters: time repetition (TR) = 1000 ms, time echo (TE) = 18 ms, flip angle = 70°, field of view (FOV) = 20 × 20 cm, matrix size = 64 × 64, axial-oblique plane with 16 slices, and a voxel size of 3.125 × 3.125 × 5 mm with a 1 mm gap. Structural images were 3D SPGR that were acquired with the following parameters: TR = 25, TE = 4, flip angle = 40°, FOV = 24 × 18 cm, 124 slices, resulting in voxel dimensions of 0.9375 × 0.9375 × 1.5 mm thick, taken axially.
fMRI Data Analysis
The fMRI data were analyzed using SPM99 (Wellcome Department of Cognitive Neurology, London, UK). The data of each participant were corrected for slice acquisition timing and motion, and normalized to the Montreal Neurological Institute (MNI) echo planar image (EPI) template, resampled to 2 × 2 × 2 mm voxels, and spatially smoothed (Gaussian kernel, full-width at half maximum = 8 mm). SPM maps were computed for contrasts between the experimental conditions and fixation, and between the two groups (excluding possible group differences in deactivation relative to the fixation condition and using a random effects model). An uncorrected height threshold of P = 0.005 and an extent threshold of 10 voxels were used.
Functional Connectivity
The functional connectivity was computed (separately for each participant) as a correlation between the average time course of all the activated voxels in each member of a pair of regions of interest (ROIs). Thirteen functional ROIs were defined to encompass the main clusters of activation in the group activation map for each group in all Condition–Fixation contrasts. The functional ROIs included 6 bilateral ROIs (frontal pole [FP, BA10], middle frontal gyrus [MFG, BA9], IFG [BA44, 45], lateral premotor areas [LPM, BA6], inferior parietal lobe [IPL, BA40], and fusiform gyrus [FFG, BA19, 37]) and a medial ROI, the medial frontal gyrus (MedFG, BA8). These labels were assigned with reference to the parcellation of the MNI single subject T1-weighted data set carried out by Tzourio-Mazoyer et al. (2002). A sphere was defined for each cluster of activation (with a radius ranging from 4 to 10 mm) that best captured the activation in the map for each group. The ROIs used in the analysis were each the union of the 6 spheres (3 experimental conditions for 2 groups). This common set of 13 ROIs was used for the 2 groups.
The activation time course for each ROI was extracted separately for each participant, and was based on the normalized and smoothed images, which had been low-pass filtered and had the linear trend removed. Furthermore, the participant’s activation time course was based on only the activated voxels within the ROI. The correlation between the time courses of two ROIs was computed on only the images belonging to the experimental condition and excluded the fixation condition, so it reflects the correlation between the activation in 2 areas while the participant is performing the task. The analysis of an ROI pair eliminated any participant who had fewer than 23 activated (2 × 2 × 2 mm) voxels (approximately equivalent to 3 functional voxels) in one of the ROIs. Fisher’s r to z′ transformation was applied to the correlation coefficients, and these transformed correlations were used in further analyses; to provide the input for the factor analysis, the z′ scores were transformed back to correlation coefficients.
Factor Analysis
To characterize the underlying cortical networks and to cluster the activated areas in terms of the interarea functional connectivity, an exploratory factor analysis was performed on the mean correlation coefficients for the 13 functional ROIs (e.g., McLaughlin et al. 1992; Peterson et al. 1999; Koshino et al. 2005). The mean z′-transformed functional connectivities (averaged across individual participants) were converted back to correlation coefficients, and a correlation matrix was created for each group for each condition, on which an exploratory factor analysis was performed. The factor extraction was done with principal components analysis and the varimax method was used for factor rotation. In order to check the independence of factors assumption of the varimax method, the factor analyses were also done using an oblique rotation (promax), and the results were almost identical and the correlations between factors did not reach a meaningful significance level. Therefore, only the varimax-based results are presented. Factors that had eigenvalues of one or above were retained (using the Kaiser–Gutman criterion), and ROIs that had a factor loading of 0.4 or greater were considered for interpretation.
Results
Behavioral Results
The autism and control groups showed similar behavioral performance, with high levels of accuracy and fast response times in both groups, as shown in Table 2. There was no reliable group difference in either the error rate or reaction time, (F1,20 = 0.18 and F1,20 = 3.09, respectively). There was a significant effect of working memory load on error rates (F2,40 = 10.77, P = 0.0002) and on reaction time (F2,40 = 15.56, P < 0.0001), with no significant interaction between group and load in either measure.
Table 2.
Mean response time (ms) and error rate (%)
| 0-back | 1-back | 2-back | ||
|---|---|---|---|---|
| Response time (ms) | ||||
| Autism | Mean | 589 | 561 | 673 |
| (SE) | 29.4 | 37.9 | 52.4 | |
| Control | Mean | 719 | 697 | 810 |
| (SE) | 57.4 | 67.6 | 79.6 | |
| Error rate (%) | ||||
| Autism | Mean | 4.0 | 5.7 | 12.5 |
| (SE) | 1.9 | 3.1 | 2.9 | |
| Control | Mean | 4.5 | 5.7 | 15.9 |
| (SE) | 2.5 | 2.9 | 3.8 |
Brain Activation Results
Both groups showed activation in regions associated with working memory tasks, such as the MFG, IFG, and the inferior parietal regions. Both groups also exhibited activation in the fusiform areas that are associated with face perception, although their coordinates of peak activation were somewhat different, as described below. The activation in the frontal regions for the autism group was mostly limited to the right hemisphere, whereas the frontal activation of the control group was bilateral. The autism group did not show activation in the superior and middle gyri of the right posterior temporal lobes, areas associated with processing of social information (e.g., Ojemann et al. 1992; Haxby et al. 1994; Puce et al. 1998; Allison et al. 2000; Critchley et al. 2000; Narumoto et al. 2001; Pelphrey et al. 2004), whereas the control group did display activation in these locations. In general, the level of activation in a number of areas increased with working memory load for both groups.
