Abstract
Alterations in inhibitory interneurons contribute to cognitive deficits associated with several psychiatric and neurological diseases. Phasic and tonic inhibition imparted by γ-amino-butyric acid (GABA) receptors regulates neural activity and helps to establish the appropriate network dynamics in cortical circuits that support normal cognition. This review highlights basic science demonstrating that inhibitory signaling is altered in aging, and discusses the impact of age-related shifts in inhibition on different forms of memory function, including hippocampus-dependent spatial reference memory and prefrontal cortex (PFU)-dependent working memory. The clinical appropriateness and tractability of select therapeutic candidates for cognitive aging that target receptors mediating inhibition are also discussed.
Keywords: GABAA α5, GABAB receptor, hippocampus, memory, tonic inhibition, prefrontal cortex
Memory decline in normal aging
Successes in modern medicine have resulted in marked improvements in somatic and peripheral health, and an unprecedented extension of the human lifespan. Such advances, however, are currently outpacing our ability to maintain optimal brain function and cognition later in life. This problem is accentuated by the striking rise in incidence of the age-associated neurological disorder, Alzheimer’s disease (AD), characterized by a precipitous decline in cognitive functioning (for example, loss of memory). It is important to recognize, however, that aging is accompanied by several non-pathological brain changes that, even in the absence of AD, can significantly and deleteriously influence cognitive capacities. Although the contemporaneous decline of cognition in normal aging is less severe than in AD, this type of cognitive decline will affect the vast majority of seniors and can be sufficiently severe as to negatively impact on quality of life and endanger personal independence and well-being [1]. With the current emphasis on developing disease-modifying strategies for treatment of AD, other therapeutic approaches that may broadly improve cognitive function across aged populations are often neglected in research and drug-discovery efforts. Such interventions could provide significant individual, medical, and economic benefits, both by treating non-AD-related cognitive dysfunction and by potentially extending the window of good cognitive functioning in individuals with prodromal AD.
While aging is clearly a brain-wide process, changes within the hippocampus and PFC are at the forefront of cognitive aging research because these regions are vital for dissociable forms of memory that are vulnerable to decline at advanced ages. The hippocampus is a region in the medial temporal lobe that is crucial for long-term memory formation and maintenance. Hippocampal memory can be characterized as ‘declarative’, referring to the ability to remember factual information that can literally be declared (e.g., ‘Who is the president of the United States?’), ‘episodic’ (e.g., remembering the details of a past birthday), or ‘spatial’ (e.g., remembering the location of one’s house or workplace). In contrast to hippocampus-dependent memory, neuronal networks in PFC subserve a form of short-term memory known as ‘working memory’ which involves maintaining information in mind for a relatively brief duration. This temporary representational knowledge is essential for our ability to seamlessly plan and execute behavior. Notably, given its importance in guiding our current actions, a foundational characteristic of PFC function is the ability to readily and rapidly update information being held in working memory stores in response to environmental demands. This balance between information maintenance and updating has led to the conceptualization of the PFC as a ‘mental sketchpad’.
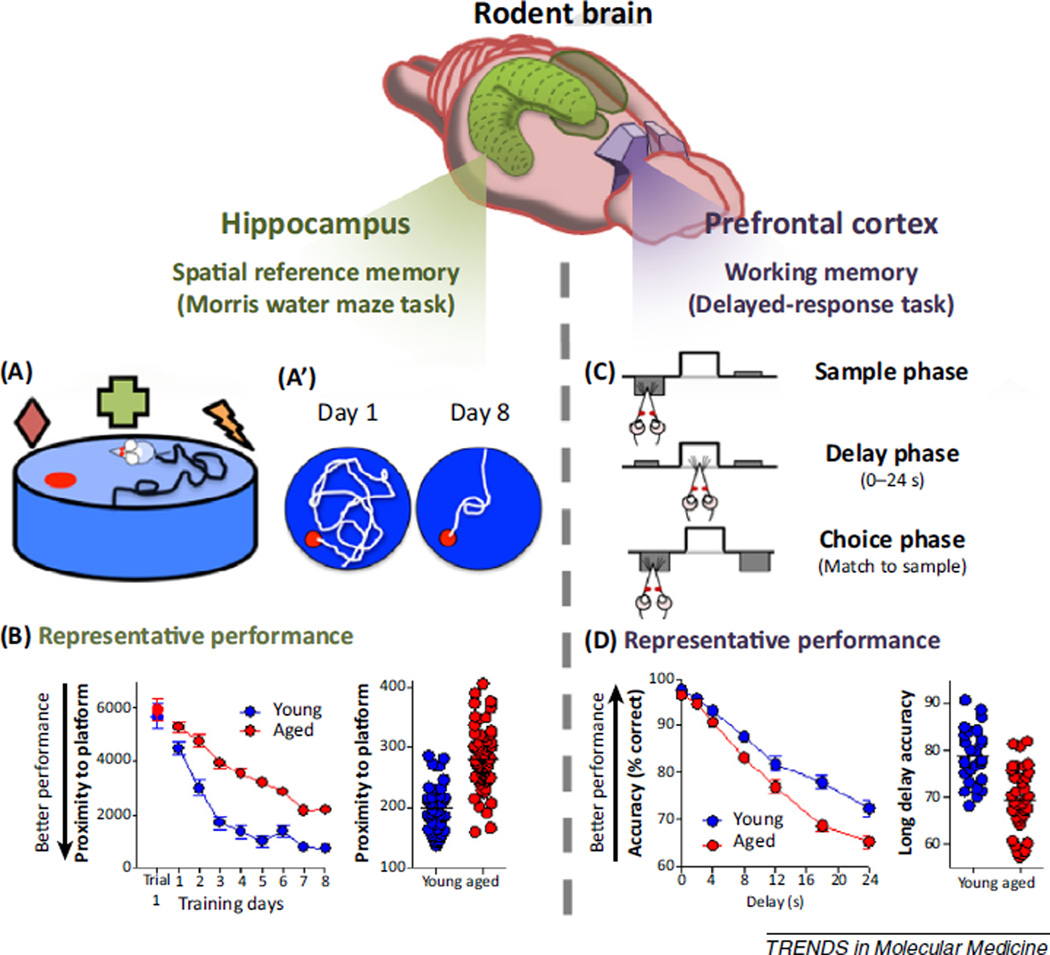
Laboratory rodents are experimentally tractable and translationally appropriate as animal models to investigate the neurobiology underlying age-related memory decline. Aged rats exhibit many of the characteristic cognitive deficits prominent in human aging, including impaired memory functions that are supported by the hippocampus and PFC [2–6]. Behavioral analyses that provide an index of memory function can be paired with anatomical, molecular, electrophysiological, and pharmacological endpoints, allowing mechanisms of brain aging to be directly evaluated against cognitive abilities [7–14]. The Morris water maze is the ‘gold standard’ assessment for evaluating hippocampal memory in rodents. In this spatial navigation task (Figure 1A), rats are placed in a pool of water and must rely on visual cues situated around the maze to learn and remember the location of an escape platform hidden beneath the surface of the water [15]. Damage to the hippocampal formation severely impairs performance on this task, as is evidenced by longer and less-direct swim paths to reach the escape platform [16–18]. Aged rats also are reliably impaired on the water maze task (Figure 1B), with performance mimicking that of rats with hippocampal damage [19–21]. More recently, humans have been tested on virtual navigation assessments that have been reverse-engineered from rodent water maze task designs. Older individuals reliably perform worse than younger adults on these virtual spatial reference memory tasks, underscoring the translational potential of molecular mechanisms identified with the aid of rodent water maze performance [22–24]. It is notable that, despite the fact that many aged rats show pronounced impairment in spatial reference memory as assessed by the water maze, the numbers of principal cells of the hippocampus are remarkably preserved with age [25–27]. This observation has directed mechanistic investigation of age-related memory decline away from neurodegenerative processes and toward molecular and structural factors that can influence neuronal communication and plasticity.
Figure 1.