A 2 (group) × 3 (working memory load conditions) analysis of variance was performed on the beta values for each ROI. Because no group × working memory load interaction was obtained for any ROI (indicating that the group differences were similar across the three working memory load conditions), the data were collapsed across the three load conditions in the analyses below and are shown in the collapsed form for each participant group separately in Figure 2 and Table 3, depicting the findings described above.
Figure 2.
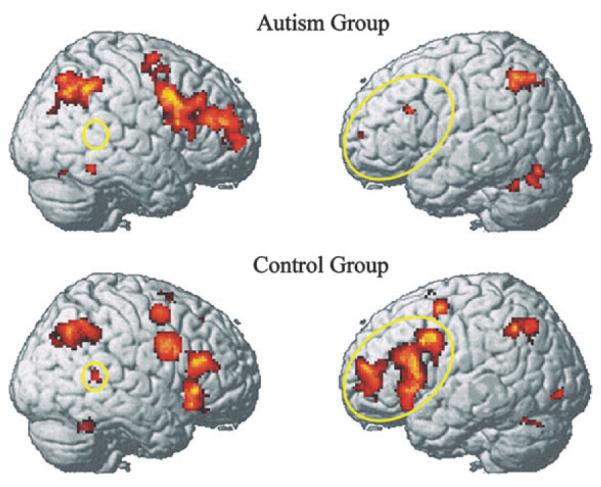
Reduced activation in left IFG and right superior/middle temporal gyri in autism (yellow ellipses) during the n-back task with faces (contrast with Fixation baseline). The data are collapsed across the three working memory conditions.
Table 3.
Areas of activation for each group for the contrast between the working memory tasks (collapsed across three conditions) with the Fixation baseline
| Location of peak activation | Brodmann’s area |
Cluster size |
t(10) | MNI coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Autism | ||||||
| L inferior frontal | 46 | 40 | 4.06 | −50 | 22 | 26 |
| R precentral | 9 | 2201 | 5.98 | 42 | 8 | 36 |
| R supplementary motor area | 6 | 64 | 4.63 | 4 | 8 | 62 |
| R middle cingulate | 32 | 208 | 6.52 | 6 | 22 | 44 |
| L inferior parietal | 40 | 581 | 6.39 | −38 | −54 | 50 |
| R inferior parietal | 40 | 1169 | 6.65 | 52 | −44 | 44 |
| R fusiform/R inferior temporal | 20 | 45 | 4.70 | 52 | −50 | −16 |
| L fusiform | 37 | 53 | 4.35 | −36 | −54 | −28 |
| L cerebellum | 19 | 136 | 4.87 | −34 | −70 | −28 |
| Control | ||||||
| L middle frontal | 10 | 709 | 7.63 | −34 | 54 | 14 |
| L middle frontal | 6 | 189 | 6.71 | −32 | 2 | 58 |
| R orbital frontal | 47 | 2281 | 8.57 | 38 | 24 | −12 |
| L supplementary motor area | 8 | 1717 | 8.08 | −2 | 20 | 50 |
| L supplementary motor area | 6 | 41 | 4.30 | −8 | −6 | 62 |
| L precentral | 9 | 3942 | 13.44 | −36 | 4 | 30 |
| R middle cingulate | 33 | 395 | 7.08 | 6 | 10 | 26 |
| L inferior parietal | 7, 40 | 735 | 9.34 | −32 | −58 | 42 |
| R angular gyrus | 7, 39 | 1585 | 8.80 | 36 | −64 | 42 |
| R middle temporal | 22 | 60 | 6.91 | 58 | −44 | 8 |
| L fusiform | 37 | 34 | 6.45 | −44 | −62 | −24 |
| R fusiform | 37 | 115 | 4.85 | 42 | −50 | −26 |
| L inferior occipital | 18 | 52 | 6.37 | −34 | −88 | −6 |
| L putamen, L amygdala | 34 | 50 | 5.52 | −24 | 6 | −14 |
| R thalamus | 44 | 3.86 | 20 | −12 | 8 | |
Note: L = left, R = right. The threshold used for significant activation was P < 0.005 for a spatial extent of at least 10 voxels, uncorrected for multiple comparisons. Region labels apply to the entire extent of the cluster. The t-values and MNI coordinates are for the peak activated voxel in each cluster only.
Group subtraction indicated the regions in which the 2 groups differed reliably. The autism group showed less activation than the control group in the right temporal area (superior and middle temporal gyrus), suggesting a deficit in theory of mind processing in this group. The autism group also showed less activation than the control group in the left frontal area (IFG and MFG), which may be indicative of less verbal coding or verbally mediated processing of the faces in the autism group. On the other hand, the only areas in which the autism group showed greater activation than the control group were areas in the right hemisphere: one of the superior frontal regions, LPM, and superior parietal regions. These results of the group subtraction are shown in Table 4 and Figure 3.
Table 4.