Using rodents to assess age-related decline of hippocampus- and prefrontal cortex (PFU)-dependent memory. (Above) The schematic shows the hippocampus (green) and prefrontal cortex (purple) in rodent brain, which serve distinct types of memory (spatial reference memory and working memory, respectively). (Below) (A) The functionality of the rat hippocampus can be evaluated in spatial learning tasks such as the Morris water maze. The schematic shows the water maze task apparatus in which rats use spatial cues (represented by colored shapes) to learn and remember the location of a stationary escape platform (red circle) hidden beneath the surface in a large tank of water. A’ shows representative swim paths from the first (day 1) and last (day 8) training days. After training, rats with intact hippocampal function take a more direct path to the platform. (B) Representative performance of young adult and aged rats across 8 days of training (three trials per day). While both young and aged rats find the platform more efficiently over time, aged rats are less accurate in their search, indicating attenuated hippocampus-dependent learning and memory function. Notably, deficits are not uniform among the aged rodent population (represented on vertical scatterplot). A subset of aged rats perform on a par with young rats, demonstrating a relative preservation of memory function. (C) The functionality of the rat prefrontal cortex can be evaluated using delayed-response tasks. This task assesses working memory, a flexible form of memory that maintains information for a brief duration (seconds) to help guide current and future action. The schematic shows a food-motivated, delayed-response task that contains three phases per trial. In the sample phase, rats are presented with one of two sample levers (left or right). Once the rat chooses the sample lever, a delay phase is initiated which ranges from 0 to 24 s. Following the delay interval, both levers are extended and the rat must remember and choose the lever presented in the sample phase to receive a food reward. The lever position in the sample phase is varied randomly across trials such that the rat must rely on trial-unique information to make the correct choice. Rats can perform many such trials in a single session (>100), and the mean accuracy of performance at different delay intervals provides an index of working memory function. (D) Both young and aged rats perform comparably and with a high degree of accuracy when the delay between the sample and choice phases is brief. At longer delay intervals aged rats are disproportionately and significantly impaired. There are individual differences in performance among aged rats, with some aged rats maintaining working memory function on a par with young rats and some demonstrating marked impairment. Data reproduced from [14,20,35].
Although less widely used, similar approaches can be employed to gain insight into the molecular underpinnings of PFC-dependent memory [3]. The rodent PFC is less anatomically elaborate than in primates; however, rats are capable of many complex behaviors and there is evidence for anatomical and functional homology between rodent and primate PFC (reviewed in [28–31]). Such findings provide a strong rationale for using aged rodents to investigate the neural mechanisms of working memory decline. Importantly, under normal circumstances the PFC and hippocampus work together to broadly support memory function spanning across both short and long durations. Support for regional investigation of molecular mechanisms that contribute to age-related memory decline is derived from substantial empirical data across species which highlight that the roles of hippocampus and PFC in memory can be behaviorally dissociated, as can memory impairments that emerge in aged populations. In particular, delayed-response tasks are often used to assess PFC-dependent working memory abilities in humans, nonhuman primates, and rodents (recently reviewed in [3,32,33]). These tasks evaluate memory for a position or object over a short duration, with the accuracy of the memory typically being tested in a choice setting. In contrast to spatial reference memory (which involves retaining information about a single location across days), the to-be-remembered information in a delayed-response task varies from trial to trial, requiring the subject to continually update the representation being held in mind or remembered. Figure 1C shows a delayed-response task in which rats are required to remember the position of a response lever over delay intervals that range from 0 to 24 s. Damage to the medial PFC (mPFC), the rodent homolog of primate dorsolateral PFC, significantly impairs performance on this task in a delay-dependent manner [34]. By contrast, lesions of the hippocampus do not disrupt performance and, if anything, facilitate working memory using the task shown in Figure 1C [34]. These data strongly suggest that any decline in delayed-response performance detected in aged rats is likely to be mediated by the PFC and not by the hippocampus. Indeed, while young and aged rats show comparable performance on the delayed-response task at short delays, aged rats are disproportionately impaired as the delays over which the subject must remember the lever location become longer [35] (Figure 1D). These data are highly consistent with findings from aged nonhuman primates and humans, which denote both robust age-related behavioral deficits using delayed-response tasks [36–39], and a decline in the electrophysiological signatures of working memory maintenance in the aged prefrontal cortex [40].
In agreement with the unique contributions of PFC and hippocampus to memory functions highlighted above, the neural substrates that are believed to enable long-term memory in hippocampus (Box 1) and working memory in PFC (Box 2) are also somewhat distinct. Notably, however, both require sustained excitation of pyramidal neurons as well as coordinated signaling from inhibitory interneurons that synthesize γ-aminobutyric acid (GABA). Under normal circumstances, interneurons regulate the excitation of individual pyramidal cells, and synchrony in neural networks, to establish the complex network dynamics that enable cognition [41,42]. Disruption of inhibitory signaling within cortical circuits is a central feature of cognitive disabilities across several psychiatric and neurodegenerative diseases [43,44]. Moreover, such cognitive deficits can be reproduced in experimental animal models in which normal GABAergic signaling has been disrupted [45,46]. This review will focus on age-related changes in inhibitory signaling that accompany aging, with an emphasis on changes that are likely to affect function through extrasynaptic or presynaptic high-affinity GABAergic receptors. These data will be reviewed in the context of both hippocampus- and PFC-dependent memory, and as the potential basis for therapeutic interventions for optimizing cognitive functioning at advanced ages.
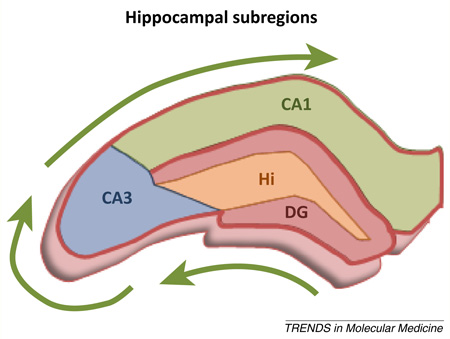
Box 1. The role of interneurons in hippocampal circuits that support spatial reference memory.
The hippocampus is anatomically subdivided into discrete subregions based on cellular and synaptic composition. Information flow is unidirectional: the dentate gyrus (DG) is the major input region of the hippocampus, and its constituent granule cells synapse upon CA3 pyramidal neurons that in turn project to CA1 pyramidal neurons (Figure I). Thus, DG–CA3–CA1 constitutes the classic trisynaptic pathway within which durable modifications to synaptic connections enable learning and memory. Importantly, the mechanisms of long-term synaptic modifications that support memory require highly-precise temporal and spatial dynamics that are established in part via GABAergic interneurons distributed throughout hippocampal subfields. Notably, the hilus contains interneurons that modulate both recurrent excitation between dentate granule cells, and glutamatergic mossy fiber projections from the DG to CA3 pyramidal cells. Recent work using optogenetic approaches demonstrates that reducing activity of hilar interneurons impairs spatial reference memory in rodents [122]. At the cellular level, optogenetic inhibition of hilar interneurons increases activity of DG granule cells. These data directly support a role for interneurons in memory and further indicate that alterations in interneuron function in the hippocampus of aged rodents could contribute to memory decline [71,123–125]. Future work using similar approaches to regulate other distinct subclasses of interneurons will be important for elucidating their role in different aspects of memory. With respect to this issue, it is notable that interneurons may not follow the unidirectional pathways described above and, instead, may help to integrate the hippocampus by making connections among all three subdivisions [126].
Figure I. Schematic depicts the unidirectional flow of information (green arrows) among synaptic connections between the dentate gyrus (DG; red), CA3 (blue), and CA1 (green). The hilus (Hi; orange) contains interneurons that innervate not only granule cells in the DG but also pyramidal neurons in the CA3.
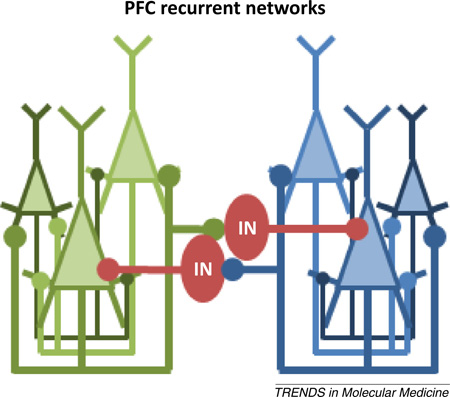
Box 2. The role of interneurons in PFC circuits that support working memory.
Unlike long-term memory, which requires persistent synaptic modification over days, weeks, and years, working memory requires neural activity that is only transiently stable (on the order of seconds), and that enables the information being held to be readily updated or modified. The cellular organization of the PFC is not unidirectional; instead, it is theorized to be comprised of many interconnected microcircuits that respond to specific, preferred stimuli (Figure I). Within this recurrent network, transient activation by a preferred stimulus will excite all other interconnected neurons and sustain activity that persists in the absence of continued sensory stimulation. This persistent firing is presumed to form the basis of mental representation that is working memory [127,128]. Lateral inhibition provided by interneurons (IN) enhances the relative strength of microcircuits that encode salient information by inhibiting neighboring microcircuits encoding irrelevant, competing representations [129]. Indeed, alterations to PFC interneurons are widely believed to cause disordered working memory in schizophrenic patients [44]. Prefrontal interneurons comprise diverse subtypes that differ based on spiking frequency, synaptic interactions, and molecular markers [129–131]. An important area of future work will need not only to clearly delineate what subtypes of interneurons are most affected by age or disease but also to determine the specific contributions of these individual interneuron subclasses to microcircuit function and, ultimately, to memory.
Figure I. Schematic depiction of two theoretical PFC microcircuits (denoted in green or blue) composed of reciprocally interconnected pyramidal neurons (triangles) that respond to a common stimulus; their sustained activation is presumed to enable the maintenance of a stable mental representation. Collaterals from each microcircuit also innervate an interneuron (IN, red circle) that laterally inhibits adjacent columns encoding competing representations.
Overview of inhibitory signaling systems
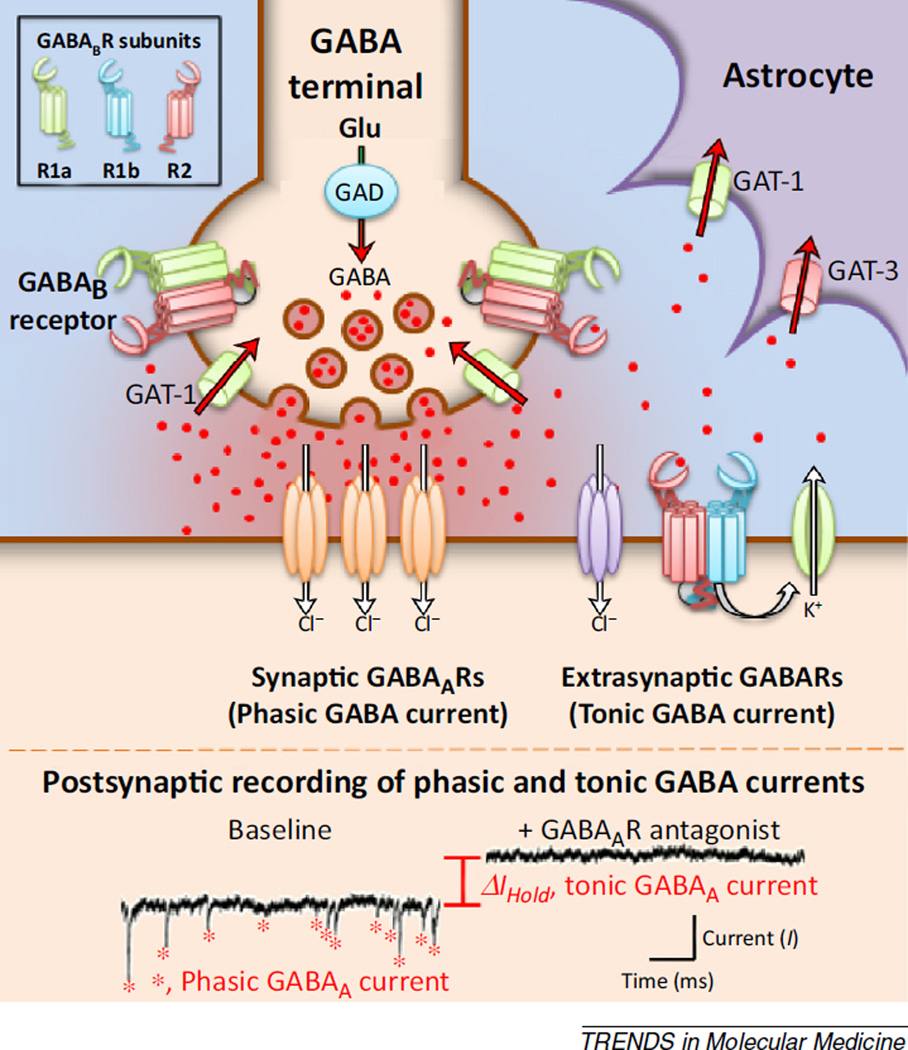
GABA is the primary inhibitory neurotransmitter in the mammalian central nervous system. It is synthesized in presynaptic terminals from L-glutamic acid via a reaction that depends on glutamic acid decarboxylase (GAD; Figure 2). It is loaded into synaptic vesicles by the vesicular GABA transporter (VGAT), and released in an activity-dependent manner when action potentials depolarize the terminal, causing calcium influx and subsequent exocytosis. High concentrations of GABA are transiently produced in the synaptic cleft following action potential-mediated exocytosis, and some synaptically released GABA is believed to escape (or ‘spillover’) from the synaptic cleft into the extracellular space [47,48]. The exact concentration of this extrasynaptic or ambient GABA depends on a complex interrelationship between this process of spillover and uptake, the latter of which is mediated by both neuronal and non-neuronal active transport mechanisms. Of these, GAT-1 is a primary neuronal GABA transporter, while GAT-3 is commonly associated with non-neuronal cells [49,50].
Figure 2.
Extrasynaptic GABAARs and GABABRs mediate tonic inhibition. The schematic shows a GABAergic synapse and diagrams key aspects of GABAergic signaling. GABA is synthesized from glutamate (Glu) in the presynaptic terminal by the enzyme glutamic acid decarboxylase (GAD), and is removed from the extracellular space following synaptic release via membrane-bound transporters, including the GABA transporter GAT-1, that are localized to neurons and astrocytes, and GAT-3, primarily localized to astrocytes. During activity-dependent release, GABA acts at chloride-permeant synaptic receptors (ionotropic GABAARs, shown in orange) positioned at postsynaptic sites that closely appose GABA terminals. In addition, GABA can spill over from the synaptic zone to activate high-affinity extrasynaptic GABAergic receptors, including some subtypes of GABAARs (purple receptors) as well as metabotropic GABABRs (green/blue/red receptors). Notably, GABABRs are heterodimers, containing an obligate R2 subunit and a variable R1a or R1b subunit (see inset at top left). GABABRs that contain R1a are preferentially targeted to presynaptic terminals where they serve as autoreceptors and regulate GABA release by blocking action potential-dependent exocytosis of GABA-containing vesicles. GABABRs that contain R1b are preferentially targeted to dendrites where they contribute to tonic inhibition through opening of potassium channels. The bottom panel shows representative traces from whole cell patchclamp recordings from a cortical neuron. The trace under baseline conditions shows phasic inhibitory postsynaptic currents (red stars) produced by spontaneous vesicular release of GABA and subsequent activation of synaptic GABAARs. Application of a GABAAR antagonist eliminates these phasic currents and also produces a change in the holding current (ΔIHold) of the postsynaptic cell. The change in holding current is produced by the blockade of extrasynaptic GABAARs tonically activated by low levels of ambient GABA.