Areas of activation emerging from the group subtraction
| Location of peak activation | Brodmann’s area |
Cluster size |
t(20) | MNI coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| (Control > autism) | ||||||
| LMFG | 9 | 133 | 4.30 | −50 | 12 | 36 |
| RMFG | 10 | 21 | 3.54 | 42 | 42 | 0 |
| LIFG | 47 | 74 | 4.09 | −24 | 8 | −14 |
| RIFG | 47 | 66 | 3.63 | 42 | 28 | −6 |
| MedFG | 8 | 128 | 3.94 | −6 | 20 | 48 |
| Anterior cingulate | 33, 24 | 791 | 5.64 | 6 | 12 | 24 |
| Cingulate gyrus | 32 | 57 | 3.58 | −4 | 26 | 34 |
| LIPL | 40 | 42 | 3.85 | −42 | −38 | 26 |
| LIPL | 40 | 18 | 3.50 | −58 | −38 | 22 |
| RIPL | 40 | 33 | 3.97 | 42 | −28 | 24 |
| R temporal pole | 22 | 20 | 3.30 | 52 | 10 | −4 |
| R superior temporal gyrus | 22 | 140 | 4.22 | 46 | −26 | −6 |
| R middle temporal gyrus | 39 | 76 | 5.07 | 44 | −66 | 20 |
| L fusiform gyrus | 19 | 14 | 3.55 | −26 | −60 | −12 |
| L parahippocampal gyrus | 28 | 12 | 3.88 | −22 | −20 | −20 |
| L parahippocampal gyrus | 21 | 3.28 | −42 | −42 | −6 | |
| L caudate | 63 | 3.79 | −18 | −14 | 26 | |
| R caudate | 44 | 3.46 | 16 | −10 | 28 | |
| R mammillary body | 27 | 3.57 | 4 | −14 | −12 | |
| L thalamus | 57 | 3.64 | −2 | −22 | 8 | |
| (Autism > control) | ||||||
| R MFG | 8 | 44 | 3.56 | 34 | 28 | 44 |
| R LPM area | 6 | 76 | 4.38 | 32 | −12 | 56 |
| R superior parietal lobe | 7 | 18 | 3.81 | 28 | −70 | 48 |
Notes: L = left, R = right. The threshold for significant activation was P < 0.005 for a spatial extent of at least 10 voxels, uncorrected for multiple comparisons. Region labels apply to the entire extent of the cluster. The t-values and MNI coordinates are for the peak activated voxel in each cluster only.
Figure 3.
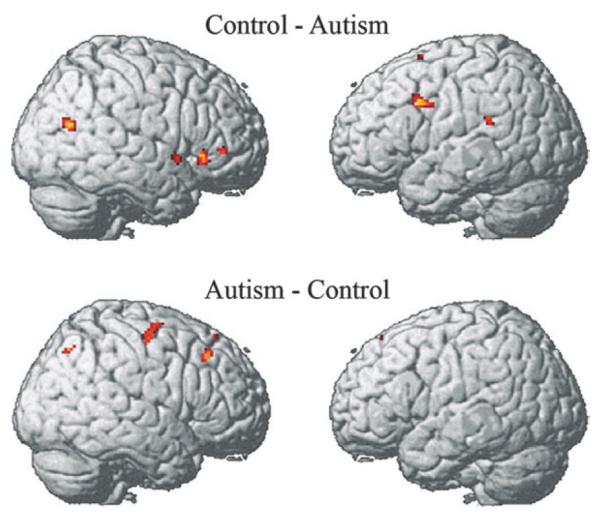
Group contrast showing areas where the control group showed more activation than the autism (top) and autism group exhibited more activation than the control (bottom). The data were collapsed across 3 working memory load conditions.
Fusiform Activation
Although there was not a reliable group difference in the right fusiform activation (though there was a reliable difference on the left), there were several interesting results in this area that provide clues to differences between the 2 groups. First, there was a difference in the location of the right fusiform activation between the control and autism groups, which replicates a previous fusiform activation finding in a study of face perception in autism (Schultz et al. 2000). The autism group’s fusiform activation was more lateral and more inferior than the control group’s, displaced away from an area typically activated during face perception toward an area typically activated during object perception, as shown in Figure 4. (Although the peak of the control group’s activation in our study and in the Schultz et al. (2000) study was in the fusiform gyrus, in both cases the cluster extended to the cerebellum.) To assess the reliability of the activation location difference between the 2 groups, the center of mass of the activated voxels (at P < 0.05 corrected threshold) within each participant’s fusiform activation cluster was computed. (Two participants with autism and one control participant did not have fusiform activation and their data were excluded from this analysis.) One-tailed t-tests examined the hypothesis of the autism group’s activation being more lateral than the control group’s (autism mean x-coordinate = 45.6, control mean x-coordinate = 43.1, t(17) = 1.76, P = 0.048) and more inferior (autism mean z-coordinate = −23, control mean z-coordinate = −25.6, t(17) = 2.39, P = 0.015). There was no difference between groups in the y-coordinate (autism mean = −50.2, control mean = −50.4).
Figure 4.
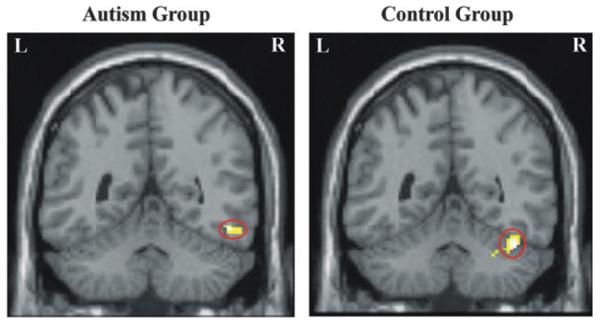
Right fusiform gyrus activation in autism and control groups.
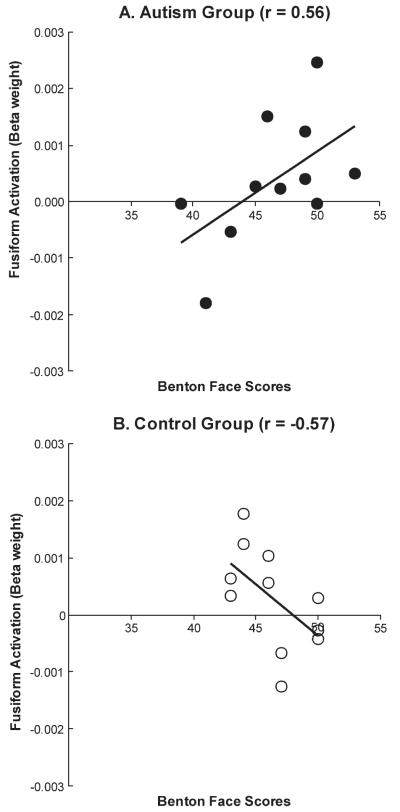
A second finding related to the fusiform activation was a correlation in both participant groups (but in opposite directions) between the amount of fusiform activation and Benton Facial Recognition Test (BFRT) scores (Benton et al. 1994). The activation measure used here was the right fusiform activation beta weight from the SPM analysis of individual participants. The autism and control groups differed reliably in the direction of the correlation between their Benton score and their right fusiform activation (z = 2.77, P < 0.01). The autism group showed a marginally reliable positive correlation between Benton score and right fusiform activation [r = 0.56, t(9) = 2.05, P = 0.070], whereas the control group showed a marginally reliable negative correlation [r = −0.57, t(9) = −2.09, P = 0.066], as shown in Figure 5. In control subjects, the higher the Benton performance level, the smaller the amount of activation was, which is the usual finding relating skill and activation in normal populations, reflecting something like efficiency of processing. However, among participants with autism, the higher the performance, the greater was the amount of activation. A similar relation was found in another impaired population, such that among reading-impaired children, the better they can read, the greater was their activation in parietotemporal areas during reading (Meyler et al. in press). In the impaired population, the correlation may reflect something like increasing activation with increasing intactness. A third finding concerning the fusiform activation in autism, as described below, pertains to its unusual functional connectivity with other areas.
Figure 5.
Correlation between Benton Face Recognition test scores and right fusiform activation in (A) autism and (B) control groups.
Functional Connectivity
The magnitude of the functional connectivity (correlation of the time course of activation) between activated brain areas was compared between the two groups. (Compared with several other previous studies, which used an EPI acquisition sequence, the functional connectivities here, which were acquired with a spiral sequence, were generally lower for both groups. In fact, two other studies using a spiral pulse sequence like this study produced generally lower functional connectivities than studies using an EPI sequence. We speculate that the spiral-based data here are less sensitive to group differences in functional connectivity than are our EPI-based studies.) There was a reliably lower level of functional connectivity in autism than in the control group between the left frontal regions (IFG and MFG) and the left and right fusiform areas [t(20) = 2.59, P < 0.05]. When the fronto-fusiform connectivity was compared between the two groups separately for right and left fusiform areas, the group difference was found to be statistically reliable for the connectivity between the left fusiform gyrus and left frontal regions [t(20) = 2.29; P < 0.05]. These results are interesting in light of several possible functions of the left frontal regions that might be performed in coordination with face processing in the n-back task, such as verbal processing (left IFG) and working memory maintenance and updating (MFG). This lower functional connectivity between frontal and fusiform areas provides further evidence for atypical processing of faces in autism.
We examined whether this functional connectivity difference could be arising from a possible difference in the number of activated voxels between the 2 groups or from variability in the number of voxels contributed across subjects for the 2 groups. The numbers of activated voxels within the defined spheres of functional ROIs (left IFG and left fusiform gyrus) did not differ significantly between the autism and control groups (P > 0.1). This greatly decreases the probability that the connectivity difference resulted from fewer voxels in participants with autism. In order to evaluate whether connectivity differences could be influenced by differences between the groups in the variability of the number of activated voxels, we conducted Fmax tests on this measure for all of the ROIs. Although the group with autism showed somewhat greater between-subject variability in the number of voxels activated than the controls for most of the ROIs, the difference in variance between groups did not approach significance in any ROI.
We also examined whether the medications taken by some participants with autism affected the results or not. It should be noted here that the medications mentioned in this study were not taken on the day of the MRI scan. Nevertheless, we compared the data of participants for those on and not on medication and have not found differences in this study nor in our past published fMRI studies of functional connectivity in different subject samples. From a theoretical perspective, functional connectivity likely relates to the development of structural connections as well as their capacity to dynamically bring different systems on line to address task demands. Medications that reduce anxiety and behaviorally enhance cognitive function might be expected to increase functional connectivity rather than reduce it if they impact functional connectivity at all.
Unlike several other studies (Kana et al. 2006; Just et al. 2007) which found reduced frontal–parietal functional connectivity in autism, there was no reliable group difference in frontal–parietal connectivity in this study of face working memory, although the difference between the groups was in the expected direction (autism mean over all frontal–parietal ROI pairs = 0.24; control mean = 0.26 [t(20) < 1]). (The functional connectivities were also considerably lower in absolute magnitude for both groups compared with the other studies, suggesting that the sensitivity of the measurement may have been lower here. Another possible reason for the lack of reliable frontal–parietal underconnectivity in this study is that the task may not have drawn heavily enough on the parietal lobe. In the previous studies that did find reliable frontal–parietal underconnectivity (such as a study of the Tower of London task), the parietal areas and the frontal–parietal functional connectivity played a larger role.)