Receptors for GABA (GABARs) are broadly divided based on whether they are ionotropic (GABAARs) or metabotropic (GABABRs), but activation of either subtype is predominantly inhibitory in mature neurons of the adult nervous system. Ionotropic GABAARs are permeant to chloride ions, and thus activation of GABAARs typically produces inhibition either by producing a hyperpolarizing current or through a process known as shunting inhibition. Hyperpolarization predominates in cells that have active transport mechanisms to expel chloride, while shunting inhibition plays a more prominent role in cells in which chloride is passively distributed. By contrast, inhibition through metabotropic GABABRs is produced through several possible Gi/o-dependent signaling cascades, typically leading to either opening of potassium-selective ion channels (Figure 2) or inhibition of calcium-selective channels.
The broad inhibitory actions of both GABAARs and GABABRs can further be divided into several more-specific categories typically referred to as phasic inhibition, tonic inhibition, and presynaptic inhibition. Phasic inhibition refers to inhibition produced by transient activation of GABARs in the synaptic cleft after activity-dependent exocytosis of GABA. This type of inhibition is mediated primarily by ionotropic GABAARs that contain the γ2 subunit [51,52], although GABABRs can also carry a somewhat slower component of the fast synaptic response. Tonic inhibition refers to inhibition mediated by activation of extrasynaptic GABARs that are expressed outside of the synaptic cleft. Across brain regions, this type of inhibition is often mediated by ionotropic GABAARs that contain the δ subunit [53,54]. In addition, the α5 subunit is robustly expressed in hippocampus [55–57], and extrasynaptic GABAARs containing the α5 subunit have been shown to mediate tonic inhibition in both hippocampus proper and the dentate gyrus [58,59]. Beyond GABAARs, extrasynaptic GABABRs also support tonic inhibitory currents in some brain regions, including PFC [48]. Finally, presynaptic inhibition refers to inhibition of activity-dependent exocytosis of neurotransmitters following activation of GABARs expressed on the presynaptic terminal. GABAergic autoreceptors are expressed on axon terminals that release GABA, while GABAergic heteroreceptors are expressed on other types of axon terminals [60,61].
Accumulating data highlight tonic inhibition as a particularly important regulator of neuronal activity and network dynamics in cortical circuits. Indeed, extrasynaptic GABARs are already attractive therapeutic targets for treatment of a broad range of neuropsychiatric disorders including depression, schizophrenia, and Parkinson’s disease [62–66]. Recent evidence further indicates that GABARs that mediate tonic inhibition are altered in aged hippocampus and PFC, suggesting that normalizing inhibitory signaling at these extrasynaptic receptors may also be of utility in cognitive dysfunction that accompanies the normal aging process.
GABAergic signaling alterations in aged hippocampus and impact on spatial memory
Molecular and cellular alterations in hippocampal GABA signaling
Inhibitory interneurons of the hippocampus undergo marked molecular and phenotypic alterations across the lifespan. In particular, beginning in middle-age, many interneurons cease to express GAD [67]. This reduction would be expected to confer less GABA synthesis and a consequent increase in hippocampal excitability. Consistent with this interpretation, and with data from neuropsychiatric conditions in which aberrant excitation is believed to contribute to memory impairment [68–70], the magnitude of decline in GAD-expressing neurons in the hilar region of dentate gyrus strongly predicts the severity of spatial memory deficits among aged rats [71]. Electrophysiological studies provide further evidence for anomalous GABAergic signaling in the aged hippocampus. Specifically, whole cell patch-clamp electrophysiology has been used to isolate and evaluate GABA-mediated currents in individual cells in young and aged hippocampal slices. Studies employing such methods demonstrate a reduced frequency of spontaneous inhibitory postsynaptic potentials (IPSPs) in granule cells of the aged dentate gyrus [72]. Moreover, both the amplitude and frequency of GABAAR-mediated currents, as well as the amplitude and duration of the slow GABABR-mediated IPSP, are attenuated in aged CA1 pyramidal neurons [73,74].
Although parallel in vitro electrophysiological studies have not been conducted in CA3 pyramidal neurons, substantial evidence affirms that aging is accompanied by attenuated GABAergic signaling and aberrant excitability in this hippocampal subregion. Specifically, expression of gabra5, that encodes the α5 GABAAR subunit [75], is reduced in CA3 of aged rats in comparison to young adults. As described above, GABAARs containing the α5 subunit are localized extrasynaptically, and these receptors can contribute to tonic inhibition in the hippocampus. In addition to GABAARs, expression of the GABABR1 subunit is also reduced throughout the hippocampus of memory-impaired aged rats. Notably, however, lower expression of GABABR1 is only accompanied by a reduction in GABABR coupling to its effector G protein in CA3, suggesting that age-related GABABR signaling dysfunction may be particularly pronounced in this hippocampal subregion [11,13]. Functional consequences for these age-related alterations in GABARs findings are illustrated by in vivo electrophysiological studies in which CA3 pyramidal neuron activity was monitored in behaving young and aged rats. Specifically, these studies revealed an age-related increase in the average and maximal firing rates of CA3 pyramidal neurons which was coupled to reduced specificity and plasticity associated with the encoding of spatial information [76,77].
GABAergic neuropharmacology and memory function
The findings described above indicate that GABA signaling is attenuated in the aged hippocampus, and provide a compelling rationale for targeting aberrant excitation as a treatment to improve memory function. Building on this rationale, the commonly prescribed anti-epileptic levetiracetam has been recently shown to enhance the retention of newly learned information over a 24 h time-period in aged rats [78]. While levetiracetam is known to reduce hippocampal excitation, it should be noted that the specific mechanism of action for such effects remains unclear and may not involve direct actions at GABARs. Indeed, it has been suggested that this drug may regulate cellular excitation by inhibition of neurotransmitter release via modulation of calcium channels [79,80]. Notably, however, other pharmacological approaches that more specifically target extrasynaptic GABARs also benefit memory function in rodent models of age-related memory decline. In particular, administration of positive allosteric modulators of α5-containing GABAARs improves performance of aged rats in several tests of hippocampus-dependent memory [81]. As the expression of α5-containing GABAARs is largely restricted to the hippocampal formation, these findings provide initial evidence that normalizing hippocampal hyper-excitability via enhancement of tonic inhibition may be beneficial for memory function in aging.
GABAergic signaling alterations in aged prefrontal cortex: impact on working memory
Molecular and cellular alterations in PFC GABA signaling
In agreement with findings from hippocampus, there is a reduction in the number of GAD-expressing cells in aged PFC. Notably, however, such reductions are not unique to interneurons in PFC because a subtle reduction in the number of excitatory pyramidal neurons has also been reported in this brain region [82–84]. Moreover, the density of both symmetric (i.e., inhibitory) and asymmetric (i.e., excitatory) synapses decreases with age in the primate PFC [85]. Consistent with the structural data, magnetic resonance spectroscopy of the human brain reveals that both PFC GABA and glutamate concentrations decline in an age-dependent manner, beginning in middle-age [86]. While this body of work strongly supports an age-related reduction in overall PFC neurotransmission, these data do not reveal whether aging shifts PFC neural circuits towards greater or less excitability.