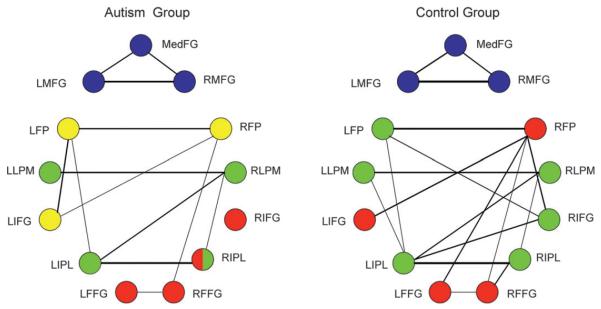
Factor Analysis
The factor analyses showed differences between the two groups in the way that activating regions were grouped into networks by virtue of the similarities among their activation time courses, as follows. 1) A greater number of factors/subnetworks emerged for the autism group (4 factors) compared with the control group (3 factors). These differing numbers of factors accounted for almost the same total amount of variance in the 2 groups. The factor structure is shown in Table 5 and Figure 6. (In Fig. 6, the factor rendered in blue can be referred to as a frontal factor; green: frontal–parietal; red: fusiform; yellow: an additional frontal factor in the autism group.) Thus the autism group had smaller and more numerous networks. The differential network topologies in the two groups suggest a possible account for the impairments in global coherence and integration in autism. 2) In the autism group, the fusiform areas were grouped with the right parietal area and the right inferior frontal area. For the control group, the fusiform area was grouped with 2 frontal areas, the right FP and left IFG. This differential grouping indicates that face processing in autism is performed by a different network, one which excludes a left frontal region.
Table 5.
Factor structure and factor loadings emerging from the factor analysis (Varimax rotation) of the autism and control groups’ functional connectivities
| ROI | Autism |
Control |
|||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F1 | F2 | F3 | |
| MedFG | 0.72 | 0.67 | |||||
| LMFG | 0.83 | 0.87 | |||||
| RMFG | 0.83 | 0.83 | |||||
| LLPM | 0.72 | 0.49 | |||||
| RLPM | 0.77 | 0.65 | |||||
| LFP | 0.76 | 0.58 | |||||
| RFP | 0.72 | 0.67 | |||||
| LIFG | 0.78 | 0.74 | |||||
| RIFG | 0.50 | 0.71 | |||||
| LIPL | 0.58 | 0.71 | |||||
| RIPL | 0.50 | 0.61 | |||||
| LFFG | 0.68 | 0.74 | |||||
| RFFG | 0.73 | 0.52 | |||||
| Eigenvalue | 2.07 | 1.97 | 1.85 | 1.83 | 2.72 | 2.29 | 2.07 |
Note: L = left, R = right. The abbreviations for the ROIs are as follows: MedFG (BA8); MFG (BA9); LPM (BA6); FP (BA10); IFG (BA44, 45); IPL (BA40); and FFG, fusiform gyrus (BA19, 37).
Figure 6.
A graphical depiction of the combined results of the factor analyses and functional connectivity, showing that the autism group had a smaller number of functional connections among their brain regions than the control group. Node colors correspond to factors (blue: frontal factor; green: frontal–parietal; red: fusiform; yellow: an additional frontal factor in the autism group). The internode link thicknesses depict functional connectivity strength, with only those connectivities above some fixed threshold being depicted. FFG = fusiform gyrus.
3) The autism group’s frontal–parietal network was small and relatively unintegrated with frontal regions, consisting of only 4 regions, bilateral inferior parietal and bilateral LPM, 2 of which are premotor. By contrast, the control group’s frontal–parietal network consisted of both parietal regions and a larger number of frontal regions, namely left FP, right IFG, and bilateral premotor regions. In other words, the autism group had fewer frontal and parietal regions activating as a network. 4) The autism group had a second frontal network that consisted of the left and right FPs and the left IFG.
Discussion
The results of the study crystallized some previous findings about brain activation in autism in the context of working memory for faces. First, the abnormal fusiform activation associated with face processing in autism was embedded in a larger context of smaller and less synchronized cortical networks, particularly involving synchronization with frontal regions. The smaller networks suggest limitations in autism on the integration of the coactivating cortical processing sites. Second, the fusiform activation itself was at a slightly different location in the group with autism. In the autism group, the amount of activation was higher for participants with higher Benton face-recognition scores, whereas in the control group, the participants with higher Benton face-recognition scores tended to show lower fusiform activation. Third, the results showed that the autism group did not bring affective/social processing into play in the face working memory task, and used less verbal processing, compared with the control group. Instead, the autism group relied more on visually oriented feature processing (indicated by their activation of areas associated with visual processing).
In the introduction to this article, we raised a set of questions concerning the strategies that the 2 participant groups might differentially use in accomplishing the face working memory task. One possible strategy was the use of verbal coding of faces. In the present study, the autism group exhibited lower activation in left hemisphere regions, specifically left IFG, indicating less use of verbal coding by this group in contrast to the control group, which did use this strategy. A second hypothesis was that the autism group may not use an affective or socially oriented strategy to approach this task. One source of evidence that the autism participants did not rely on a socially oriented strategy in this task is the autism group’s lower level of activation in regions related to social and theory of mind processing, namely, right superior and middle temporal gyri (e.g., Allison et al. 2000; Castelli et al. 2002; Frith and Frith 2003; Pelphrey et al. 2003; Schultz et al. 2003). A second source of evidence consistent with this hypothesis was the location of the autism group’s fusiform activation, displaced toward the inferior temporal gyrus, an area associated with object processing (a finding in agreement with previous studies of face processing in autism; Schultz et al. 2000). In the present study, the task was to construct a short-term representation of the faces, and no processing of social aspects of faces was required. Yet, the control group appeared to process some social information associated with the faces, which indicates that their processing of social aspects of faces is rather automatic, despite there being no necessity to perform such processing. Overall, these results suggest that the participants with autism might be processing faces while attributing little if any social meaning to them.
If the participants with autism tend not to use verbal or social-affective strategies, then the obvious question is, how are they managing to perform this task at least as proficiently as the control participants? That leads us to our third hypothesis, that the autism group might be using a more visually oriented approach, relying more on posterior regions of the brain in this task. The results provided support for this hypothesis. The individuals with autism showed lower activation in the left hemisphere, more reliance on the posterior regions corresponding to the perceptual system rather than the frontal regions of the brain corresponding to the executive functions, and lower functional connectivity. Functional connectivity between the fusiform gyrus and the IFG was lower for the autism group than for the control group. The factor analysis also showed that the posterior regions are not well synchronized with the frontal regions for the autism group. The data also suggest that in autism, the posterior perceptual network does not include the regions for processing face and social stimuli. Instead, the faces seem to be treated more like objects by the autism group, both in terms of the precise fusiform location activation and in terms of the lack of social activation. The asocial processing of the faces in autism appears to rely differentially more on posterior perceptual systems.
Processing Styles and Underconnectivity
The processing style of the autism group that is inferred from the activation results in this study can be related to under-connectivity between frontal and more posterior regions. The activation results suggested that the processing of the autism group was a-social and a-verbal, and instead was visually oriented. Underconnectivity could undermine several of the possible processing styles that were previously described. For example, a verbal coding approach in this face processing task would require the linguistic processes associated with the left IFG to be coordinated with the fusiform face processing, a processing style that would be impaired by underconnectivity with frontal regions. Similarly, a social coding approach might require a network consisting of the MedFG and the right superior temporal gyrus to relate theory of mind processing to the face processing of the fusiform area, again a processing style that might be undermined by underconnectivity between frontal areas and others. On the other hand, the visual processing style, which might entail coordination between occipital and parietal regions, would be less dependent on the connectivity with frontal areas, and the activation patterns suggested that this was the dominant processing style used in the autism group. It is important to note that according to this account, the use of a visual processing style here is not a choice based on preference but on relative unavailability of the resources needed to support some of the other processing styles.
The underconnectivity might shed light on another characteristic of the unique processing of autism, which is related to feedforward and feedback loops in the brain. Because of the underconnectivity between frontal and posterior brain networks in autism, results of information processing in the posterior regions may not be transmitted to the frontal regions in an appropriate and systematic fashion (improper feedforward or bottom-up processing), and conversely, feedback or top-down information from the anterior regions may not be sufficiently transmitted to the posterior regions. This would give rise to a cycle of poor information transmission between the anterior and posterior parts of the brain, and could result in increased reliance on the posterior regions. This dependence on visually based processing could become more problematic as task difficulty increases and the need for higher-level strategies and concepts grows. When tasks are easy, the transmission of intermediate processing results back and forth between the anterior and posterior regions may be less important; therefore, individuals with autism may show a similar level of performance to control participants. However, as task difficulty increases, more communication of intermediate results (i.e., more inter-center coordination) may be needed and individuals with autism may show greater performance deficits in more difficult tasks. For example, the strength in visually based processing in autism is often accompanied by deficits in configural processing (Dakin and Frith 2005; Behrmann et al. 2006).
Describing the psychological processing in autism as being of a different “processing style” is probably accurate, but it is scientifically unsatisfying. The description simply begs the question of why people with autism should “prefer” a given style of processing. It is surely not simply a matter of preference or taste or even of choice, but rather a consequence of neural circuitry that expresses itself in this manner. The question then is why a given “style” of processing should emerge in the disorder of autism. In the section below, we propose that the underlying disorder in the neural systems in autism, particularly underconnectivity of cortical areas with frontal areas, favors some “processing styles” over others.
Cortical Underconnectivity and Face Processing in Autism
Face processing in a working memory task provides an appropriate venue to examine the relation between cognitive and social deficits in autism. The finding of atypical face processing in people with autism has been attributed to either a social or a perceptual deficit depending on the theoretical perspective taken. The social account is that disrupted social motivation and interaction is the primary determinant of difficulty in face processing (e.g., Dawson et al. 2002, 2005). A competing account attributes atypical face processing to a perceptual deficit in configural processing in autism (e.g., Dakin and Frith 2005; Behrmann et al. 2006). Although the social and cognitive explanations are posed as alternatives to each other, we suggest that there is a core deficit at the neural systems level that unifies these 2 accounts.
The social and perceptual deficits that underpin the 2 accounts are demonstrably present in the data reported here. The clearest neural evidence of a social deficit in this study was the underactivation of the right posterior superior temporal area associated with theory of mind processing. The evidence of a cognitive deficit was manifested in the left inferior frontal underactivation. In the theoretically contested territory of face processing, the multiple facets of abnormal fusiform activation in autism included its displaced location and reversed direction of correlation with Benton Face Recognition scores. One of the most interesting new findings was that the abnormality of the fusiform activation was found within the context of an abnormal cortical network. As the perspective of underconnectivity theory predicted, the autism group exhibited functional under-connectivity between fusiform face processing and frontal processing in the service of working memory for faces.
As new evidence emerges with studies of a broader range of tasks, underconnectivity theory has become progressively refined to better specify the brain locations and circumstances that characterize functional underconnectivity in autism. One of these refinements is a broadening of the scope of the theory to encompass a larger set of psychological processes. For example, the social deficit observed in autism may be due to underconnectivity among the cortical regions supporting theory of mind processing (Castelli et al. 2002), and/or to underconnectivity between emotional processing (possibly amygdala-centered) and perceptual processing in areas such as the fusiform gyrus (Schultz 2005). Similarly, the perceptual deficit in face processing, which can be construed as atypically little global processing relative to local processing, might be due to the underconnectivity among the regions that perform the global integration. Broadening the scope of any theory of autism is essential if it is to encompass the diversity of the symptoms of autism, including the recent findings of postural deficits (Minshew et al. 2004). The diversity itself is indicative of a system-wide neural systems disorder that is not localized to one brain area nor to one type of thinking. Underconnectivity is an early attempt at a neural systems disorder theory of autism.