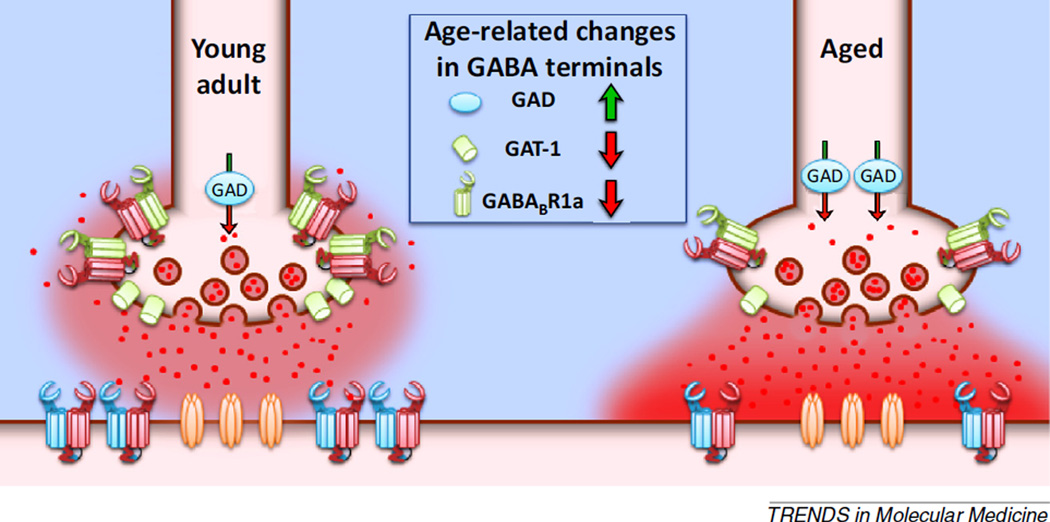
More recent findings, however, are beginning to reveal age-related cellular and molecular changes in PFC, that, unlike in hippocampus, support increased inhibition with advancing age. For example, one study evaluating GABA-signaling protein expression in microdissected rat mPFC showed an increase in expression of GAD, the enzyme important for GABA synthesis, and a decrease in expression of the GABA transporter, GAT-1 [14], in aged PFC relative to young adult (Figure 3). These biochemical data suggest that there is increased production but decreased clearance of extracellular GABA in the aged PFC, findings which together suggest that aged pyramidal neurons in this brain region may be subject to increased inhibition.
Figure 3.
Age-related dysregulation of tonic GABA signaling at prefrontal cortex (PFC) synapses. The schematic shows representative presynaptic terminals from young adult and aged rat PFC that illustrate age-related changes in GABAergic signaling protein expression. Presynaptically, expression of glutamic acid decarboxylase (GAD) is upregulated in the aged PFC, and there is a concurrent reduction in expression of GABA transporter GAT-1 and GABABR1a [11,14]. These changes suggest an imbalance in the capacity to produce GABA relative to the ability to clear synaptic GABA or inhibit its further release. This biochemical evidence is corroborated by electrophysiological findings that suggest that the loss of autoreceptors manifests as a reduction in presynaptic GABA tone that normally limits further GABA release [89]. Consequently, there is an increase in extracellular GABA that increases the tonic activation of postsynaptic GABA receptors [89]. Together, these alterations would be expected to confer an agerelated increase in basal inhibition of PFC pyramidal neurons.
Support for this interpretation comes from whole cell patch-clamp electrophysiology studies that have directly assessed GABA activity at PFC synapses. Using this approach, one study found an age-related increase in miniature, but not spontaneous, inhibitory postsynaptic currents (IPSCs) in pyramidal neurons from aged rats that exhibited behavioral impairments [87]. Moreover, in primates, the frequency of spontaneous, but not miniature, ISPCs reportedly increases with age [88]. While both studies provide support for an age-related increase in inhibitory transmission within the PFC, the mechanism for this enhancement is less clear. Recent work has begun to address this issue, building on data from young animals showing that GABABRs can mediate tonic inhibition of PFC pyramidal neurons [48]. Specifically, studies compared tonic activation of presynaptic GABAB autoreceptors expressed on inhibitory inputs to pyramidal cells in young and aged rats [89]. The GABABR agonist baclofen significantly reduced evoked IPSC amplitude in both young and aged PFC neurons, but the magnitude of this baclofen-mediated inhibition was attenuated in aged neurons. These findings suggest that there is an age-related reduction in functional GABAB autoreceptors localized to inhibitory inputs onto pyramidal neurons, and this possibly contributes to dysregulated autoregulation of GABA release (Figure 3). Subsequent experiments revealed further age-related differences in GABAB autoreceptors. Specifically, in young neurons, application of a GABABR antagonist shifted evoked IPSC amplitudes above baseline levels, indicating the presence of tonic GABAB autoreceptor activity. This GABABR antagonist-mediated shift was significantly attenuated in aged neurons, indicating that there is an age-related loss of tonic inhibition at these GABAB autoreceptors. Broadly, this body of work shows that aging is associated with a reduction in GABABR autoregulation of GABA release, which would be expected to increase basal inhibition of pyramidal neurons. This conclusion has been directly supported by findings from other experiments in which selective antagonists of GABAA and GABAB receptors reveal an increase in tonic activation of these extrasynaptic receptors on PFC pyramidal neurons [89] (Figure 3).
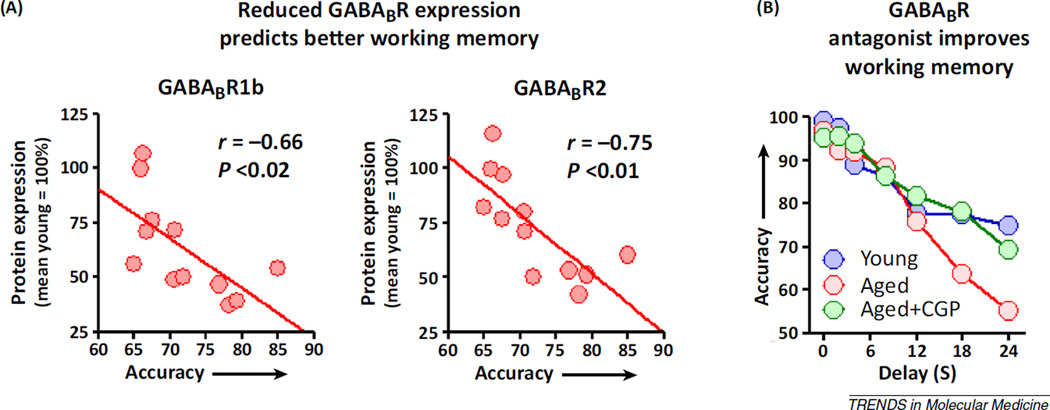
Overall, the convergent findings from the biochemical and electrophysiological experiments support an increase in tonic inhibition of aged PFC pyramidal neurons. Potentially in response to increased GABA availability, both the GABABR1 and GABABR2 subunits, which together form the functional GABABR complex [90,91], are significantly reduced in the aged PFC, as is maximal baclofen-stimulated GTP exchange, a measure of GABABR:G-protein coupling [11,14]. Notably, downregulation of GABABRs in aging might be expected to compensate for age-related elevations in extracellular GABA, thereby preserving an optimal level of excitation required for working memory (Box 2) [92]. Consistent with this interpretation, the expression of both subunits constituting postsynaptic GABABRs (R1b and R2) strongly predicts working memory abilities, such that lower GABABR expression is associated with greater accuracy on a delayed-response task (Figure 4A) [14].
Figure 4.
Targeting GABABR signaling to improve working memory. (A) Likely in response to excess GABABR-mediated inhibition, expression of the subunits that together form postsynaptic GABABRs (GABABR1b and GABABR2) is reduced in the aged medial prefrontal cortex (mPFC) [11,14]. The downregulation of postsynaptic GABABRs receptor might serve to compensate for the excess inhibition described above as well as help to preserve the persistent activity of pyramidal neurons that is required for working memory maintenance. In support of this hypothesis, the bottom-right panel shows that, among aged rats, lower GABABR1b and GABABR2 expression strongly predicts better working memory. (B) If the downregulation of GABABRs is a crucial mechanism for preserved working memory in aging, then blockade of GABABR signaling should restore working memory in impaired aged subjects. The line graph shows performance of a cohort of aged rats (red circles) that are impaired relative to young adult rats (blue circles) on the delayed-response task. Using a within-subjects design, in which the same aged subjects shown in red were administered a GABABR antagonist CGP55845 (CGP) directly into the PFC (green circles), aged rat performance is restored to that of young adults. Data reproduced from [14].
GABAergic neuropharmacology and memory function
The molecular and electrophysiological analyses described above demonstrate that tonic inhibition is increased in the aged PFC, and that lower expression of the subunits that comprise postsynaptic GABABRs is strongly associated with better working memory abilities among aged rats. Together these findings provide a solid rationale for blocking excess GABA signaling at extrasynaptic GABABRs to improve working memory function in aging. As an initial validation of this approach, direct administration of a GABABR antagonist into the mPFC of aged rats significantly improves working memory function on a delayed-response task, such that aged rats reach a level of performance on a par with young controls [14] (Figure 4B). These findings were replicated in aged rats using a systemic route of administration, extending the preclinical validation of GABABRs as a target for age-related decline in working memory. It is notable that, unlike many pharmacotherapies that can improve cognition, the benefit of GABABR antagonists was restricted to aged rats. Indeed, systemic administration of a GABABR antagonist in this study impaired working memory abilities in young rats [14]. These data, considered together with the electrophysiological studies described above, suggest that the differential behavioral effects of GABABR antagonists in young and aged rats may be mediated by the relative actions of the antagonist in blocking tonic GABA at presynaptic versus postsynaptic sites. Because antagonists exert no direct biological actions, any behavioral output conferred by a GABABR antagonist will result from displacement of endogenous GABA at these receptors. Specifically, experiments described above suggest that the primary GABAergic tone in young rats is present on presynaptic GABABRs. The antagonist acting at these receptors would be expected to block the autoregulation of GABA release, and result in increased extracellular GABA and increased pyramidal neuron inhibition. By contrast, this presynaptic GABAergic tone is lost in aging, suggesting that a GABABR antagonist administered in the aged PFC preferentially blocks activation of postsynaptic GABABRs and thus reduces pyramidal neuron inhibition (Figure 3).
Targeting inhibitory signaling in humans as a treatment for age-related cognitive decline
Given its history as a treatment for epilepsy, and the preclinical data demonstrating spatial memory improvement in aged rodents, levetiracetam represents a tractable clinical candidate for treating age-related decline of hippocampus-dependent memory. Human neuroimaging studies support a relationship between increased hippocampal excitability and memory dysfunction in aging [93,94]. In particular, patients diagnosed with mild cognitive impairment (MCI), who have clinically detectable memory loss and an increased propensity to develop AD, show significantly increased hippocampal activity compared to normal controls [95–98]. A recent study investigating the effects of levetiracetam in MCI patients reported normalization of DG/CA3 activity and improved recall in a memory task [99]. These data provide an initial proof-of-concept that reducing hippocampal activity may be useful in treating some forms of age-related memory decline.
There have also been initial studies investigating GABABRs as a target for alleviating memory decline, with a focus on MCI and AD [100]. One GABABR antagonist reached clinical trials for cognition, and this compound was initially tested in MCI patients [101,102]. Patients were treated for 2 months and efficacy was evaluated periodically over the course of the study. Individuals given the GABABR antagonist showed significant improvement in working memory, psychomotor speed, and attention compared to placebo controls. An expanded Phase II trial was then conducted in patients meeting the diagnostic criteria for AD, but failed to meet its clinical endpoint. Importantly, the AD patient population may not be the most appropriate for GABABR antagonists because these drugs are likely to have their greatest effects in individuals lacking pronounced neurodegeneration [103,104]. Nevertheless, the data from MCI patients are consistent with recent observations in aged rats, and further underscore the potential efficacy of GABABR antagonists as a treatment for PFC-mediated cognitive decline in normal aging.
Concluding remarks and future perspectives
The divergent changes in GABAergic signaling that occur in aged hippocampus and PFC offer unique challenges from the perspective of treating memory decline. Based on the mechanistic data described above, therapeutics that target inhibitory changes in one brain region might be expected to exacerbate age-related dysregulation of GABAergic signaling in another. Indeed, there is a precedent suggesting that different therapeutic strategies may be required for improving memory functions supported by the hippocampus and PFC, specifically with respect to therapeutics that target cAMP/protein kinase A (PKA) signaling. Whereas cAMP activators hold promise for the alleviation of hippocampus-dependent memory impairment [105,106], overactivation of cAMP/PKA signaling in the PFC likely contributes to the manifestation of agerelated working memory decline [107–109]. As such, guanfacine, a negative regulator of cAMP/PKA signaling via α2-adrenergic receptors, has emerged as a promising clinical candidate for the improvement of PFC-dependent working memory in older individuals [40,110,111]. Notably, however, the cognitive benefit of guanfacine does not extend to hippocampus-dependent memory [112]. With respect to the region-specific shifts in tonic inhibition described herein, there are some clear directions for future investigation that could clarify strategies for normalizing inhibitory signaling and improving memory function in aging. First, GABA receptor subtypes that are differentially expressed in hippocampus and PFC could be targeted to specifically correct inhibitory signaling disruptions in one brain region while minimizing the influence on the other. For example, given the selective expression of the α5 GABAAR subunit in hippocampus, systemic administration of positive α5 allosteric modulators is likely to specifically influence GABAergic signaling within this brain region. Additional information regarding concurrent changes to excitatory signaling might reveal other receptor candidates to target for normalizing age-related signaling dysfunction. Indeed, while this review has focused on inhibitory signaling alterations, such changes are tightly linked to excitatory neurotransmission, and it is essential to determine whether there are endogenous, adaptive changes to excitatory signaling pathways in aging that attempt to counteract or rebalance the normal excitatory–inhibitory dynamic. Such compensatory mechanisms may represent important therapeutic targets for treating memory dysfunction.
It should be noted that, although GABABRs are widely distributed in brain, a body of work in rodent models indicates that GABABR antagonists do not impair performance on hippocampus-dependent memory assessments and that these drugs can actually enhance performance under some conditions [113,114]. The mechanisms for such enhancement are not well understood, but it is plausible that these compounds attenuate aberrant excitation via modulation of GABABR-mediated autoregulation of GABA release. Notably, the hippocampus and PFC are interconnected, and neurobiological alterations in one region are likely to impact upon the other. For example, working memory aids in the initial encoding of episodic or reference memory. At the same time, retrieval of information from long-term memory into working memory stores is important for appropriate guidance of ongoing behavior. An important topic for future investigation concerns better understanding how modulation of inhibition in hippocampus or PFC impacts on the functional connectivity between these regions and within the larger brain circuits that support cognition. Such data may provide insights regarding the clinical approaches that are likely to have the greatest impact in treating cognitive impairment in aging [115,116].
Beyond strategies that uniquely regulate inhibitory/ excitatory dynamics in either the PFC or hippocampus, additional investigation directed toward uncovering the causative mechanisms responsible for age-related inhibitory signaling dysfunction may reveal new targets for treating age-related memory decline. For example, emerging evidence suggests that GABAergic interneurons may be particularly vulnerable to the effects of psychogenic stress [117,118]. Because dysregulation of the hypothalamus–pituitary–adrenal (HPA) axis and protracted exposure to glucocorticoids is a well-documented feature of normal aging [8,119–121], normalization of glucocorticoid signaling represents one avenue to explore for the prevention of inhibitory signaling dysfunction across both PFC and hippocampus.
Highlights.
Prefrontal cortex and hippocampus support memory functions that decline with age.
GABA-mediated inhibition plays a crucial role in neural circuits that support memory.
Aging results in altered GABAergic signaling in prefrontal cortex and hippocampus.
Treatments that normalize GABA signaling may improve memory in aging.
Acknowledgments
We thank Dr Barry Setlow for critical comments on the manuscript. Supported by AG029421 (J.L.B.), the McKnight Brain Research Foundation (J.L.B., C.J.F.), and a McKnight Brain Institute Fellowship (J.A.M.).