Limitations of the Theory
Although the underconnectivity perspective is providing an extremely useful theoretical framework for investigating autism (helping to frame issues, formulate hypotheses, and relate findings across studies and methodologies), the theory itself does not currently provide the answers to some central questions about autism. We view the current form of the underconnectivity theory as preliminary, and anticipate that future research will result in considerable refinement and expansion. Below we discuss some of the key questions that are currently unaddressed by the theory, but which any comprehensive theory of autism must ultimately account for.
Other Types of Connectivity Disturbances besides those Measured by fMRI
We expect that alterations in connectivity among neural systems and in local connectivity are likely to emerge with further study, including instances of increased connectivity between some areas. Moreover, other technologies in addition to fMRI are being brought to bear on issues of neural connectivity in autism, such as histology, electrophysiology, morphometry, and DTI. One of the many uncertainties to be addressed by some combination of approaches concerns the nature of and relationship between white matter and gray matter abnormalities, and the nature of and relationship between functional and anatomical abnormalities in autism.
Participant Samples
Another lacuna is the absence of functional connectivity studies of a wider range of people with autism, such as those who are not high functioning. Even in this study of high functioning autism, there are only 11 participants with autism. Another limitation is that several studies that appear to provide independent evidence for a hypothesis may draw on the same group of participants. It is important to note that most of our previously published findings of functional underconnectivity in autism had minimal overlap (one participant) with the current study (Just et al. 2004, 2006; Kana et al. 2006, in press). However, one previous finding of functional underconnectivity (Koshino et al. 2005) had a 50% overlap of the participants with autism. Such issues point to the importance of extension of research to larger and more diverse participant groups with autism.
The Generality of Connectivity Disturbances in Autism
There are many other uncertainties about the generality of the underconnectivity findings. Although we have reported under-connectivity in a number of different types of thinking that span the autism syndrome (executive, perceptual, language, social perception, inhibition), there has been no study of connectivity in many other types of relevant tasks. The tasks yet to be so investigated include tasks without frontal involvement, tasks with nonvisual input modalities (auditory, haptic, olfactory), and motor tasks. The ontology of the underconnectivity is another unresearched issue. The theory provides a framework for formulating such relevant questions about neural connectivity in autism, without prejudging the answers, which are a matter for empirical investigation. In fact, a more accurate term to describe this approach may well be “neural systems connectivity theory,” to embrace the range of possible findings.
Summary
The results from the present study place face processing deficits in autism within a larger context of cognitive and social deficits in autism. In autism, the fusiform activation associated with face processing was less synchronized with frontal areas, was displaced in location, and was unaccompanied by activation in areas associated with social processing. It would be desirable to have these findings replicated in future studies with larger sample size using different face processing tasks, but the new findings converge well with previous autism findings in other types of tasks. The underconnectivity theory framework accounts not only for the current fMRI results, but also for the more general tendency of individuals with autism to rely more on visual processing and posterior brain regions and less on the cognitive, verbal, and social functions underpinned by frontal brain regions. Conceptualizing autism as a neural systems disorder characterized by brain connectivity abnormalities provides a useful step toward a unified account of the diversity of deficits in autism.
Acknowledgments
This research was supported by the Collaborative Program of Excellence in Autism Grant HD35469 from the National Institute of Child Health and Human Development. The authors would like to thank Sarah Schipul and Stacey Becker for assistance with the data analysis.
Footnotes
Conflict of Interest: None declared.
References
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Oxford University Press; New York: 1986. [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SCR. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Leonard GL, Kimchi R, Luna B, Humphreys K, Minshew NJ. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006;44:110–129. doi: 10.1016/j.neuropsychologia.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Cook EH, Anderson GM, Rubenstein JLR, Greenough WT, Beckel-Mitchener A, Courchesne E, Boulanger LM, Powerll SB, Levitt PR, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychol. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Cogn Brain Res. 2003;17:651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, Rogers SJ. Intact and impaired memory functions in autism. Child Dev. 1996;67:1816–1835. [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher K, Varney NR, Spreen O. Contributions to neuropsychological assessment. Oxford University Press; New York: 1994. [Google Scholar]
- Blackstock EG. Cerebral asymmetry and the development of early infantile autism. J Autism Childh Schizophr. 1978;8:339–353. doi: 10.1007/BF01539636. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Dev Psychopathol. 2002;14:209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. NeuroImage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuro-Report. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local-overconnectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SCR, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48:497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Dawson G. Lateralized brain dysfunction in autism: evidence from the Halstead-Reitan Neuropsychological Battery. J Autism Dev Disord. 1983;13:269–286. doi: 10.1007/BF01531566. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol. 2005;27:403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Alyward E, Richards T. Defining the broader phonotype of autism: genetic, brain, and behavioral perspectives. Dev Psychopathol. 2002;14:581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- Frith C. What do imaging studies tell us about the neural basis of autism? Novartis Found Symp. 2003;251:149–166. [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Happé F. Autism: beyond “theory of mind”. Cognition. 1994;50:115–132. doi: 10.1016/0010-0277(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Mostofsky SH, Cutting LE, Mahone EM, Astor BC, Denckla MB, Landa RJ. Subtle executive impairment in children with autism and children with ADHD. J Autism Dev Disord. 2005;35:279–293. doi: 10.1007/s10803-005-3291-4. [DOI] [PubMed] [Google Scholar]