References
- 1.Wagster MV, et al. The 87% J. Gerontol. A. Biol. Sci. Med. Sci. 2012;67:739–740. doi: 10.1093/gerona/gls140. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, et al. Characterizing cognitive aging in humans with links to animal models. Front. Aging Neurosci. 2012;4:21. doi: 10.3389/fnagi.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bizon JL, et al. Characterizing cognitive aging of working memory and executive function in animal models. Front. Aging Neurosci. 2012;4:19. doi: 10.3389/fnagi.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster TC, et al. Characterizing cognitive aging of spatial and contextual memory in animal models. Front. Aging Neurosci. 2012;4:12. doi: 10.3389/fnagi.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 2012;13:240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu. Rev. Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- 7.Smith TD, et al. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J. Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bizon JL, et al. Hypothalamic–pituitary–adrenal axis function and corticosterone receptor expression in behaviourally characterized young and aged Long–Evans rats. Eur. J. Neurosci. 2001;14:1739–1751. doi: 10.1046/j.0953-816x.2001.01781.x. [DOI] [PubMed] [Google Scholar]
- 9.Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur. J. Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H-Y, et al. Muscarinic receptor-mediated GTP-Eu binding in the hippocampus and prefrontal cortex is correlated with spatial memory impairment in aged rats. Neurobiol. Aging. 2007;28:619–626. doi: 10.1016/j.neurobiolaging.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 11.McQuail JA, et al. GABAB receptor GTP-binding is decreased in the prefrontal cortex but not the hippocampus of aged rats. Neurobiol. Aging. 2012;33:1124.e1–1124.e12. doi: 10.1016/j.neurobiolaging.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bañuelos C, et al. Age-related changes in rostral basal forebrain cholinergic and GABAergic projection neurons: relationship with spatial impairment. Neurobiol. Aging. 2013;34:845–862. doi: 10.1016/j.neurobiolaging.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McQuail JA, et al. Hippocampal Gαq/11 but not Gαo-coupled receptors are altered in aging. Neuropharmacology. 2013;70:63–73. doi: 10.1016/j.neuropharm.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banñuelos C, et al. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J. Neurosci. 2014;34:3457–3466. doi: 10.1523/JNEUROSCI.5192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris RGM. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981;12:239–260. [Google Scholar]
- 16.Morris RG, et al. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 17.Moser E, et al. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J. Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffenach H-A, et al. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher M, et al. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 20.Bizon JL, et al. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol. Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McQuail JA, Nicolle MM. Spatial reference memory in normal aging Fischer 344 × Brown Norway F1 hybrid rats. Neurobiol. Aging. 2015;36:323–333. doi: 10.1016/j.neurobiolaging.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Driscoll I, et al. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm. Behav. 2005;47:326–335. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Moffat SD, Resnick SM. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav. Neurosci. 2002;116:851–859. doi: 10.1037//0735-7044.116.5.851. [DOI] [PubMed] [Google Scholar]
- 24.Moffat SD, et al. Extrahippocampal contributions to age differences in human spatial navigation. Cereb. Cortex. 2007;17:1274–1282. doi: 10.1093/cercor/bhl036. [DOI] [PubMed] [Google Scholar]
- 25.West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol. Aging. 1993;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen T, et al. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol. Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- 27.Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 29.Uylings HBM, et al. Do rats have a prefrontal cortex? Behav. Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Floresco SB, Jentsch JD. Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology. 2011;36:227–250. doi: 10.1038/npp.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol. Learn. Mem. 2011;96:417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez JS, Paule MG. Working memory delayed response tasks in monkeys. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edn. CRC Press; 2009. [PubMed] [Google Scholar]
- 33.Hara Y, et al. Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age. 2012;34:1051–1073. doi: 10.1007/s11357-011-9278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sloan HL, et al. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav. Brain Res. 2006;171:116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 35.Beas BS, et al. Distinct manifestations of executive dysfunction in aged rats. Neurobiol. Aging. 2013;34:2164–2174. doi: 10.1016/j.neurobiolaging.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J. Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnsten AFT, Goldman-Rakic PS. Analysis of alpha-2 adrenergic agonist effects on the delayed nonmatch-to-sample performance of aged rhesus monkeys. Neurobiol. Aging. 1990;11:583–590. doi: 10.1016/0197-4580(90)90021-q. [DOI] [PubMed] [Google Scholar]
- 38.Bachevalier J, et al. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol. Aging. 1991;12:99–111. doi: 10.1016/0197-4580(91)90048-o. [DOI] [PubMed] [Google Scholar]
- 39.O’Donnell KA, et al. Preservation of prefrontal cortical volume in behaviorally characterized aged macaque monkeys. Exp. Neurol. 1999;160:300–310. doi: 10.1006/exnr.1999.7192. [DOI] [PubMed] [Google Scholar]
- 40.Wang M, et al. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr. Opin. Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 42.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleschevnikov AM, et al. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J. Neurosci. 2004;24:8153–8160. doi: 10.1523/JNEUROSCI.1766-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez-Burgos G, et al. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enomoto T, et al. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol. Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 46.Auger ML, Floresco SB. Prefrontal cortical GABA modulation of spatial reference and working memory. Int. J. Neuropsychopharmacol. 2014 doi: 10.1093/ijnp/pyu013. Published online October 31, 2014. http://dx.doi.org/10.1093/ijnp/pyu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J. Physiol. 2007;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, et al. GABAB receptor-dependent modulation of network activity in the rat prefrontal cortex in vitro. Eur. J. Neurosci. 2010;31:1582–1594. doi: 10.1111/j.1460-9568.2010.07191.x. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Burgos G, et al. GABA transporter GAT1 prevents spillover at proximal and distal GABA synapses onto primate prefrontal cortex neurons. J. Neurophysiol. 2009;101:533–547. doi: 10.1152/jn.91161.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kersanté F, et al. A functional role for both γ-aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. J. Physiol. 2013;591:2429–2441. doi: 10.1113/jphysiol.2012.246298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schweizer C, et al. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol. Cell. Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 52.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 53.Brickley SG, et al. Adaptive regulation of neuronal excitability by a voltage- independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- 54.Stell BM, et al. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sur C, et al. Autoradiographic localization α subunitcontaining GABAA receptors in rat brain. Brain Res. 1999;822:265–270. doi: 10.1016/s0006-8993(99)01152-x. [DOI] [PubMed] [Google Scholar]
- 56.Turgeon SM, Albin RL. GABAB binding sites in early adult and aging rat brain. Neurobiol. Aging. 1994;15:705–711. doi: 10.1016/0197-4580(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 57.Wisden W, et al. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glykys J, et al. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor α5 subunit–deficient mice. J. Neurophysiol. 2006;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- 60.Vigot R, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waldmeier PC, et al. Roles of GABAB receptor subtypes in presynaptic auto- and heteroreceptor function regulating GABA and glutamate release. J. Neural Transm. 2008;115:1401–1411. doi: 10.1007/s00702-008-0095-7. [DOI] [PubMed] [Google Scholar]
- 62.Brickley SG, Mody I. Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deidda G, et al. Modulation of GABAergic transmission in development and neurodevelopmental disorders: investigating physiology and pathology to gain therapeutic perspectives. Front. Cell. Neurosci. 2014;8:119. doi: 10.3389/fncel.2014.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavlov I, Walker MC. Tonic GABAA receptor-mediated signalling in temporal lobe epilepsy. Neuropharmacology. 2013;69:55–61. doi: 10.1016/j.neuropharm.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Rudolph U, Möhler H. GABAA receptor subtypes: therapeutic potential in down syndrome, affective disorders, schizophrenia, and autism. Annu. Rev. Pharmacol. Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whissell PD, et al. Altered expression of δGABAA receptors in health and disease. Neuropharmacology. 2015;88:24–35. doi: 10.1016/j.neuropharm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Stanley DP, Shetty AK. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J. Neurochem. 2004;89:204–216. doi: 10.1111/j.1471-4159.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 68.Ylinen AM, et al. Enhanced GABAergic inhibition preserves hippocampal structure and function in a model of epilepsy. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7650–7653. doi: 10.1073/pnas.88.17.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lukoyanov NV, et al. Selective loss of hilar neurons and impairment of initial learning in rats after repeated administration of electroconvulsive shock seizures. Exp. Brain Res. 2004;154:192–200. doi: 10.1007/s00221-003-1658-3. [DOI] [PubMed] [Google Scholar]