- Griffith EM, Pennington BF, Wehner EA, Rogers SJ. Executive functions in young children in autism. Child Dev. 1999;70:817–832. doi: 10.1111/1467-8624.00059. [DOI] [PubMed] [Google Scholar]
- Hall GB, Szechtman H, Nahmias C. Enhanced salience and emotion recognition in autism: a PET study. Am J Psychiatry. 2003;160:1439–1441. doi: 10.1176/appi.ajp.160.8.1439. [DOI] [PubMed] [Google Scholar]
- Happé F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, Dolan R, Frackowiak R, Frith C. ‘Theory of mind’ in the brain: evidence from a PET scan study of Asperger syndrome. NeuroReport. 1996;8:197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- Haxby J, Horwitz B, Ungerleider L, Maisog J, Pietrini P, Grady C. The functional organization of human extrastriate cortex: a PET-RCBF study of selective attention to faces and locations. J Neurosci. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Rapoport SI, Grady CL. Hemispheric differences in neural systems for face working memory: a PET-rCBF study. Hum Brain Mapp. 1995;3:68–82. [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, et al. Dissociation of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS., Jr Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism: intercorrelations of regional glucose utilization. Arch Neurol. 1988;45:749–755. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- Hubl D, Bolte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, Poustka F, Dierks T. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32:477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? J Child Psychol Psychiatry. 1997;38:527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.08.004. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. NeuroReport. 2007;8:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Defining and quantifying the social phenotype in autism. Am J Psychiatry. 2002;159:895–908. doi: 10.1176/appi.ajp.159.6.895. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Goldberg MC. Language, social and executive functioning in high functioning autism: a continuum of performance. J Autism Dev Disord. 2005;35:557–573. doi: 10.1007/s10803-005-0001-1. [DOI] [PubMed] [Google Scholar]
- Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, Eddy WF, Sweeney JA. Neocortical system abnormalities in autism. Neurology. 2002;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Steinberg B, Christensen B, Law I, Parving A, Friberg L. Potential language and attentional networks revealed through factor analysis of rCBF data measured with SPECT. J Cereb Blood Flow Metab. 1992;12:535–545. doi: 10.1038/jcbfm.1992.77. [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Lee D, Hoeft F, Whitfield-Gabrieli S, Gabrieli JDE, Just MA. Brain activation during sentence comprehension among good and poor readers. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm006. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Muenz LR, Payton JB. Neuropsychological functioning in nonmentally retarded autistic individuals. J Clin Exp Neuropsychol. 1992;14:749–761. doi: 10.1080/01688639208402860. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder. J Int Neuropsychol Soc. 1997;3:303–316. [PubMed] [Google Scholar]
- Minshew NJ, Luna B, Sweeney JA. Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology. 1999;52:917–922. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63:2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Mottron L, Burack JA. Enhanced perceptual functioning in the development of autism. In: Burack JA, Charman N, Yirmiya N, Zelazo PR, editors. The development of autism: perspectives from theory and research. Erlbaum; Mahwah, NJ: 2001. pp. 131–148. [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack JA. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mundy P, Neal R. Neural plasticity, joint attention, and a transactional social orienting model of autism. Int Rev Ment Retard. 2001;23:139–168. [Google Scholar]
- Narumoto J, Okada T, Sadato N, Fukui K, Yonekura Y. Attention to emotion modulates fMRI activity in human right superior temporal sulcus. Cogn Brain Res. 2001;12:225–231. doi: 10.1016/s0926-6410(01)00053-2. [DOI] [PubMed] [Google Scholar]
- Ojemann J, Ojemann G, Lettich E. Neuronal activity related to faces and matching in human right nondominant temporal cortex. Brain. 1992;115:1–13. doi: 10.1093/brain/115.1.1. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL. Further evidence of intact working memory in autism. J Autism Dev Disord. 2001;31:257–263. doi: 10.1023/a:1010794902139. [DOI] [PubMed] [Google Scholar]
- Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev. 2004;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeown M, Goldstein J, Allison T, McCarthy G. Brain activity evoked by perception of human walking: controlling for meaningful coherent motion. J Neurosci. 2003;23:6819–6825. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Soc Biol Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Pierce K, R-A, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Piggot J, Kwon H, Mobbs D, Blasey C, Lotspeich L, Menon V, Bookheimer S, Reiss AL. Emotional attribution in high-functioning individuals with autistic spectrum disorder: a functional imaging study. J Am Acad Child Adolesc Psychiatry. 2004;43:473–480. doi: 10.1097/00004583-200404000-00014. [DOI] [PubMed] [Google Scholar]
- Prior MR, Bradshaw JL. Hemisphere functioning in autistic children. Cortex. 1979;15:73–81. doi: 10.1016/s0010-9452(79)80008-8. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci. 1998;18:2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ. Lateralization in individuals with high-functioning autism and Asperger’s disorder: a frontostriatal model. J Autism Dev Disord. 2002;32:321–332. doi: 10.1023/a:1016387020095. [DOI] [PubMed] [Google Scholar]
- Ring HA, Baron-Cohen S, Wheelwright S, Williams SCR, Brammer M, Andrew C, Bullmore ET. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of Embedded Figures Task performance. Brain. 1999;122:1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the ‘new psychophysiology’. Int J Psychophysiol. 2006;63:164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Russell F, Jarrold C, Henry L. Working memory in children with autism and with moderate learning difficulties. J Child Psychol Psychiatry. 1996;37:673–686. doi: 10.1111/j.1469-7610.1996.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger Syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van Der Gaag C, Marois R, Skudlarski P. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B. 2003;358:415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuro-Image. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Harriri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Warrington EK. Recognition memory test. Western Psychological Services; Los Angeles: 1984. [Google Scholar]
- Whitehouse AJO, Maybery MT, Durkin K. Inner speech impairments in autism. J Child Psychol Psychiatry. 2006;47:857–865. doi: 10.1111/j.1469-7610.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ. Impaired memory for faces and social scenes in autism: clinical implications of memory dysfunction. Arch Clin Neuropsychol. 2005;20:1–15. doi: 10.1016/j.acn.2002.08.001. [DOI] [PubMed] [Google Scholar]