- 70.Zhou J-L, et al. Effect of levetiracetam on visual-spatial memory following status epilepticus. Epilepsy Res. 2007;73:65–74. doi: 10.1016/j.eplepsyres.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 71.Spiegel AM, et al. Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J. Comp. Neurol. 2013;521:3508–3523. doi: 10.1002/cne.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patrylo PR, et al. Dentate filter function is altered in a proepileptic fashion during aging. Epilepsia. 2007;48:1964–1978. doi: 10.1111/j.1528-1167.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 73.Potier B, et al. Age-related alterations of GABAergic input to CA1 pyramidal neurons and its control by nicotinic acetylcholine receptors in rat hippocampus. Neuroscience. 2006;142:187–201. doi: 10.1016/j.neuroscience.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 74.Potier B, et al. Alterations in the properties of hippocampal pyramidal neurons in the aged rat. Neuroscience. 1992;48:793–806. doi: 10.1016/0306-4522(92)90267-6. [DOI] [PubMed] [Google Scholar]
- 75.Haberman RP, et al. Prominent hippocampal CA3 gene expression profile in neurocognitive aging. Neurobiol. Aging. 2011;32:1678–1692. doi: 10.1016/j.neurobiolaging.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson IA, et al. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol. Aging. 2003;24:297–305. doi: 10.1016/s0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- 77.Wilson IA, et al. Age-associated alterations of hippocampal place cells are subregion specific. J. Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koh MT, et al. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2009;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poulain P, Margineanu DG. Levetiracetam opposes the action of GABAA antagonists in hypothalamic neurones. Neuropharmacology. 2002;42:346–352. doi: 10.1016/s0028-3908(01)00185-x. [DOI] [PubMed] [Google Scholar]
- 80.Surges R, et al. Is levetiracetam different from other antiepileptic drugs? Levetiracetam and its cellular mechanism of action in epilepsy revisited. Ther. Adv. Neurol. Disord. 2008;1:13–24. doi: 10.1177/1756285608094212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koh MT, et al. Selective GABAA α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology. 2013;64:145–152. doi: 10.1016/j.neuropharm.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stranahan AM, et al. Aging reduces total neuron number in the dorsal component of the rodent prefrontal cortex. J. Comp. Neurol. 2012;520:1318–1326. doi: 10.1002/cne.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yates MA, et al. Regional variability in age-related loss of neurons from the primary visual cortex and medial prefrontal cortex of male and female rats. Brain Res. 2008;1218:1–12. doi: 10.1016/j.brainres.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith DE, et al. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J. Neurosci. 2004;24:4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peters A, et al. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grachev ID, Apkarian AV. Aging alters regional multichemical profile of the human brain: an in vivo1H-MRS study of young versus middle-aged subjects. J. Neurochem. 2001;76:582–593. doi: 10.1046/j.1471-4159.2001.00026.x. [DOI] [PubMed] [Google Scholar]
- 87.Bories C, et al. Differential balance of prefrontal synaptic activity in successful versus unsuccessful cognitive aging. J. Neurosci. 2013;33:1344–1356. doi: 10.1523/JNEUROSCI.3258-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luebke JI, et al. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125:277–288. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 89.Carpenter HE, et al. Age-related changes in GABA-B receptor mediated inhibitory tone in the medial prefrontal cortex. [November 9–13, 2013];Proceedings Neuroscience. 2013 2013 A91.02. [Google Scholar]
- 90.Robbins MJ, et al. GABA(B2) is essential for G-protein coupling of the GABA(B) receptor heterodimer. J. Neurosci. 2001;21:8043–8052. doi: 10.1523/JNEUROSCI.21-20-08043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Margeta-Mitrovic M, et al. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 92.González-Maeso J, et al. Agonist-induced desensitization and endocytosis of heterodimeric GABAB receptors in CHO-K1 cells. Eur. J. Pharmacol. 2003;481:15–23. doi: 10.1016/j.ejphar.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 93.Yassa MA, et al. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yassa MA, et al. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dickerson BC, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dickerson BC, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Celone KA, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J. Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sperling RA, et al. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bakker A, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Froestl W. Chemistry and pharmacology of GABAB receptor ligands. Adv. Pharmacol. 2010;58:19–62. doi: 10.1016/S1054-3589(10)58002-5. [DOI] [PubMed] [Google Scholar]
- 101.Froestl W, et al. SGS742: the first GABA(B) receptor antagonist in clinical trials. Biochem. Pharmacol. 2004;68:1479–1487. doi: 10.1016/j.bcp.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 102.Bullock R. SGS-742 Novartis. Curr. Opin. Investig. Drugs. 2005;6:108–113. [PubMed] [Google Scholar]
- 103.Golde TE, et al. Anti-Aβ therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron. 2011;69:203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reiman EM, et al. Considerations in the design of clinical trials for cognitive aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2012;67:766–772. doi: 10.1093/gerona/gls124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arnsten AFT, et al. Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. Trends Mol. Med. 2005;11:121–128. doi: 10.1016/j.molmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 106.Rose GM, et al. Phosphodiesterase inhibitors for cognitive enhancement. Curr. Pharm. Des. 2005;11:3329–3334. doi: 10.2174/138161205774370799. [DOI] [PubMed] [Google Scholar]
- 107.Ramos BP, et al. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- 108.Ramos BP, et al. α2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learn. Mem. 2006;13:770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang M, et al. α2A-adrenoceptors strengthen working memory networks by inhibiting cAMP–HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 110.Arnsten AF, et al. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J. Neurosci. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chapman SB, et al. Clinical trials: new opportunities. J. Gerontol. A. Biol. Sci. Med. Sci. 2012;67:773–780. doi: 10.1093/gerona/gls126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sirviö J, et al. The effects of guanfacine, alpha-2 agonist, on the performance of young and aged rats in spatial navigation task. Behav. Neural Biol. 1991;56:101–107. doi: 10.1016/0163-1047(91)90327-m. [DOI] [PubMed] [Google Scholar]
- 113.Helm KA, et al. GABAB receptor antagonist SGS742 improves spatial memory and reduces protein binding to the cAMP response element (CRE) in the hippocampus. Neuropharmacology. 2005;48:956–964. doi: 10.1016/j.neuropharm.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 114.LaSarge CL, et al. Blockade of GABA(B) receptors completely reverses age-related learning impairment. Neuroscience. 2009;164:941–947. doi: 10.1016/j.neuroscience.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 116.Eyler LT, et al. A review of functional brain imaging correlates of successful cognitive aging. Biol. Psychiatry. 2011;70:115–122. doi: 10.1016/j.biopsych.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu W, et al. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology. 2010;35:1693–1707. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gilabert-Juan J, et al. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct. Funct. 2013;218:1591–1605. doi: 10.1007/s00429-012-0479-1. [DOI] [PubMed] [Google Scholar]
- 119.Lupien S, et al. Basal cortisol levels and cognitive deficits in human aging. J. Neurosci. 1994;14:2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Montaron MF, et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol. Aging. 2006;27:645–654. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 121.Anderson RM, et al. Adrenocortical status predicts the degree of age-related deficits in prefrontal structural plasticity and working memory. J. Neurosci. 2014;34:8387–8397. doi: 10.1523/JNEUROSCI.1385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Andrews-Zwilling Y, et al. Hilar GABAergic interneuron activity controls spatial learning and memory retrieval. PLoS ONE. 2012;7:e40555. doi: 10.1371/journal.pone.0040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Andrews-Zwilling Y, et al. Apolipoprotein E4 vauses age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Palop JJ, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Knoferle J, et al. Apolipoprotein E4 produced in GABAergic interneurons causes learning and memory deficits in mice. J. Neurosci. 2014;34:14069–14078. doi: 10.1523/JNEUROSCI.2281-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sik A, et al. Inhibitory CA1-CA3–hilar region feedback in the hippocampus. Science. 1994;265:1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- 127.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 128.Arnsten AFT, et al. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang X-J, et al. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J. Neurosci. 1995;15:2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]